Abstract
Human subjects with impaired baroreflex function cannot buffer rises or falls in BP, thus allowing BP effects of endogenous or environmental stimuli that previously escaped detection to emerge dramatically. Studies in these patients led us to discover that water ingestion induced a robust increase in BP and vascular resistance. Here, using a mouse model of baroreflex impairment, we show that the increase in blood pressure after water ingestion is mediated through sympathetic nervous system activation and that the osmosensitive transient receptor potential vanilloid 4 channel (Trpv4) is an essential component of the response. Although portal osmolality decreases after water ingestion in both wild-type and Trpv4−/− mice, only the wild-type animals show a pressor response. The same volume of physiologic saline does not elicit an increase in BP, suggesting osmolality as the stimulus. The osmopressor response to water, and Trpv4 thus represent new factors now implicated in the physiology of BP regulation.
Keywords: Trpv4, blood pressure, osmopressor, sympathetic nervous system, baroreflex
Introduction
Studies in patients with baroreflex impairment led us to discover that water ingestion increases BP and vascular resistance. We found that ingestion of 16 oz (473 mL) water induces a profound increase in systolic BP averaging ~40 mmHg, with occasional increases >75 mmHg. The effect appears within 10 minutes, is maximal at 25–40 minutes, and largely dissipates by 90 minutes after ingestion.. While water’s effect was greatest in individuals with impaired baroreflex buffering, it was also present in healthy persons. In healthy young subjects with intact baroreflexes, water elicits an increase in peripheral vascular resistance without an increase in BP because of a compensatory reduction in cardiac output (1). Importantly, water ingestion raises plasma norepinephrine (NE) but not renin or vasopressin, supporting a sympathetic nervous system mechanism (2,3). Furthermore, induction of reversible autonomic blockade with the autonomic ganglionic blocking drug trimethaphan, abolishes water’s pressor action (2).
While these studies in human subjects suggest a sympathetic nervous system mechanism for water’s pressor action, we sought to discover the physiological and molecular basis of the response. There has been increasing interest in splanchnic neural and vascular mechanisms in blood pressure control in a number of animal models (4). The transient receptor potential cation channel family, especially the vanilloid family (TRPV), constitute potential mediators of the pressor response to water. These channels mediate the effects of many environmental factors, including osmolality and stretch, on neuronal and cardiovascular function (5). Trpv4, in particular, is sensitive to osmotic perturbations (6,7) and is found along the GI tract, mesenteric vessels, liver, cholangiocytes, dorsal root ganglia, and other locations throughout the body (8,9,10). Activation of Trpv4 results in release of nitric oxide, calcitonin gene related peptide, and substance P, all of which can act as neurotransmitters (11,12). In heterologous expression systems, Trpv4 activation occurs in response to hypo-osmotic stimuli. However, Trpv4−/− mice have an impaired response to both hypo- and hyper-osmotic stimuli (13). Using a mouse model system, the studies presented here address the location and nature of the pressor response to water as well as potential molecular mediators of the response.
Materials and Methods
All protocols were approved by the Vanderbilt University Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
Mice
Wild-type C57BL/6J mice (n=53) (Jackson Laboratories, ME) were used in all experiments unless otherwise noted. Dopmaine beta hydroxylase knockout mice (Dbh−/−) (n=5) were developed by standard gene-targeting methods as described by Thomas et. al. (14). Trpv4−/− mice (n=21) were developed and provided by Wolfgang Liedtke (Duke University) (13). Animals were 3–12 months of age at time of experiment.
Continuous BP Recordings
Mice were anesthetized with 4% isoflurane and maintained on 1% isoflurane in oxygen delivered from a precision vaporizer. Body temperature was maintained at 36–37 °C with an isothermal pad (Braintree Scientific, Inc., Braintree, MA). Drugs were administered through a venous catheter in the left jugular vein; and BP measured through a catheter in the right femoral artery (Micro-Renathane, Braintree Scientific Inc.) connected to a pressure transducer (DTX Plus-4812, Becton-Dickinson) and carrier amplifier (Gould Instruments). BP signals were recorded using a WINDAQ data acquisition system (DATAQ). Data were analyzed by PVwave (Visual Numerics, San Ramon, CA, USA).
Gastric/Duodenal Cannulation
An upper abdominal midline incision was made to expose the stomach for gastric and duodenal cannulation. The fundus was punctured at the greater curvature with blunt forceps. A PE-50 catheter was inserted into the gastric lumen or passed just beyond the pyloric sphincter into the duodenum. Sutures placed around the blunted and flanged end of the catheter secured the catheter in place and prevented retrograde flow of GI fluids. Normal saline or water was infused into the stomach or duodenum at a volume of 25 µL/g body weight over a 6-minute period.
Subdiaphragmatic vagotomy
The esophagus was isolated and secured at two points with silk sutures; one suture was placed where the thoracic esophagus emerges through the diaphragm, the other placed closer to the gastro-esophageal junction. The sutures acted as retractors to separate the esophagus from other structures in the area as well as to prevent leakage of GI contents into the peritoneum. The esophagus was severed just below the diaphragm, along with the dorsal and ventral vagus nerves.
Baroreflex Impairment Model Preparation
Our method is similar to previously described methods of baroreflex deafferentation (15,16). Briefly, a ventral midline incision was made in the neck, exposing the carotid bifurcation and allowing isolation of afferent components of the baroreflex. The superior cervical ganglion and superior laryngeal nerves were isolated and removed, along with the carotid sinus nerve. In addition, the adventitia and associated connective tissue were stripped from the carotid sinus regions. Baroreflex impairment was confirmed by comparing ΔHR/ΔBP upon phenylephrine challenge before and after deafferentation. (Figure S1; please see http://hyper.ahajournals.org)
Conscious BP measurements for restraint stress test
Mouse BP radiotelemetry (Data Sciences International, St. Paul, MN, USA) has been described previously (17). Briefly, the left carotid artery was isolated, a vessel clamp was placed 8–10 mm below the bifurcation to occlude blood flow, and the lumen was cut to allow insertion of the transmitter catheter to the point of the bifurcation. The transmitter body was placed in a lateral subcutaneous pocket. The overlying muscles and skin were secured via sutures.
Restraint stress test
A universal mouse restrainer (Braintree Scientific, Inc.) was used to induce restraint stress in telemetered mice. Mice were placed in the restrainer for 2 minutes. Beat-to-beat BP and HR measurements were recorded using the DSI ART Gold software (Data Sciences International). Data were analyzed by PVwave (Visual Numerics, San Ramon, CA, USA).
Drugs
Prazosin hydrochloride (Sigma) was dissolved in distilled water with the application of heat. Final dilutions were made with saline, and given intraperitoneally at 0.5 mg/kg in 100 µL water/saline. This dose was chosen because it sufficiently blocked the alpha-1 pressor effect of phenylphrine. Water was infused intraduodenally 20 minutes after i.p. prazosin. Phenylephrine hydrochloride (Sigma) was dissolved in saline and given intravenously.
Osmolality Measurement
Blood was drawn from the portal vein and carotid artery 10 minutes after duodenal infusion of water or saline, and centrifuged right after collection (600g for 10 minutes) to reduce cell lysis. Plasma osmolality was measured using the Vapro Vapor Pressure Osmometer 5520 (Wescor Inc., Utah).
Statistics
The data in this study consist of multiple blood pressure measurements prior to and after the intervention. We have used a response-feature approach to account for the repeated measures aspects of our data while avoiding complex longitudinal models (18). Average blood pressures were derived for each mouse during the baseline and post-treatment intervals ( and , respectively). The average change from baseline for the ith mouse was . Blood pressures were recorded continuously throughout the experiment. The baseline interval was from ten minutes before the onset of treatment until the start of infusion. Infusion lasted 6 minutes. The post-treatment interval was from the end of infusion and lasted 34 minutes. Tests of the change in blood pressure in response to treatment within each treatment group were assessed by comparing with using the Wilcoxon signed-rank test (WSR test). The difference in blood pressure response to infusion between separate treatment or genetic groups was assessed by comparing in the two groups using a Mann-Whitney U test (MWU test). Data are presented as the mean ± standard deviation. SPSS was used for all statistical calculations. All P values were derived with respect to two-sided alternative hypotheses.
Results
Baroreflex Deafferentation Unmasks Pressor Response
To unmask and better characterize the response in mice, we used a model of baroreflex failure (baroreflex deafferentation). Unless otherwise noted, BP data shown here are from animals after bilateral baroreflex deafferentation. Effective baroreflex deafferentation was documented in each animal by the absence of opposing changes in heart rate in response to alterations in BP (Figure S1; please see http://hyper.ahajournals.org). Our mouse model responded to water with an increase in BP similar in magnitude and time course to that observed in human subjects with baroreflex impairment (Figure S2; please see http://hyper.ahajournals.org).
Location of Water’s Action
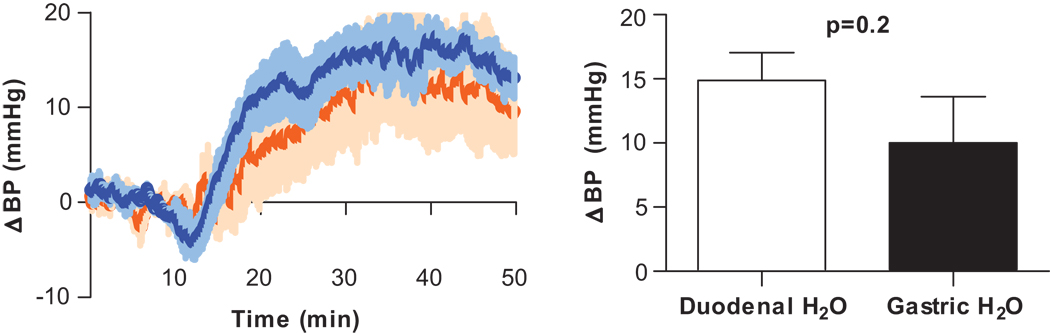
Neurons in the pharyngeal and esophageal areas are sensitive to both mechanical and chemical stimuli (including water), and can elicit cardiovascular reflexes when activated (19,20). To investigate pharyngeal and esophageal involvement in water’s pressor effect, water (25 µL/g in 6 minutes) was infused directly into the stomach via an intragastric tube. BP began to increase near the end of water infusion, but maximal pressure was not reached until 20 minutes later (10.0±13.2 mmHg, p<0.05). The experiment was repeated with direct duodenal infusion of water (catheter advanced beyond the pyloric sphincter). Intraduodenal infusion of water resulted in robust BP elevation (14.9±7.4, p<0.005) of similar magnitude to intragastric infusion (p=0.2 between groups) (Fig. 1).
Figure 1. Location of water’s action.
Change in BP after intragastric or intraduodenal infusion of water or saline (25 µL/g). Infusions occurred between 10–16 minutes. Both gastric (orange, n=7) and duodenal (blue, n=11) infusion of water resulted in a robust increase in BP.
Role of Plasma Volume in Pressor Response
To address the possibility that water increases BP by increasing plasma volume, saline (150 µL, ~10% of circulating blood volume) was infused intravenously in mice. Intravenous saline caused a small, transient spike in BP, with values returning to baseline within 2 minutes (Figure S3; please see http://hyper.ahajournals.org). This intravenous saline did not elicit the persistent pressor effect seen with water.
Role of Sympathetic Nervous System Efferents in Pressor Response to Water
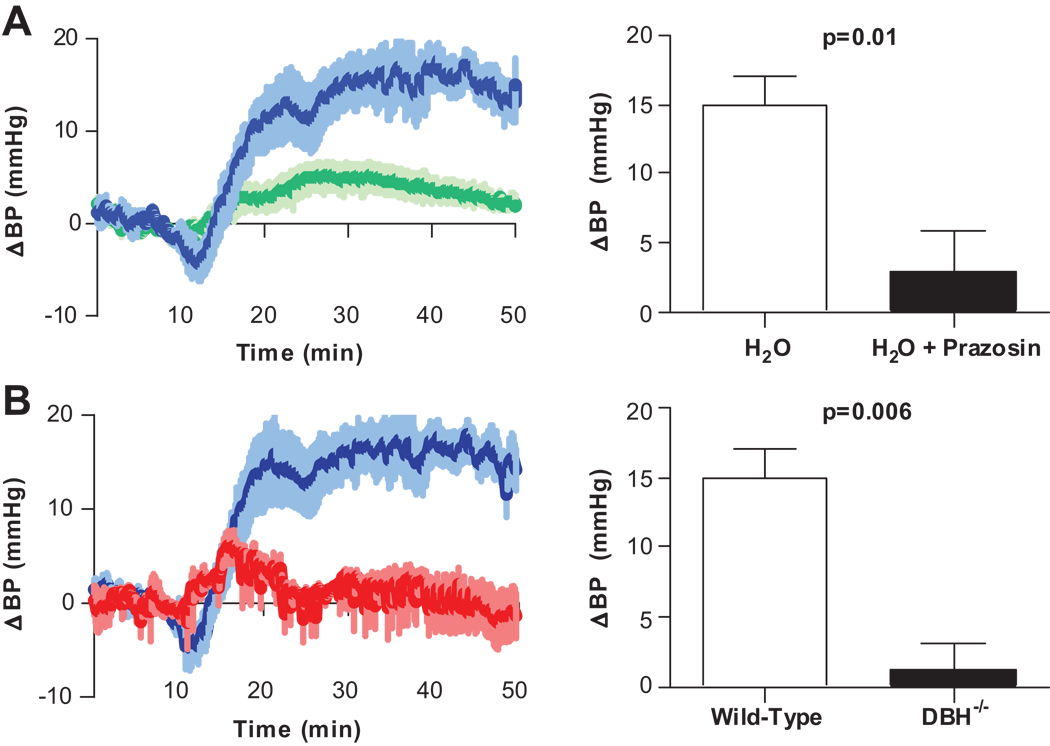
To test the hypothesis that sympathetic activity underlay the pressor effect of water, mice were given the α1 adrenoreceptor antagonist prazosin prior to water infusion. Pre-treatment with prazosin resulted in loss of the pressor response to water (3.8±2.7 vs. 14.9±7.4 mmHg, p=0.01) (Fig. 2A). Additionally, Dopamine β hydroxylase (Dbh) knockout mice with no detectable NE in blood, urine or tissue, did not produce an increase in BP in response to water infusion (1.3±1.2 mmHg, p = 0.36) (Fig. 2B).
Figure 2. Osmopressor response is mediated by sympathetic nervous system activation.
Change in BP after duodenal infusion of water (25 µL/g). A, The pressor response to water was greatly attenuated with prazosin (0.5 mg/kg IP) prior to water infusion (green, n=5). B, Dbh−/− mice display no response to intraduodenal water (red, n=5).
Vagal Involvement in Pressor Response to Water
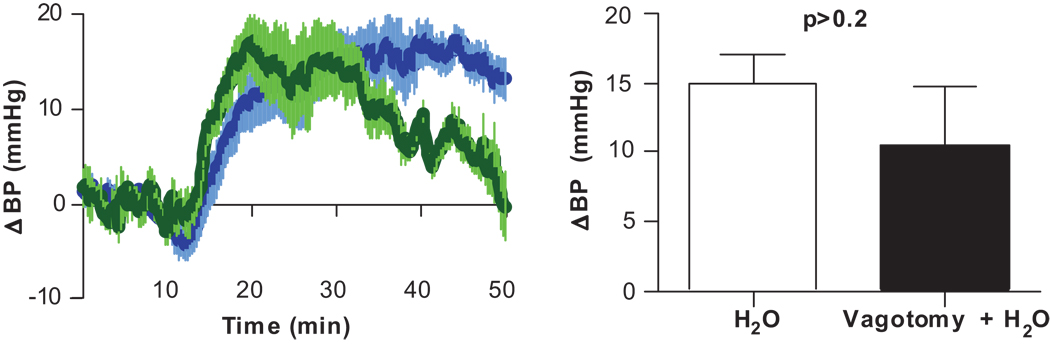
To investigate vagal afferent involvement in the response to water, surgically baroreflex-impaired mice underwent bilateral subdiaphragmatic vagotomy prior to water infusion. The vagotomized mice responded to water infusion with a similar increase in BP as intact animals (10.5±4.8 vs. 14.9± 7.4 mmHg, p=0.2) (Fig. 3).
Figure 3. Vagal afferents are not essential for water’s pressor effect.
Change in BP after duodenal infusion of water (25 µL/g) in vagotomized (green, n=6) and inact (blue, n=11) mice.
Effect of Gastric Luminal Stretch and Osmolality on BP
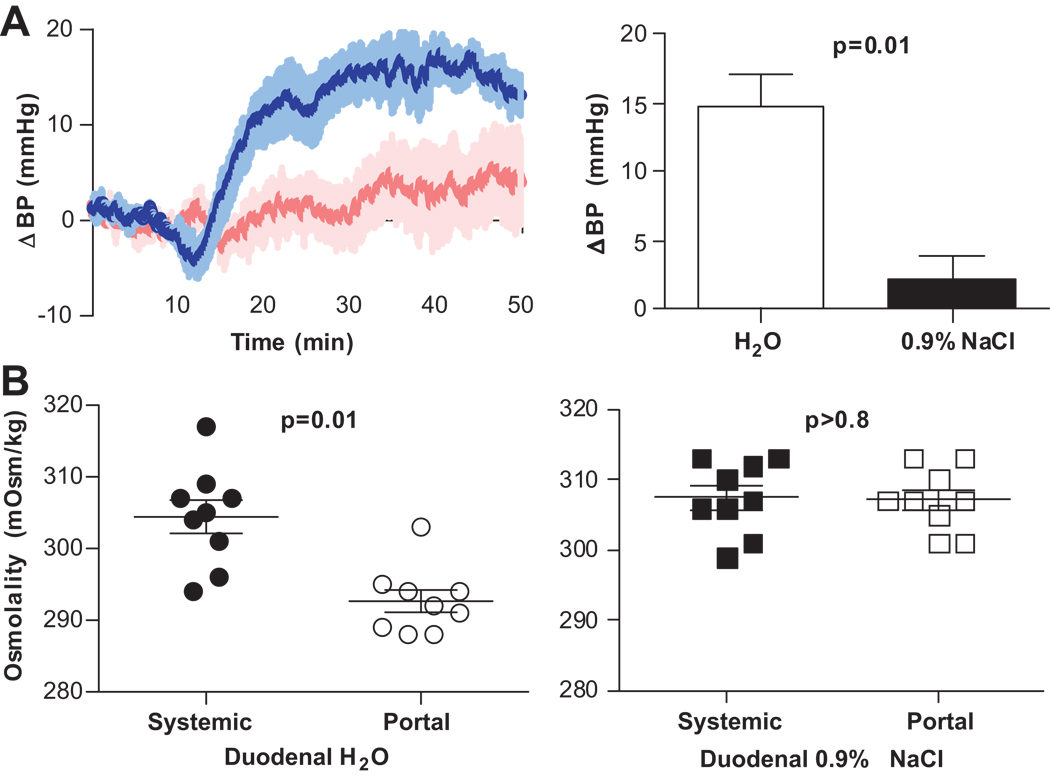
To differentiate between luminal stretch and osmolality as triggers of the water response, mice were given equivolume doses (25ul/g) of saline or water. In contrast to water, intraduodenal saline infusion did not elicit an increase in BP (2.3±8.1 mmHg, p=0.6), (Fig. 4A).
Figure 4. Effect of osmolality on BP.
Change in BP after intraduodenal infusion of water (blue, n=11) or saline (pink, n=6) (25 µL/g). Infusions occurred between 10–16 minutes A, Attenuation of the pressor response during saline infusion implicates hypo-osmolality as the stimulus. B, Systemic (●, ■) and portal (○, □) osmolality 10 minutes after intraduodenal infusion of water (●, ○, n=9) or saline(■, □, n=9). The decrease in portal osmolality after water, but not after saline infusion is consistent with portal/hepatic osmosensor involvement in the pressor response to water.
Portal vs. Systemic Osmolality after Water or Saline Infusion
To determine the effects of intraduodenal water or saline infusion on osmolality in mice, both portal and systemic osmolality were measured 10 minutes after the infusion. Water infusion lowered portal osmolality relative to systemic osmolality (292.7±4.7 mOsm/kg and 304.4±6.9 mOsm/kg, respectively, p = 0.002), whereas saline infusion did not lower portal osmolality relative to systemic osmolality (307.1±4.4 mOsm/kg and 307.4±1.7 mOsm/kg, respectively, p = 0.88 (Fig. 4B).
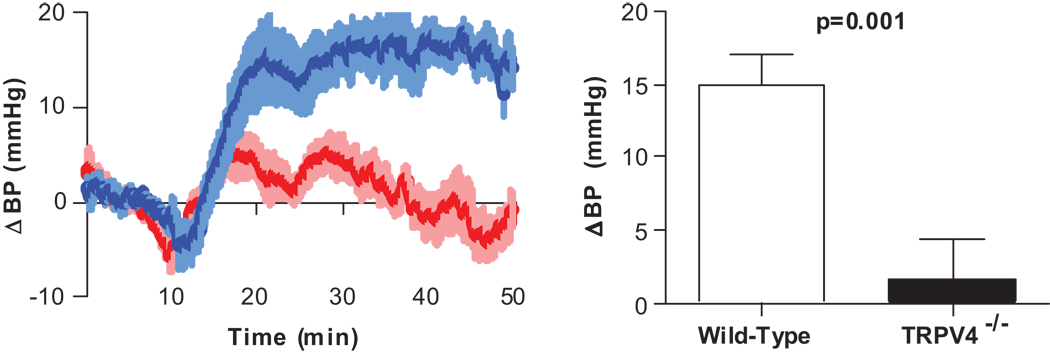
Response of Trpv4−/− Mice to Water Infusion
We used Trpv4 knockout animals to investigate the potential role of these channels in our osmopressor response. The pressor response to intraduodenal infusion of water was virtually abolished in mice lacking the Trpv4 channel compared to wild type controls (1.6±4.3 mmHg, p=0.3) (Fig. 5). To verify that the absence of the pressor response in Trpv4−/− animals was not due to a general abnormality in neural pathways or efferent sympathetic nerve function, mice were placed under restraint, which increases sympathetic output from the CNS. Both wild-type and Trpv4−/− mice showed a similar increase in BP and HR when placed under restraint, whereas Dbh−/− mice (absent sympathetic adrenergic tone) under restraint did not show increases in BP or HR (Figure S4; please see http://hyper.ahajournals.org).
Figure 5. Trpv4 is an essential mediator in the osmopressor response.
Change in BP after duodenal infusion of water (25 µL/g) in Trpv4−/− mice (red, n=11) compared to wild-type (blue, n=11).
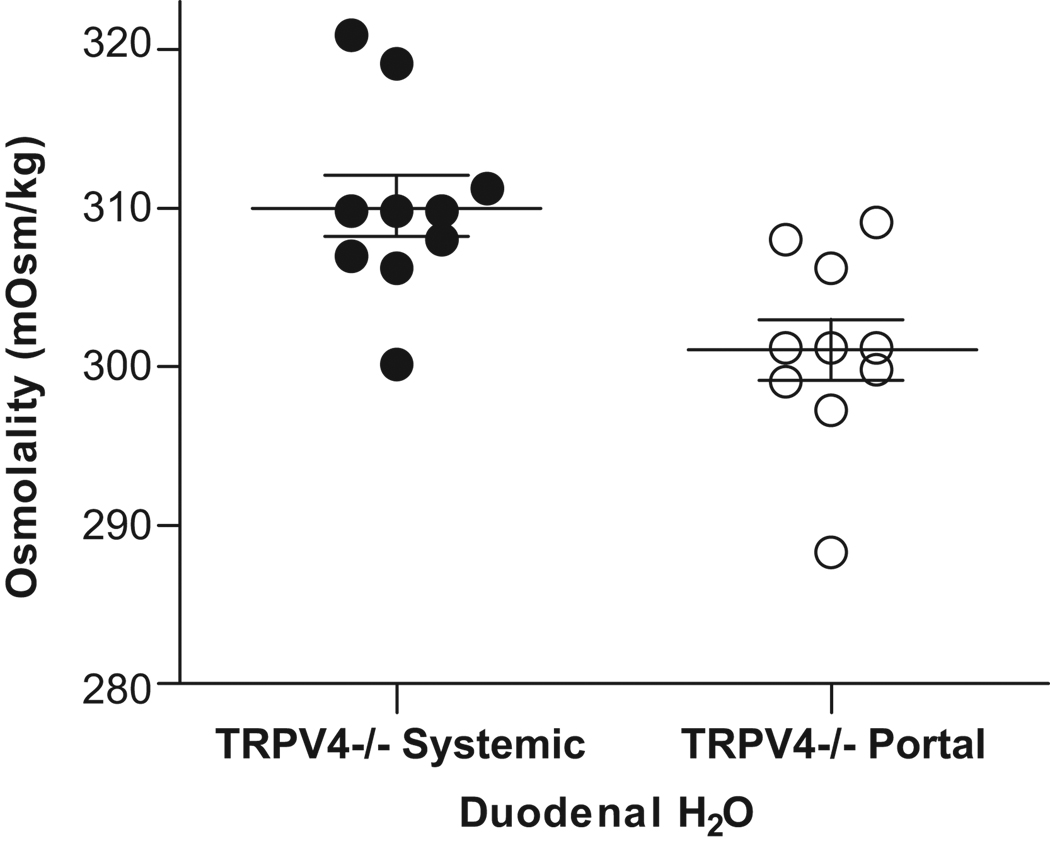
Portal vs. Systemic Osmolality in Trpv4−/− after Water Infusion
Portal and systemic osmolalities were measured 10 minutes after duodenal infusion of water in Trpv4−/− animals. Portal and systemic osmolalities in these mice were found to be 301.0±6.0 and 310.2±6.1 mOsm/kg, respectively, p=0.003 (Fig. 6).
Figure 6. Portal and systemic osmolality in Trpv4−/− after water infusion.
Systemic (●) and portal (○) osmolality 10 minutes after duodenal infusion of water (25 µL/g) in Trpv4−/− mice (n=10).
Discussion
In this study, we sought to characterize the physiological and molecular mechanism of the previously described pressor effect of water ingestion. The baroreflex deafferented mouse model allowed us to better observe water’s BP effects, without buffering by an intact baroreflex system. Our results showing the presence of a robust pressor response after both intragastric and intraduodenal infusions places the location of water’s actions at or distal to the duodenum and shows that water elicits its actions independent of pharyngeal and esophageal mechanisms. We also show that the stimulus eliciting the response is osmotic in nature since only water, and not saline, produced a pressor response. Furthermore, the absence of a pressor response with saline infusion makes luminal stretch an unlikely explanation for the increase in BP, since both fluids would have induced equal duodenal stretch. However, luminal stretch may play a role during the period of infusion when the GI tract is experiencing the highest degree of stretch. The slight, transient dip in BP immediately following the start of infusion might be explained by the acute luminal stretch experienced during infusion.
Hydration status may effect BP. However, we showed that acutely increasing plasma volume via saline infusion failed to elicit the pressor response seen with duodenal water administration in both magnitude and duration. Since intravenous administration would optimally increase plasma volume, these data effectively exclude an increase in plasma volume as the cause of the robust, sustained response observed after water ingestion.
Studies in humans provide indirect evidence that increased sympathetic nervous system activity underlie the pressor effect of water; plasma NE (2,3), muscle sympathetic nerve activity (21), and peripheral vascular resistance (1) all increase after water ingestion in human subjects. In the present study, blockade of α1 adrenoreceptors with prazosin attenuated the pressor response to water, implicating the sympathetic neurotransmitter NE in this response. Further evidence strengthening the sympathetic nervous system hypothesis was obtained from dopamine β hydroxylase (Dbh) gene knockout mice. Even though Dbh−/− mice display hypersensitivity to a variety of pressor and depressor stimuli, they were incapable of producing an increase in BP in response to water infusion (Fig. 2B). Taken together, these data show that an intact efferent sympathetic nervous system is required to elicit water’s pressor response, and specifically that sympathetic adrenergic mechanisms are critical to this response.
The neural pathways that form the loop of this pressor response to water might include brainstem centers, which receive splanchnic afferent input via the vagus nerve, or could be limited to spinal afferent nerves which can directly influence sympathetic output at the level of the spinal cord (22). However, the presence of a pressor response in bilaterally subdiaphragmatically vagotomized mice indicates that the vagus nerve is not essential for the osmopressor response. Interestingly, patients with near-complete cervical spinal cord transection also have an intact pressor response to water ingestion (23). Both of these findings suggest that the pressor response is most likely acting through a spinal reflex.
All contents absorbed by the GI tract enter the body by way of the portal blood vessels. The portal vein can exhibit a wider range of osmolality than the systemic circulation, and there is evidence of osmosensitive mechanisms in the portal system (24,25). Our data show that water infusion lowered portal osmolality relative to systemic osmolality and produced a pressor response, whereas saline infusion did not alter portal/systemic osmolality nor raise blood pressure. These data suggest that water may be acting through osmosensors in the portal/hepatic circulation. For these reasons, we have termed this pressor response of water ingestion as the “osmopressor response”.
The location of action and osmotic nature of the stimulus eliciting the pressor response made Trpv4 a top candidate as a potential mediator. Trpv4 is particularly sensitive to hypo-osmotic stimuli (6,7) and is expressed in areas optimal for sensing osmotic changes after water ingestion including mesenteric vessels and sensory afferents serving the GI tract and portal system as well as dorsal root ganglia innervating these regions (26). We show that Trpv4−/− mice have intact sympathetic efferents and can increase BP appropriately in response to non-osmotic stimuli, such as restraint stress. Additionally, we show that Trpv4−/− mice respond to water infusion by decreasing portal osmolality relative to systemic osmolality by the same magnitude (~10 mOsm/kg) as wild-type counterparts. The lack of a pressor response in Trpv4−/− mice despite a similar reduction in portal osmolality as wild-type counterparts suggest that Trpv4 may act as an osmosensor in the portal region and be an important mediator of the afferent input of the pressor response. It might be possible that spinal afferents responsible for sensing hepatic/portal environment relay this information to Trpv4-positive dorsal root ganglion neurons, which then result in the release of neuropeptides altering sympathetic output.
Perspectives
While only newly recognized, the significance of the osmopressor response may be substantial. Ingestion of water is proving to be therapeutic in the relief of episodes of debilitating hypotension or inadequate sympathetic nervous activation. Interestingly, a recent American Red Cross study documented that water ingestion can prevent reactions associated with blood donations (27).
The osmopressor response and Trpv4 are new factors now implicated in the physiology of blood pressure regulation, particularly in hypotension and fainting. They might also represent targets for future drug development to address these problems.
Supplementary Material
Acknowledgements
We thank Wolfgang Liedtke for providing Trpv4−/− animals; William Dupont for statistical consultation; and Charlene Finney for maintenance and upkeep of mice.
Sources of Funding: This work was supported by P01 HL056693 (DR), R01 HL071784 (DR), U54 NS065736 (DR), UL1 RR024975 (GB), and K23 RR020783 (SRR), all from the National Institutes of Health, Bethesda MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
Reference List
- 1.Lu CC, Diedrich A, Tung CS, Paranjape SY, Harris PA, Byrne DW, Jordan J, Robertson D. Water ingestion as prophylaxis against syncope. Circulation. 2003;108:2660–2665. doi: 10.1161/01.CIR.0000101966.24899.CB. [DOI] [PubMed] [Google Scholar]
- 2.Jordan J, Shannon JR, Black BK, Ali Y, Farley M, Costa F, Diedrich A, Robertson RM, Biaggioni I, Robertson D. The pressor response to water drinking in humans : a sympathetic reflex? Circulation. 2000;101:504–509. doi: 10.1161/01.cir.101.5.504. [DOI] [PubMed] [Google Scholar]
- 3.Raj SR, Biaggioni I, Black BK, Rali A, Jordan J, Taneja I, Harris PA, Robertson D. Sodium paradoxically reduces the gastropressor response in patients with orthostatic hypotension. Hypertension. 2006;48:329–334. doi: 10.1161/01.HYP.0000229906.27330.4f. [DOI] [PubMed] [Google Scholar]
- 4.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 5.Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 8.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. 946. [DOI] [PubMed] [Google Scholar]
- 11.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 12.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 15.Schreihofer AM, Sved AF. Use of sinoaortic denervation to study the role of baroreceptors in cardiovascular regulation. Am J Physiol. 1994;266:R1705–R1710. doi: 10.1152/ajpregu.1994.266.5.R1705. [DOI] [PubMed] [Google Scholar]
- 16.Thames MD, Miller BD, Abboud FM. Baroreflex regulation of renal nerve activity during volume expansion. Am J Physiol. 1982;243:H810–H814. doi: 10.1152/ajpheart.1982.243.5.H810. [DOI] [PubMed] [Google Scholar]
- 17.Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- 18.Dupont WD. Statistical Modeling for Biomedical Researchers: A Simple Introduction to the Analysis of Complex Data. ed. 2nd. Cambridge, U.K.: Cambridge University Press; 2009. [Google Scholar]
- 19.Cunningham ET, Jr, Ravich WJ, Jones B, Donner MW. Vagal reflexes referred from the upper aerodigestive tract: an infrequently recognized cause of common cardiorespiratory responses. Ann Intern Med. 1992;116:575–582. doi: 10.7326/0003-4819-116-7-575. [DOI] [PubMed] [Google Scholar]
- 20.Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- 21.Scott EM, Greenwood JP, Gilbey SG, Stoker JB, Mary DA. Water ingestion increases sympathetic vasoconstrictor discharge in normal human subjects. Clin Sci (Lond) 2001;100:335–342. [PubMed] [Google Scholar]
- 22.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut. 2002;51 Suppl 1:i2–i5. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tank J, Schroeder C, Stoffels M, Diedrich A, Sharma AM, Luft FC, Jordan J. Pressor effect of water drinking in tetraplegic patients may be a spinal reflex. Hypertension. 2003;41:1234–1239. doi: 10.1161/01.HYP.0000070957.08219.C9. [DOI] [PubMed] [Google Scholar]
- 24.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 25.Stricker EM, Huang W, Sved AF. Early osmoregulatory signals in the control of water intake and neurohypophyseal hormone secretion. Physiol Behav. 2002;76:415–421. doi: 10.1016/s0031-9384(02)00752-7. [DOI] [PubMed] [Google Scholar]
- 26.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007;179:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 27.Newman B, Tommolino E, Andreozzi C, Joychan S, Pocedic J, Heringhausen J. The effect of a 473-mL (16-oz) water drink on vasovagal donor reaction rates in high-school students. Transfusion. 2007;47:1524–1533. doi: 10.1111/j.1537-2995.2007.01293.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.