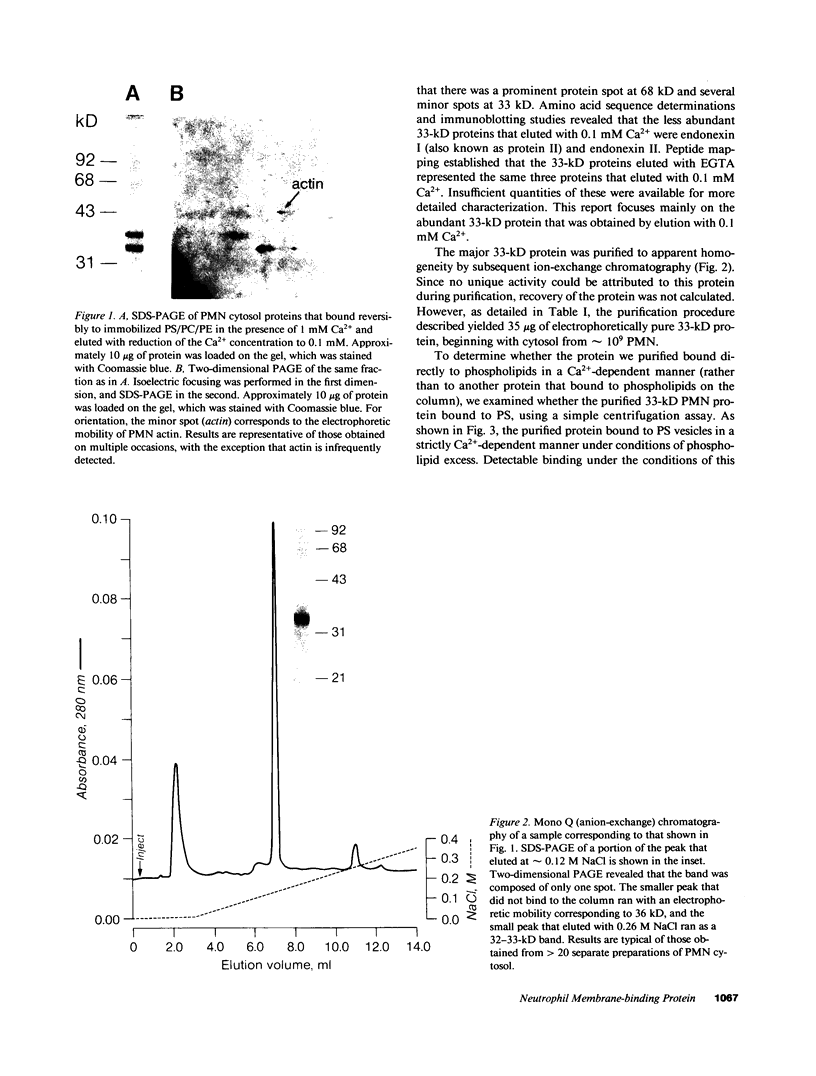
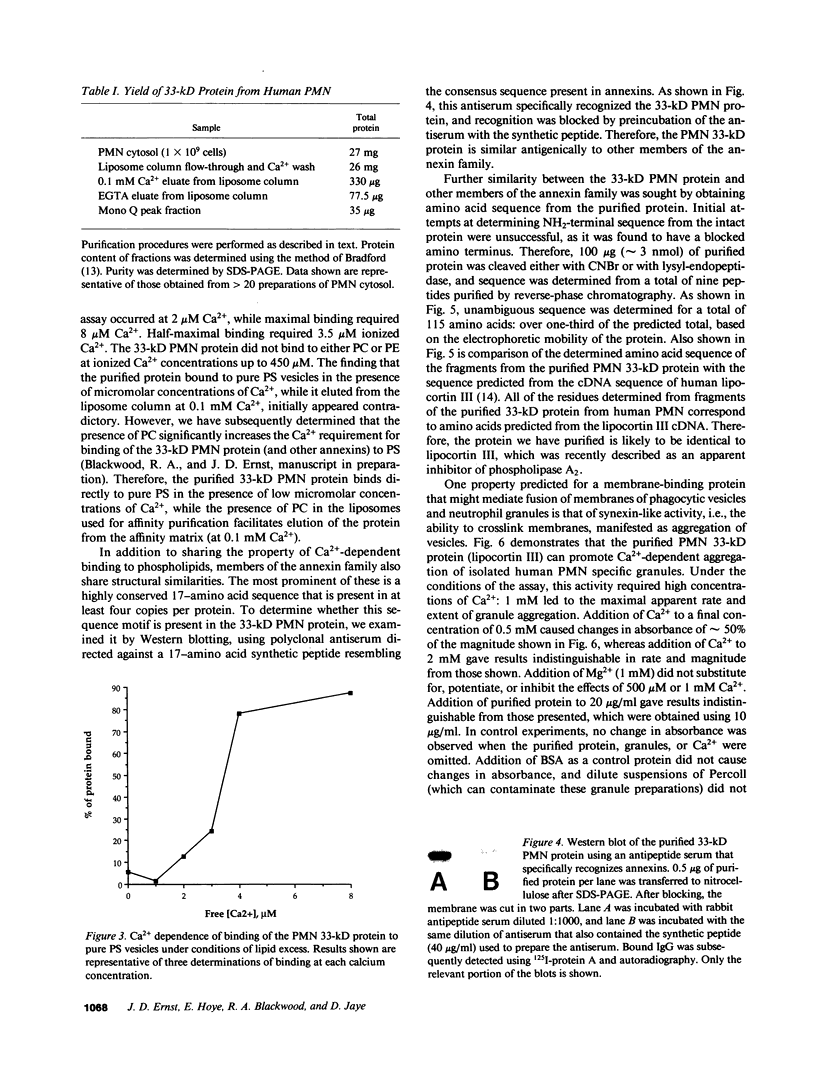
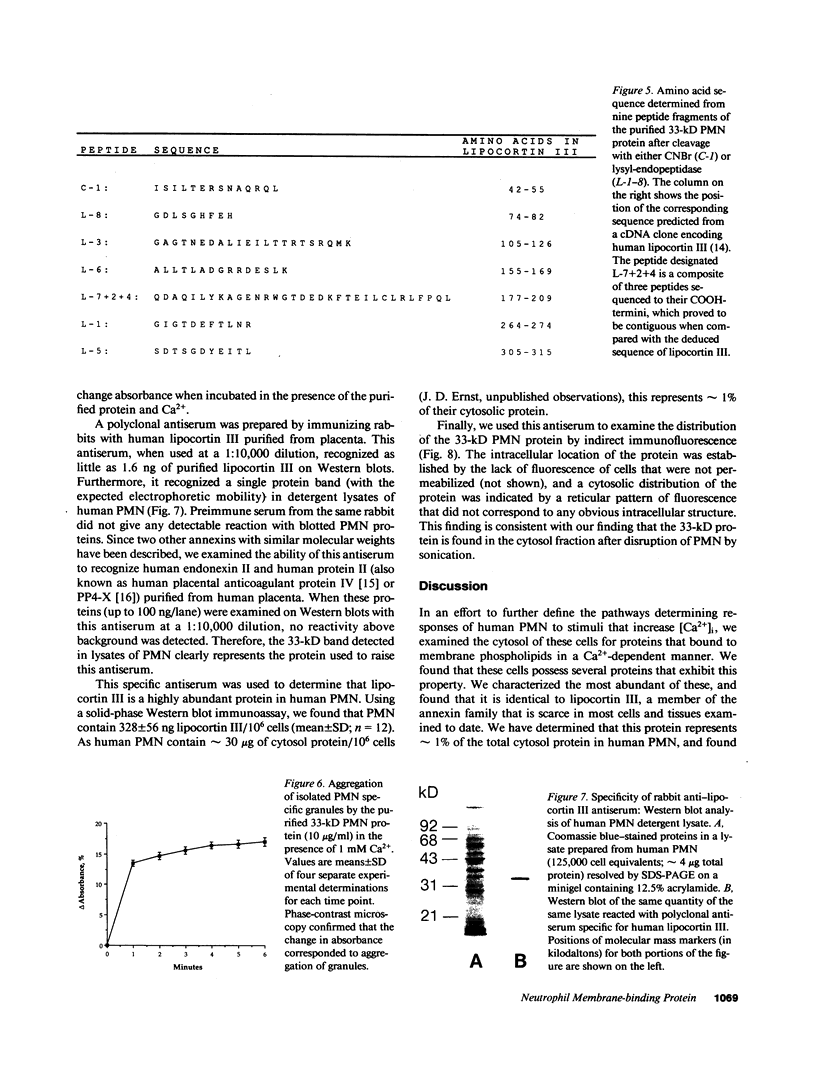
Abstract
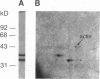
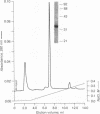
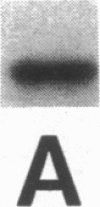
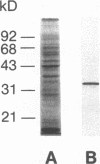
Intracellular ionized calcium has been strongly implicated in mediating several responses of human neutrophils to stimulation. However, proteins that serve as effectors of these responses have not been well characterized. To identify proteins that might serve as mediators of the effects of Ca2+ in human neutrophils, we isolated proteins that bind to membrane phospholipids in a Ca2(+)-dependent manner. The most abundant of these, a protein of 33 kD, was readily purified to homogeneity, and was found to bind to phosphatidylserine vesicles in the presence of 2 microM ionized Ca2+. In addition, this purified protein promoted Ca2(+)-dependent aggregation of isolated specific granules from human neutrophils, indicating that it might mediate membrane-membrane contact during processes such as phagosome-lysosome fusion or degranulation. This protein was localized to the cytoplasm of unstimulated neutrophils and found to account for approximately 1% of the cytosol protein. Amino acid sequence of several peptides derived from the purified protein revealed that it is identical to lipocortin III, a recently described member of the annexin family that is scarce in other cells and tissues. The abundance of this protein, together with its Ca2(+)-dependent membrane effects, suggest that it mediates membrane-localized events in stimulated neutrophils, such as phagosome-lysosome fusion or degranulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bers D. M. A simple method for the accurate determination of free [Ca] in Ca-EGTA solutions. Am J Physiol. 1982 May;242(5):C404–C408. doi: 10.1152/ajpcell.1982.242.5.C404. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Ernst J. D., Hoye E., Blackwood R. A. Use of a novel strategy for the preparation and characterization of an antipeptide antibody capable of recognizing members of the annexin family. Biochem Biophys Res Commun. 1989 Jun 30;161(3):959–964. doi: 10.1016/0006-291x(89)91336-3. [DOI] [PubMed] [Google Scholar]
- Funakoshi T., Heimark R. L., Hendrickson L. E., McMullen B. A., Fujikawa K. Human placental anticoagulant protein: isolation and characterization. Biochemistry. 1987 Aug 25;26(17):5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- Grundmann U., Abel K. J., Bohn H., Löbermann H., Lottspeich F., Küpper H. Characterization of cDNA encoding human placental anticoagulant protein (PP4): homology with the lipocortin family. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3708–3712. doi: 10.1073/pnas.85.11.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers P., Ernst J. D., Düzgünes N., Hong K. L., Fedor J., Goldstein I. M., Papahadjopoulos D. Synexin-like proteins from human polymorphonuclear leukocytes. Identification and characterization of granule-aggregating and membrane-fusing activities. J Biol Chem. 1987 Jun 5;262(16):7850–7858. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Sakata M., McMullen B. A., Miao C. H., Funakoshi T., Hendrickson L. E., Fujikawa K. Placental anticoagulant proteins: isolation and comparative characterization four members of the lipocortin family. Biochemistry. 1988 Aug 23;27(17):6268–6276. doi: 10.1021/bi00417a011. [DOI] [PubMed] [Google Scholar]