Abstract
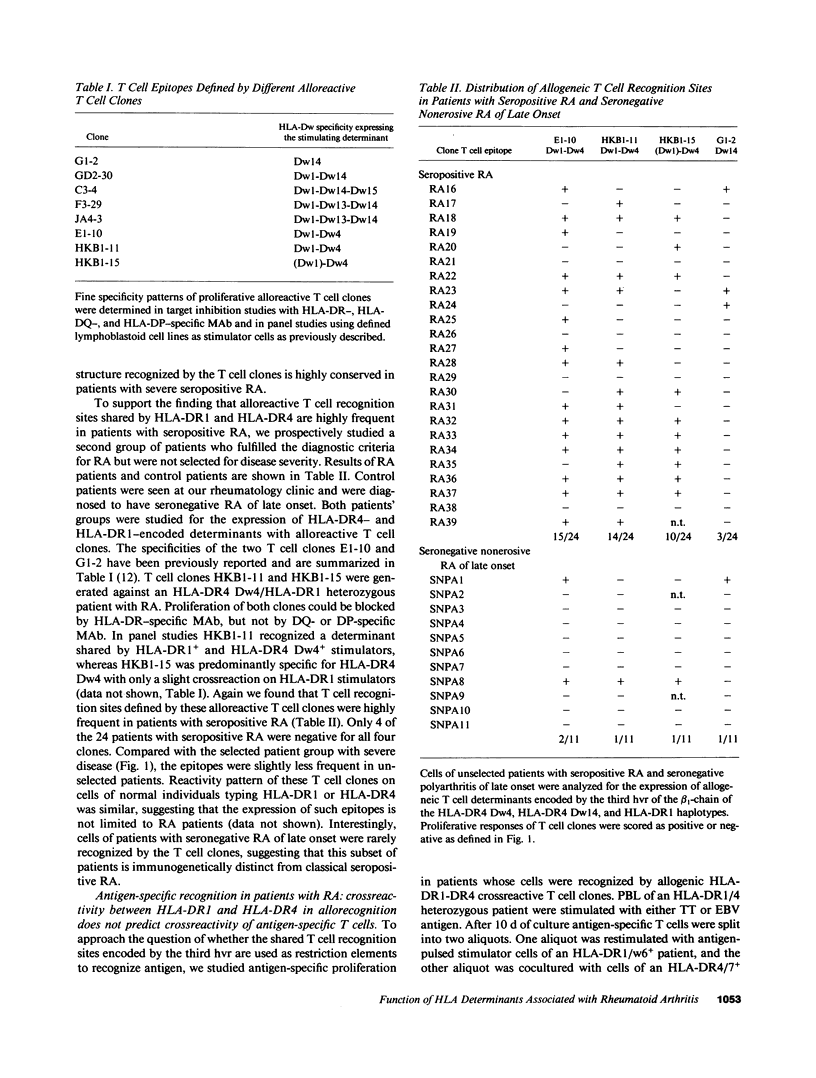
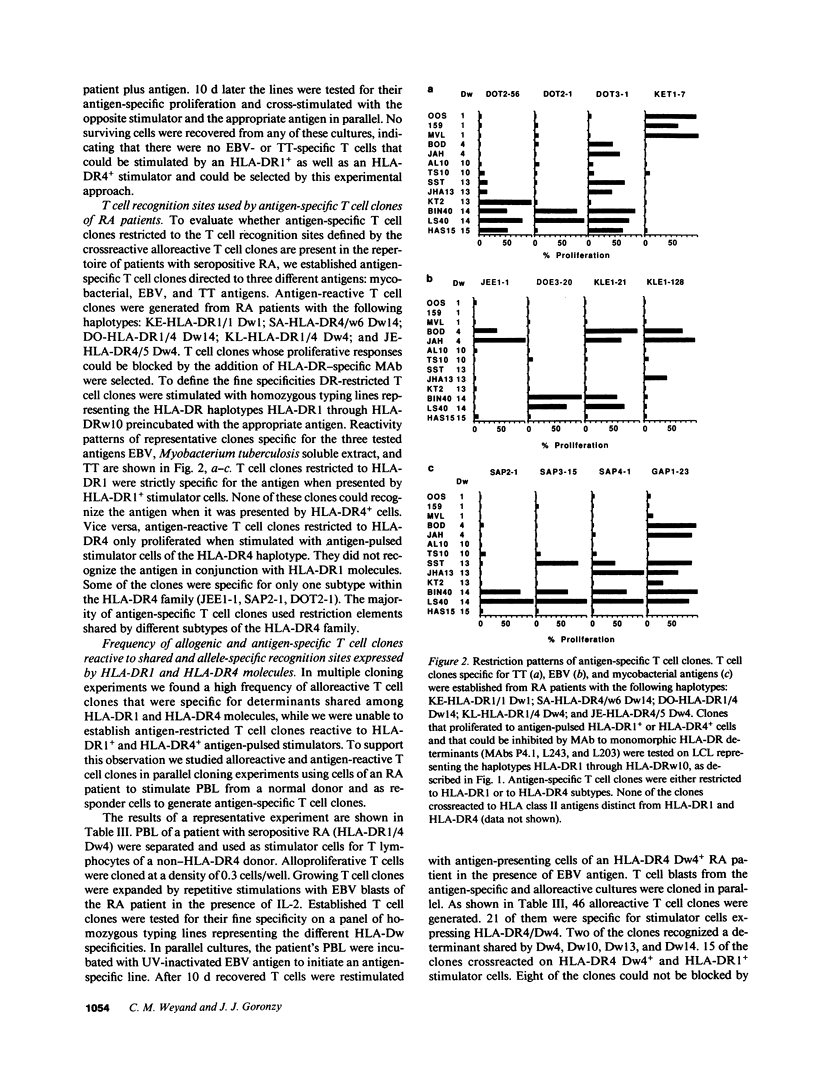
The susceptibility to develop seropositive rheumatoid arthritis (RA) has been linked to specific genomic polymorphisms within the HLA complex. Two different haplotypes have been associated with the disease, HLA-DR1 and HLA-DR4. To investigate the link between such phenotypic disease associations and potential immune mechanisms we used alloreactive and antigen-specific human T cell clones. Here we describe a panel of alloreactive T cell clones directed to polymorphic determinants encoded by the third hypervariable region (hvr) of the HLA-DR beta 1-chain. T cell determinants defined by these clones are shared among HLA-DR1, HLA-Dw4, HLA-Dw13, HLA-Dw14, and HLA-Dw15, and are frequent in a population of RA patients. To study the role of such disease-associated epitopes in antigen-restricted T cell recognition we generated T cell clones from RA patients specific for mycobacterial antigens, Epstein-Barr virus antigens, and tetanus toxoid. In all three antigenic systems T cell clones were restricted to either HLA-DR1 or HLA-DR4. These data suggest that the polymorphisms within the first and second hvr of the HLA-DR beta 1-chain that are distinct in HLA-DR1 and HLA-DR4 and not associated with the disease are crucially involved in the recognition of antigens. Polymorphic determinants encoded by the third hvr are shared among disease-associated haplotypes and may function to mediate the interaction of alloreactive T cell receptor molecules with the HLA complex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Duquesnoy R. J., Marrari M., Hackbarth S., Zeevi A. Serological and cellular definition of a new HLA-DR associated determinant, MC1, and its association with rheumatoid arthritis. Hum Immunol. 1984 Jul;10(3):165–176. doi: 10.1016/0198-8859(84)90037-5. [DOI] [PubMed] [Google Scholar]
- Fathman C. G., Goronzy J., Weyand C. Gene conversion. A mechanism to explain HLA-D region and disease association. Ann N Y Acad Sci. 1986;475:24–31. doi: 10.1111/j.1749-6632.1986.tb20853.x. [DOI] [PubMed] [Google Scholar]
- Fox R., Sportsman R., Rhodes G., Luka J., Pearson G., Vaughan J. Rheumatoid arthritis synovial membrane contains a 62,000-molecular-weight protein that shares an antigenic epitope with the Epstein-Barr virus-encoded associated nuclear antigen. J Clin Invest. 1986 May;77(5):1539–1547. doi: 10.1172/JCI112469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Shared T cell recognition sites on human histocompatibility leukocyte antigen class II molecules of patients with seropositive rheumatoid arthritis. J Clin Invest. 1986 Mar;77(3):1042–1049. doi: 10.1172/JCI112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J., Weyand C., Fathman C. G. Cloning of human alloreactive T cells. Methods Enzymol. 1987;150:333–341. doi: 10.1016/0076-6879(87)50091-x. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Shen M., Song Q. L., Merryman P., Degar S., Seki T., Maccari J., Goldberg D., Murphy H., Schwenzer J. Molecular diversity of HLA-DR4 haplotypes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2642–2646. doi: 10.1073/pnas.83.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Smith J. A., Gefter M. L. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986 Nov 20;324(6094):260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- Healey L. A., Sheets P. K. The relation of polymyalgia rheumatica to rheumatoid arthritis. J Rheumatol. 1988;15(5):750–752. [PubMed] [Google Scholar]
- Heber-Katz E., Acha-Orbea H. The V-region disease hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989 May;10(5):164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Rees A. D., Bal V., Ikeda H., Wilkinson D., De Vries R. R., Rothbard J. B. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur J Immunol. 1988 Jun;18(6):973–976. doi: 10.1002/eji.1830180623. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature. 1988 Apr 28;332(6167):840–843. doi: 10.1038/332840a0. [DOI] [PubMed] [Google Scholar]
- Mazzilli M. C., Tanigaki N., Cascino I., Costanzi Porrini S., Trabace S., Cappellacci S., Testa L., Gandini E. A mouse monoclonal antibody against a polymorphic determinant in a defined subset of DR molecules. Hum Immunol. 1986 Jun;16(2):148–156. doi: 10.1016/0198-8859(86)90043-1. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T., McDevitt H. O., Payne R. O. Increased frequency of HLA-Cw3 and HLA-Dw4 in rheumatoid arthritis. Arthritis Rheum. 1977 Jun;20(5):1037–1042. doi: 10.1002/art.1780200501. [DOI] [PubMed] [Google Scholar]
- Nepom B. S., Nepom G. T., Mickelson E., Schaller J. G., Antonelli P., Hansen J. A. Specific HLA-DR4-associated histocompatibility molecules characterize patients with seropositive juvenile rheumatoid arthritis. J Clin Invest. 1984 Jul;74(1):287–291. doi: 10.1172/JCI111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Nichol F. E., Woodrow J. C. HLA DR antigens in Indian patients with rheumatoid arthritis. Lancet. 1981 Jan 24;1(8213):220–221. doi: 10.1016/s0140-6736(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Ollier W., Venables P. J., Mumford P. A., Maini R. N., Awad J., Jaraquemada D., D'Amaro J., Festenstein H. HLA antigen associations with extra-articular rheumatoid arthritis. Tissue Antigens. 1984 Nov;24(5):279–291. doi: 10.1111/j.1399-0039.1984.tb02139.x. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Torres P., de las Aguas J. T., Fernandez R., van Eden W., de Vries R. R., Stanford J. L. Evidence for an HLA-DR4-associated immune-response gene for Mycobacterium tuberculosis. A clue to the pathogenesis of rheumatoid arthritis? Lancet. 1986 Aug 9;2(8502):310–313. doi: 10.1016/s0140-6736(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Press M., Notarfrancesco A., Genel M. Risk of hypoglycaemia with alternate-day growth hormone injections. Lancet. 1987 May 2;1(8540):1002–1004. doi: 10.1016/s0140-6736(87)92270-7. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Carson D. A., Valbracht J., Houghten R., Vaughan J. H. Human immune responses to synthetic peptides from the Epstein-Barr nuclear antigen. J Immunol. 1985 Jan;134(1):211–216. [PubMed] [Google Scholar]
- Roudier J., Rhodes G., Petersen J., Vaughan J. H., Carson D. A. The Epstein-Barr virus glycoprotein gp110, a molecular link between HLA DR4, HLA DR1, and rheumatoid arthritis. Scand J Immunol. 1988 Apr;27(4):367–371. doi: 10.1111/j.1365-3083.1988.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Schiff B., Mizrachi Y., Orgad S., Yaron M., Gazit E. Association of HLA-Aw31 and HLA-DR1 with adult rheumatoid arthritis. Ann Rheum Dis. 1982 Aug;41(4):403–404. doi: 10.1136/ard.41.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Immune response (Ir) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Blaese R. M. Defective EBV-specific suppressor T-cell function in rheumatoid arthritis. N Engl J Med. 1981 Nov 19;305(21):1238–1243. doi: 10.1056/NEJM198111193052102. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Mapping of allospecific T-cell recognition sites encoded by the HLA-DR4 beta 1-chain. Hum Immunol. 1989 Feb;24(2):133–143. doi: 10.1016/0198-8859(89)90053-0. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Fathman C. G. Human T-cell clones used to define functional epitopes on HLA class II molecules. Proc Natl Acad Sci U S A. 1986 Feb;83(3):762–766. doi: 10.1073/pnas.83.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoschke D., Segall M. Dw subtypes of DR4 in rheumatoid arthritis: evidence for a preferential association with Dw4. Hum Immunol. 1986 Jan;15(1):118–124. doi: 10.1016/0198-8859(86)90322-8. [DOI] [PubMed] [Google Scholar]


