Abstract
The innate immune response is evoked as a consequence of interactions between invading foreign infectious agents and host immune cells. A successful innate immune response is pivotal in maintaining the delicate balance between health and disease; an insufficient response results in infection, whereas an excessive response results in prolonged inflammation and tissue damage. Alterations in the state and function of the nervous system influence the immune response. The nervous system regulates innate immune responses through the release of neurotransmitters, neuropeptides and neurohormones. However, many questions related to the molecular and cellular mechanisms involved, the physiological role of the link between the immune and the nervous system, and the biological significance of neuro-immune interactions remain unresolved. The interactions between the nematode Caenorhabditis elegans and its pathogens provide insights into mechanisms of neuroendocrine regulation of immunity and address many outstanding issues related to neuro-immune interactions.
Introduction
When faced with challenge, the body responds by turning on complex adaptive pathways that are turned off when the threat passes. Failure to achieve stability in the face of acute stress (major life events or events that trigger the ‘fight-or-flight’ response) or chronic stress (the cumulative load of minor day-to-day stresses) has long-term consequences. In animals, two important systems that mediate quick and precise responses to stresses are the nervous and immune systems. These systems are connected physically and physiologically, and share many similarities that facilitate interactions between them (Box 1).
Box 1. Similarities between the nervous and immune systems.
Both the nervous system and the immune system (see Kioussis and Pachnis, 2009):
mount a variety of essential, coordinated responses to danger
connect and carry information from and to distant parts of the body
use a range of chemical signaling molecules, from small molecules, such as neurotransmitters and neuropeptides, to large proteins, such as growth factors
communicate extremely rapidly through chemical and cellular networks, by utilizing shared and conserved second messengers and signaling pathways
form specialized membrane structures called synapses that facilitate information transmission
have threshold sensitivity and show quantal responses
posses memory; however, the response kinetics are orders of magnitude different
share developmental and functional mechanisms
Physiologically and emotionally challenging experiences can be described as ‘stress’. It is known both from anecdotal evidence and published studies that sustained neuronal activities, such as experiencing prolonged psychological and emotional stresses, negatively impact the immunological state (Glaser and Kiecolt-Glaser, 2005). For example, prolonged elevated psychological stress is associated with increased risk of infection by respiratory viruses (Cohen et al., 1991; Stone et al., 1992). Interestingly, in mice, psychological stress elevates glucocorticoid levels, causing a consequent downregulation of antimicrobial peptides in the skin, and increases susceptibility to streptococcal skin infections (Aberg et al., 2007). The stress-immune connection also extends to invertebrates. In black garden ants, caste dimorphism influences the expression of innate immune genes (Graff et al., 2007). Thus, the detrimental effects of sustained stress on immune function are not limited to humans but seem to be conserved across vertebrate and invertebrate species.
Stress influences the nervous system and innate immunity through conserved mechanisms. In humans, chronic stress and depression lead to persistent activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) axis (Kudielka and Wust, 2010). Chronic secretion of glucocorticoids and noradrenaline (norepinephrine) from the HPA and SAM axes, respectively, can have profound effects on many immune functions (Sternberg, 2006). In rodents, multiple ‘emotional’ stressors (cohabitation with sick cage-mate, social subordination) and physical stressors (inescapable footshock, individual housing) increase noradrenaline turnover in the hypothalamus, indicating activation of the sympathetic nervous system (Costa-Pinto and Palermo-Neto, 2010). Unlike with humans, it is not easy or possible to ascertain the psychological state or stress of many genetically amenable model organisms, such as C. elegans and Drosophila. However, given that stress affects neuronal secretion, which in turn affects immune function, these organisms might be useful for investigating the molecular mechanisms underlying the various effects of nervous system activity on immunity. In this Perspective article, we briefly discuss the interactions between the nervous and immune systems, as well as how investigations into infection of C. elegans by various pathogens extends our knowledge of how the nervous system affects epithelial immunity. We also propose areas of research in which the C. elegans model could contribute to our understanding of neuronal regulation of immunity. Finally, we highlight areas in which other animal models are needed to compliment or extend findings in the C. elegans system to make them relevant to human disease.
Interaction between the nervous and innate immune systems
The nervous and immune systems communicate through multiple neuroanatomical and neurohormonal routes. For example, the release of immune mediators elicited by invading pathogens triggers the nervous system to initially amplify local immune responses to facilitate pathogen clearance. Subsequently, the nervous system – through systemic endocrine and regional neuronal routes – suppresses the immune response to restore the body to a homeostatic state. The mechanisms and the mode of action of various nervous-system-derived mediators in regulating innate and adaptive immunity have been studied extensively, and have been summarized in excellent reviews (e.g. Steinman, 2004; Sternberg, 2006).
Much of our current understanding about the intricate neuro-immune interactions and their implications on health and diseases comes from studies performed in rats and mice. Together with human studies, this work has established a framework for future studies on neural-immune communications. Although the nervous systems of rodents are complex, regimens that mimic conditions of chronic stress have enabled experimental assessment of the effects of chronic stress on immunity (Schmidt et al., 2010). Chronic sympathetic nervous system activation can also be achieved by generating mice that lack adrenergic receptors (Hein et al., 1999). However, whether immune functions are altered in the latter model of chronic sympathetic nervous system activation, and whether the effects mimic those induced by chronic stress, are issues that have not been addressed. The complexity of the mammalian brain is an additional challenge: the human brain contains close to 100 billion neurons that function in a highly coordinated manner to regulate organismal physiology and behavior. Technical limitations in addressing these issues hampers progress in understanding the molecular mechanisms underlying neuroendocrine regulation of immunity in vivo.
Studies using genetically tractable invertebrates such as Drosophila and C. elegans have contributed much to our understanding of innate immunity. Innate immunity is the first line of defense against pathogens and, in higher vertebrates, it influences the adaptive immune response (Hoebe et al., 2004). Innate immunity can be divided into two distinct but overlapping components: basal and inducible immunity. Basal immunity includes constitutively expressed antimicrobial effectors, such as defensins, that allow the host to instantaneously respond to invading pathogens (Zhao et al., 1996; O’Neil et al., 1999; Ooi et al., 2002). Inducible innate immunity is activated only following pathogen exposure, supplying additional effector molecules and immune effector cells to assist in host defense. Recent studies of invertebrates and vertebrates have provided compelling evidence that innate immunity is largely conserved across evolutionary lineages. Together with our knowledge of their nervous systems, Drosophila and C. elegans have great potential to be exploited as experimental systems to address issues regarding how the nervous system affects innate immunity. However, with the possible exception of the effect of circadian rhythms on immunity (Shirasu-Hiza et al., 2007), little is known about how the activity of the nervous system affects immune function in Drosophila. By contrast, multiple recent studies have exploited the relatively simple and well-defined nervous and immune systems of C. elegans to reveal distinct mechanisms of neuronal regulation of innate immunity (Kawli and Tan, 2008; Styer et al., 2008; Anyanful et al., 2009; Reddy et al., 2009; Zugasti and Ewbank, 2009). In the following sections, we summarize our current knowledge of the C. elegans innate immune system and discuss how epithelial immunity of this organism is influenced by the nervous system.
C. elegans innate immunity
Over the past decade, studies on how C. elegans defends itself from various bacterial, fungal and microsporidial pathogens have provided genetic and genomic tools to the area of host-pathogen interactions. A central mechanism of innate immunity in most studied animals involves an axis formed by recognition of pathogen-associated molecular patterns (PAMPs) by Toll-like receptors (TLRs), and the activation of nuclear factor-κB (NFκB) transcription factors. Despite lacking the TLR-NFκB axis and ‘professional’ immune cells, such as macrophages and neutrophils, C. elegans can effectively protect itself from diverse pathogens. C. elegans relies mainly on the innate immune response of epithelial cells to fight off microbial infections at the epidermis and in the intestine. As a result, C. elegans has contributed substantially to our knowledge of epithelial immunity and NFκB-independent immune pathways. Studies of C. elegans have revealed that the expression of host defense effectors is regulated by several evolutionarily conserved signaling pathways (Irazoqui et al., 2010), including a p38 mitogen-activated protein kinase (MAPK) pathway, the insulin/insulin-growth-factor-receptor (IGF-1) pathway, a transforming growth factor-β (TGFβ) pathway and a programmed cell death (PCD) pathway (Aballay and Ausubel, 2001; Tan, 2001; Kim et al., 2002; Garsin et al., 2003). Conserved transcription factors – such as the endodermal zinc finger GATA (Kerry et al., 2006; Shapira et al., 2006) and the bZIP transcription factors (Estes et al., 2010) – regulate the expression of genes encoding host effector molecules. These and other aspects of C. elegans as a model to study host-pathogen interactions have been reviewed extensively (Schulenburg et al., 2004; Gravato-Nobre and Hodgkin, 2005; Sifri et al., 2005; Irazoqui et al., 2010) and will not be elaborated on here. We instead focus on the unique features that make C. elegans an ideal model for interrogating neuronal-endocrine-immune connections.
C. elegans as a model to analyze interactions between the nervous system and epithelial immunity
Studies of C. elegans have contributed significantly to the field of neurobiology, furthering our understanding of the development, organization and functioning of the nervous system in an entire organism. Although C. elegans has a simple nervous system, a wide variety of complex behaviors and responses to intrinsic and extrinsic stimuli have been characterized and analyzed (Riddle et al., 1997). As discussed in the previous section, C. elegans has a well-developed and robust innate immune system for fighting off pathogenic challenges. Many features make C. elegans uniquely suited to investigate the complex interactions between the neuronal, endocrine and immune systems (Box 2). These features have facilitated many discoveries and provided insights into how the nervous system affects immunity of the epidermis and the intestine.
Box 2. Unique characteristics of C. elegans as a model for neuro-immune interaction studies.
C. elegans has a well-defined nervous system; the identity of each of its 302 neurons is known and the hardwiring of every neuron has been outlined, to the detail of all the 5000 synapses and 600 gap junctions between neurons being precisely mapped (Albertson and Thomson, 1976; White et al., 1986)
Genetic screens have yielded numerous mutants that affect neuronal function and have led to the delineation of pathways involved in the regulation of neuronal signaling and function (Bargmann and Kaplan, 1998)
Neuronal activation can be monitored by using cellular Ca2+ imaging techniques (Dittman, 2009) and electrophysiological methods (Raizen and Avery, 1994); sophisticated microfluidic tools facilitate the study of neuronal responses under physiological conditions at a cellular level (Ben-Yakar et al., 2009)
Laser-beam-mediated ablation or triggered cell-necrosis-mediated degeneration of individual neurons helps eliminate specific neurons post developmentally (Sulston and White, 1980; Harbinder et al., 1997)
Infections by human pathogens result in rapid mortality, providing a quick and quantitative read out for the outcome of infection (Tan et al., 1999)
Infection-induced changes in the host tissue can be observed in live animals owing to the transparent body (Nicholas and Hodgkin, 2004)
Alterations in gene expression can be monitored using quantitative reverse transcriptase PCR (qRT-PCR) (Mallo et al., 2002; Shapira et al., 2006; Troemel et al., 2006); tissue localization and distribution of genes whose expression changes during infection can be observed using GFP transgenes (Couillault et al., 2004; Alper et al., 2007) – this information can be used to dissect the upstream signaling modules (Alper et al., 2007; Kurz et al., 2007)
Bacterial colonization and persistence can be measured either by enumerating live bacteria recovered from the intestinal lumen or using GFP-tagged bacteria (Alegado and Tan, 2008)
Addition and reconstitution of metabolites and toxic substances can be easily achieved to study the effect of their loss or excess on host physiology and outcome of pathogenic challenge (Nandakumar and Tan, 2008; Green et al., 2009)
High-throughput small-molecule screens for chemical-genetic analysis of immune as well as neuronal processes can be carried out (Artal-Sanz et al., 2006; Moy et al., 2009)
Neuroendocrine regulation of the epidermal immune response
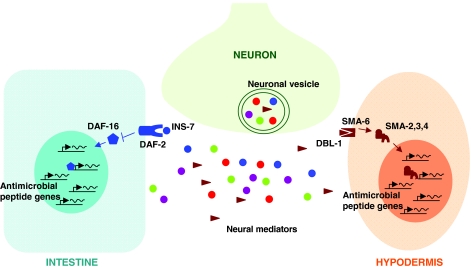
An important function of the skin (epidermis) is to provide antimicrobial defense. The C. elegans epithelial layer surrounding the animal consists of the epidermis (also known as hypodermis) and specialized cells. The epidermis secretes the cuticle to form the external surface of the worm. The epidermis also plays an important role in pathogen defense, and this is best understood from studies involving infection of C. elegans by the fungal pathogen Drechmeria coniospora. The conidia of D. coniospora germinate on contact with the worm cuticle and produce hyphae that can pierce the cuticle and the underlying epidermis. Defense against this fungus includes the induction of antimicrobial peptides encoded by both nlp-29 and the ‘cnc-2 cluster’ (Couillault et al., 2004). Induction of nlp-29 in the epidermis requires cell-autonomous p38 MAPK signaling (Pujol et al., 2008b). By contrast, induction of the epidermis-restricted cnc-2 is independent of p38 MAPK signaling and instead requires the neuronally expressed ligand DBL-1, which activates the C. elegans non-canonical TGFβ pathway (Zugasti and Ewbank, 2009) (Fig. 1). This TGFβ pathway consists of the heterodimeric receptor SMA-6–DAF-4 as well as the downstream signaling components that form the SMAD complex (SMA-2, SMA-3 and SMA-4). Thus, the C. elegans nervous system regulates epidermal immunity against fungal pathogens by producing a cytokine-like signal that induces the expression of antimicrobial peptides. In humans, TGFβ signaling plays a dual role in both pro-and anti-inflammatory responses (Wahl, 2007). However, the mechanisms that mediate these dual effects are not well understood.
Fig. 1.
Nervous system regulation of epithelial and epidermal innate immunity in C. elegans. Simplified depiction of our current understanding of mechanisms by which the nervous system interacts with and instructs the innate immune system in C. elegans. Neural mediators, which include identified neuropeptides (INS-7 and DBL-1), the neurotransmitter dopamine and as-yet-unidentified neuropeptides and neurotransmitters, are secreted from specialized membrane-bound vesicles in neurons and function in a non-cell-autonomous manner to regulate the expression of immune genes in epithelial and epidermal cells through known or unknown immune signaling pathways in C. elegans. Neuronally expressed INS-7 peptide released from dense-core vesicles activates DAF-2 (insulin/IGF-1 receptor) and suppresses expression of DAF-16-regulated antimicrobial genes (Kawli and Tan, 2008). Neuron-derived DBL-1 binds TGFβ receptor SMA-6 and activates the SMAD complex (SMA-2, -3 and -4), which regulates the expression of target genes involved in the immune response (Zugasti and Ewbank, 2009). This schematic is simplified and underestimates the regulatory complexity in space and time, because all interactions are depicted as occurring simultaneously in a single cell of a given cell type. INS-7, neuropeptide ligand for IIR-FOXO; DAF-2, IIR receptor; DAF-16, FOXO transcription factor; DBL-1, TGFβ ligand; SMA-6, TGFβ receptor; SMA-2,3,4, SMAD complex.
Recent studies of vertebrates revealed that skin is a prominent target of neuroendocrine signals that profoundly impact skin biology, including immunity (Arck et al., 2006; Peters et al., 2006). For example, the neuroendocrine system controls the production and secretion of antimicrobials by the epidermis (Aberg et al., 2007). Thus, C. elegans provides a useful system for studying the role of the neuroendocrine system in regulating the epidermal innate immune response.
Neuroendocrine regulation of intestinal immunity
Several features make C. elegans an excellent model in which to investigate interactions between pathogens and intestinal cells. The C. elegans digestive tract (gut) consists of the pharynx, intestine and rectum, with the intestine making up roughly one third of the total somatic mass of the animal. Although the entire intestine is derived clonally from a single cell (the E blastomere of the eight-cell embryo), the cells are morphologically distinct, and might have dedicated functions (McGhee, 2007). First, both extracellular and intracellular infections of the C. elegans intestinal tract have been described (Gravato-Nobre and Hodgkin, 2005; Troemel et al., 2008). Second, C. elegans intestinal epithelial cells possess morphological features that are characteristic of mammalian intestinal cells, such as apical microvilli that are anchored into a terminal web of actin and intermediate filaments. Third, C. elegans is transparent, so colonization of intestinal cells by pathogens and intracellular translocation of fluorescently labeled host proteins can be observed over the course of an infection. Not surprisingly, host-pathogen interactions involving infection of the intestinal epithelial cells are among the most extensively studied infections in C. elegans. These features, together with the findings discussed in the following sections, support the idea that C. elegans is emerging as a facile and powerful system to study how the nervous system affects epithelial immunity.
Neuronal regulation of intestinal immunity though IIR-FOXO signaling
A functional connection between secretion from neuronal dense-core vesicles and intestinal immunity was recently elucidated (Kawli and Tan, 2008). Specifically, mutants defective in neuronal secretion, such as unc-13 and unc-31 mutants, were more resistant to infection by the human pathogen Pseudomonas aeruginosa; this correlated with decreased colonization and increased clearance of the pathogen, as well as enhanced basal and induced expression of immune-related genes. By contrast, mutations in neuronal G protein or Goα-signaling components, or treatments that resulted in a sustained increase in neuronal secretion in worms, increased sensitivity to pathogens. These worms, whose immune responses are similar to those of humans undergoing prolonged psychological stress, have decreased expression of immune-related genes. It was further demonstrated that neuronal activity regulates immunity by controlling secretion of insulin-like peptide (INS-7) from dense-core vesicles. INS-7 activates insulin/IGF-1-receptor (IIR) signaling in the intestinal cells, resulting in cytoplasmic retention of the FOXO transcription factor DAF-16 and decreased expression of antimicrobial genes (Fig. 1). This mechanism of innate immune regulation by Goα signaling through its effect on neuroendocrine secretion might be conserved. For example, the function of UNC-13 following synaptic vesicle docking and prior to vesicle fusion is highly conserved between Drosophila (Aravamudan et al., 1999) and mammals (Brose and Rosenmund, 2002). Mammalian Munc13 proteins are also crucial for insulin secretion (Kang et al., 2006), raising the possibility of neuronal regulation of IIR-FOXO signaling in immunity in mammals.
The importance of neuroendocrine modulation of immune function through IIR-FOXO signaling is further underscored by the discovery that pathogens have evolved mechanisms to subvert this pathway to suppress host immunity (Evans et al., 2008). Infection of C. elegans by P. aeruginosa induces ins-7 expression. Increased INS-7 activates IIR-FOXO signaling, which accelerates the exit of the FOXO transcription factor DAF-16 from nuclei in intestinal cells and suppresses host immune-gene expression. This mechanism of immune suppression requires P. aeruginosa two-component response regulator GacA and the quorum sensing regulators LasR and RhlR. This is distinct from the suppression of host immunity by the type III secretion system, which involves the direct injection of virulence effectors into the host cytoplasm to inhibit or limit the duration of NFκB and MAPK activation. Interestingly, infection with the mouse enteric pathogen Citrobacter rodentium also relocates FOXO3a, the mammalian homolog of DAF-16, to the cytoplasm of intestinal epithelial cells in vivo. FOXO3a translocation from the nucleus to the cytoplasm is mediated by activation of the IIR-FOXO pathway (Snoeks et al., 2008). Together, these observations raise the possibility that suppression of host immunity by inactivation of DAF-16 (FOXO3a in mammals) represents a conserved mechanism that is co-opted by pathogens of invertebrate and vertebrate hosts to suppress host immunity. Further studies using C. elegans will probably uncover additional molecular genetic determinants of pathogen virulence and host immunity as they ‘battle’ over the control of the IIR-FOXO pathway.
Regulation of immunity by dopaminergic signaling
Recently, a role for the neurotransmitter dopamine was demonstrated in C. elegans immunity (Anyanful et al., 2009). Pre-exposure to a virulent or avirulent strain of enteropathogenic Escherichia coli (EPEC) significantly increases the survival of C. elegans in response to a subsequent exposure to EPEC, a phenomenon referred as ‘conditioning’. The conditioning response involves the activation of intestinal antimicrobial genes and requires the IIR-FOXO and p38 MAPK signaling pathways. Conditioning is mediated by dopamine, because mutants defective in dopamine binding, synthesis and uptake are significantly impaired in the conditioning response. C. elegans hermaphrodites have eight dopaminergic neurons, and these have sensory endings that are exposed to the external environment. The dopaminergic pathway has been implicated in the modulation of diverse forms of behavioral plasticity, including food sensing (Sawin et al., 2000; Kindt et al., 2007). Whether dopamine also mediates the detection of pathogens by sensory neurons remains to be determined. It is also currently unclear whether dopamine regulates IIR-FOXO and p38 MAPK signaling pathways directly or indirectly, and how dopamine affects intestinal gene expression is unknown.
Dopamine also plays a role in modulating immunity in mammals (Wrona, 2006): studies have shown that some neurological diseases associated with hypo- and hyperactivity of the central dopaminergic system, such as Parkinson’s disease and schizophrenia, are closely correlated with severely abnormal immune functions (Muller et al., 1993; Basu and Dasgupta, 2000). Further investigation using C. elegans as a model has the potential to shed light on the universal molecular mechanisms by which dopamine influences the immune response.
NPR-1-mediated pathogen resistance
Two independent studies have shown that the C. elegans npr-1 gene, which encodes a G-protein coupled receptor most similar to the mammalian neuropeptide Y (NPY) receptor, is required for resistance to P. aeruginosa (Styer et al., 2008; Reddy et al., 2009). However, the mechanism by which this neuronally restricted protein mediates pathogen resistance remains unresolved. Styer et al. concluded from their study that NPR-1 functions in three sensory neurons to directly regulate the intestinal expression of immune genes regulated by p38 MAPK signaling (Styer et al., 2008). By contrast, Reddy et al. proposed that the observed effect of NPR-1 on immunity was an indirect effect of the influence of npr-1 on oxygen-dependent behavioral avoidance (Reddy et al., 2009). The two studies carried out similar experiments, such as assaying susceptibility under conditions in which contributions of behavior to the outcome of infection were minimized. Yet, despite controlling for possible contribution of behavioral alterations to the outcome of the assays, these studies reported conflicting results. Whereas Styer et al. noted a significant increase in the susceptibility of the npr-1 mutant to pathogens, Reddy et al. reported that pathogen susceptibility of the npr-1 mutant was indistinguishable from that of the wild type. Whether the different conclusions reached by the two groups were due to cryptic differences in strain backgrounds or experimental conditions remains to be established. Another difference in the two studies was the conclusion reached based on the expression pattern of innate immunity genes, which was used to ascertain the effect of npr-1 on the p38 MAPK signaling pathway. Styer et al. compared the expression profile of npr-1 mutants with that of wild-type animals under conditions of P. aeruginosa infection and observed decreased expression of immunity genes in the npr-1 mutant. To rule out the possibility that varying degrees of pathogen exposure contributed to infection-related transcriptional changes, Reddy et al. evaluated the effect of NPR-1 on the basal expression of p38 MAPK 1 (PMK-1)-regulated immune genes and concluded that PMK-1-regulated gene expression was unperturbed in npr-1 mutants. Assessment of gene expression patterns under different conditions can lead to differential conclusions on the effect of npr-1 on the p38 MAPK signaling pathway. Additionally, basal expression, infection-induced expression and fold change upon infection are different analytical parameters that can lead to differential categorization of a target gene for its upstream regulatory signaling pathway.
Despite the different conclusions of these studies, the findings reveal yet another aspect of interaction between the nervous and innate immune systems. Mammalian NPY is involved in the neuronal regulation of immunity through the sympathetic nervous system (Elenkov et al., 2000). Although C. elegans lacks an identifiable NPY gene, the genome contains many predicted neuropeptides that can function as NPR-1 ligands (Bargmann, 1998; Li et al., 1999). Indeed, the FMRF-amide (Phe-Met-Arg-Phe-NH2)-related peptides [FaRPs, which are encoded by FMRF-amide-like neuropeptide (flp) genes] FLP-18 and FLP-21 can function as endogenous ligands for NPR-1 in regulating oxygen sensing (Rogers et al., 2003). The role of these and other related neuropeptides in immunity remains to be investigated. The findings of Reddy et al. also allude to the involvement of behavioral responses in protection from pathogenic challenge; however, an in-depth discussion of the interplay between behavior and immunity is beyond the scope of this article. Whether C. elegans is an appropriate model to investigate conserved NPY-mediated immune regulation remains to be established.
Future perspectives: using C. elegans to address outstanding questions regarding neuro-immune interactions
Activity of the nervous system can profoundly affect the competence of the immune system and hence dramatically influence human health. Given the complexity of the human nervous and immune systems, and genetic heterogeneity between individuals, an in-depth understanding of the mechanisms underlying neuro-immune interactions remains a herculean task. Any therapeutic strategy to intervene requires in-depth understanding of the cellular and molecular mechanisms underlying neuro-immune interactions, some of which are probably conserved across evolution. For the conserved mechanisms, animal models might prove to be important. Features and tools that make C. elegans an attractive model system for genetic analysis of neuro-immune interactions in vivo, as listed in Box 2, have helped to obtain new insights. In the following sections, we propose some additional questions pertaining to neuroendocrine interaction with epithelial immunity that C. elegans might be particularly well suited to address.
Neural mediators of immunity
The C. elegans and vertebrate nervous systems have many parallels and a few striking differences. Genes that function in neurotransmitter biosynthesis and synaptic release, as well as neurotransmitter receptors, are highly conserved between C. elegans and mammals (Bargmann, 1998). The similarities extend to mechanisms of synthesis, release and signaling by these neurotransmitters (Richmond, 2005; Chase and Koelle, 2007; Rand, 2007). Recent studies have implicated two neuronally expressed peptides, INS-7 and DBL-1, as well as the neurotransmitter dopamine, in the regulation of epithelial immune responses to bacterial and fungal pathogens (Kawli and Tan, 2008; Anyanful et al., 2009; Zugasti and Ewbank, 2009). A role for an NPY-like receptor, NPR-1, in pathogen resistance has also been proposed (Styer et al., 2008; Reddy et al., 2009), but the ligand that mediates this process remains to be determined. Potential neural mediators of epithelial immunity in C. elegans that warrant investigation include additional neuropeptides and neurotransmitters.
Neuropeptides
In general, neuropeptides are short sequences of amino acids that are packaged as precursor peptides and processed in dense-core vesicles prior to secretion by neurons (Li and Kim, 2008). In mammals, many neuropeptides, including endogenous opioids (endorphins, enkephalins, dynorphins), substance P and NPY, have been implicated in immune regulation (Zakharova and Vasilenko, 2001; Sternberg, 2006). The C. elegans genome seems not to encode these peptides nor their receptors (Bargmann, 1998); however, it contains 113 predicted genes encoding at least 250 distinct neuropeptides (Li and Kim, 2008) that fall into three large families: (1) INS, the insulin-like peptides (Pierce et al., 2001); (2) FLP (the FaRPs) (Li, 2005); and (3) NLP, the neuropeptide-like proteins that make up a highly diverse group of non-insulin, non-FLP peptides (Nathoo et al., 2001). To date, only INS-7 has been shown to signal through the nervous system to intestinal cells to modulate immune function (Kawli and Tan, 2008). NPR-1 is a neuropeptide receptor and, thus far, FLP-18 and FLP-21 have been shown to function as ligands of NPR-1 for oxygen sensing (Rogers et al., 2003). However, a flp-21 mutant had no detectable immune defects, indicating that FLP-21 is dispensable for NPR-1-mediated pathogen resistance (William C. Chen and M.-W.T., unpublished). The existence of a large neuropeptide gene family in C. elegans leaves open the possibility that additional neuropeptides modulate the immune response.
In addition to their function as signaling molecules, some neuropeptides, such as substance P and NPY, also act as antimicrobials against a wide range of Gram-positive and Gram-negative bacteria and yeasts, demonstrating yet another conserved means of neural-immune cooperation to maximize host survival (Shimizu et al., 1998; Hansen et al., 2006; El Karim et al., 2008). The amphipathic nature and cationic charge might confer antimicrobial ability to some of the neuropeptides (Brogden et al., 2005). In addition to the neurons, the olfactory epithelium, gingival cavity and gastrointestinal tract also secrete some neuropeptides (Dawidson et al., 1997; Marutsuka et al., 2001; Linden et al., 2002; El Karim et al., 2006). C. elegans NLP-31 was shown to have anti-fungal and modest antibacterial activity (Couillault et al., 2004). Interestingly, NLP-29 and CNC-2 (a member of the NLP family), which are expressed in the epidermis, also contribute to anti-fungal defense: overexpression of nlp-29 and cnc gene clusters enhances resistance to the fungus Drechmeria (Couillault et al., 2004; Pujol et al., 2008a; Zugasti and Ewbank, 2009). Whether other C. elegans neuropeptides possess antimicrobial activity remains to be determined.
Neurotransmitters
Neurotransmitters are small chemical messengers that are packaged into specialized membrane-bound vesicles in neurons for immediate release in response to appropriate stimuli. Neurotransmitters, including acetylcholine (ACh), dopamine, serotonin and noradrenaline, have been implicated in the interaction between the nervous and the immune systems in mammals (Mossner and Lesch, 1998; Elenkov et al., 2000; Rosas-Ballina and Tracey, 2009). With the exception of dopamine, which was mentioned earlier, we discuss here the possible role of these neurotransmitters in innate immunity.
Adrenaline and noradrenaline
The classic fight-or-flight response activation of the sympathetic nervous system results in the release of catecholamines, such as adrenaline (epinephrine) and noradrenaline, from the adrenal medulla and sympathetic nerve terminals. These catecholamines mediate their effects through adrenoceptors to elicit a wide range of physiological changes that are able to aid an animal in the face of imminent danger. Noradrenaline and adrenaline are important efferent immune modulators because they can modulate a range of immune-cell activities, including cell proliferation, cytokine and antibody production, lytic activity, and migration (Elenkov et al., 2000). How these neurotransmitters affect immunity of the epithelial cells remains unclear. Although adrenaline and noradrenaline are absent in invertebrates, their functional counterparts in insects and C. elegans are tyramine and octopamine, respectively (Horvitz et al., 1982; Roeder et al., 2003; Alkema et al., 2005). It will be interesting to determine whether these catecholamines function in the C. elegans epithelial immune response.
Acetylcholine
The cholinergic system plays an important role in the inflammatory response (Rosas-Ballina and Tracey, 2009). ACh is a component of the cholinergic system, which acts through muscarinic and nicotinic ACh receptors (mAChRs and nAChRs, respectively) and acetylcholinesterase (AChE). Specifically, ACh functions as an anti-inflammatory signal by binding to nAChRs on macrophages and inhibiting NFκB signaling. The ACh level is controlled by AChE, which hydrolyses ACh. Recently, a mechanism by which a microRNA modulates cholinergic signaling to affect immune response was elucidated in mice (Shaked et al., 2009). First discovered in C. elegans, microRNAs are small non-coding regulatory RNAs that have emerged as important post-transcriptional modulators of gene expression in many tissues, including the nervous and immune systems. For example, in response to inflammatory stimuli, leukocytes induce miR-132 expression: miR-132 binds to the 3′-untranslated region of AChE transcripts, resulting in decreased levels of AChE and a corresponding increase in ACh. Thus, induction of miR-132 expression reduces inflammation.
Although the cholinergic pathway is well characterized in C. elegans, its role in immunity has not been determined. Similarly to that in mice, C. elegans cholinergic signaling is regulated by a microRNA, miR-1 (Simon et al., 2008). miR-1 affects both the amount of ACh released by presynaptic neurons (via a retrograde signal that involves the myogenic transcription factor mef-2) as well as postsynaptic sensitivity of muscle cells to ACh. Interestingly, we have observed that mef-2 mutants are sensitive to P. aeruginosa (Marianne C. Campbell and M.-W.T., unpublished). It is therefore tempting to speculate that worms could use miR-1 to regulate MEF-2 activity and use presynaptic levels of ACh to influence neuro-immune communication.
Serotonin
Serotonin is a monoamine neurotransmitter that is widely distributed in the gut and the central nervous system (CNS), and functions in various neuro-immune interactions. During the inflammatory response, serotonin triggers the release of various chemotactic factors from peripheral blood leukocytes and endothelial cells to attract immune cells to the site of inflammation to clear pathogens (Das et al., 1997). Additionally, serotonin function is required for optimal macrophage activity in both innate and adaptive immunity (Mossner and Lesch, 1998). Whether serotonin affects the C. elegans immune response to pathogenic challenge remains to be fully resolved. C. elegans can detect and avoid pathogenic bacteria, and this ‘associative learning’ is mediated by serotonin (Zhang et al., 2005). A serotonin biosynthetic mutant worm that does not produce detectable serotonin is indistinguishable from wild type with respect to its sensitivity to infection by P. aeruginosa (Zhang et al., 2005; Kawli and Tan, 2008). However, in a separate study using conditions that prevented pathogen avoidance, the same mutant was more susceptible (Shivers et al., 2009). Further studies that compare expression of immune-related genes in the epithelial cells in serotonin-deficient and wild-type animals should help to determine the direct role of serotonin in C. elegans immunity.
γ-aminobutyric acid and glutamate
γ-aminobutyric acid (GABA) and glutamate play a central role in HPA-stress integration. In response to stress, the HPA is activated and immunity is suppressed. Central glutamate systems activate the HPA, whereas GABA inhibits the HPA (Makara and Stark, 1974; Feldman and Weidenfeld, 1997). During stress, GABA also directly influences epithelial immunity. For example, in human volunteers, anxiety and stress are associated with lower levels of salivary immunoglobulin A (IgA). IgA secretion by the oral mucosa offers protection against oral and upper respiratory tract infections. GABA administration prior to stressful stimuli exposure elevates IgA secretion under stress (Abdou et al., 2006).
C. elegans uses both GABA and glutamate as neurotransmitters. C. elegans mutants that are deficient in GABA (due to genetic lesion in the unc-25 gene, which encodes a GABA biosynthetic enzyme) are sensitive to infection by P. aeruginosa. However, the observed pathogen sensitivity is probably indirect and due to a requirement of GABA for defecation, because most defecation-defective animals exhibit increased pathogen sensitivity regardless of their immune status (Shapira and Tan, 2008). The role for GABA in C. elegans immunity has not been completely ruled out because the expression of epithelial immunity genes in GABA-deficient animals has not been determined. In addition, the role of glutamate in C. elegans immunity has not been ascertained.
The importance of neurotransmitters in signaling host stress and immune activation is further highlighted by the presence of diverse mechanisms that pathogens have evolved to detect and respond to them. For example, EPEC can respond to host-derived noradrenaline by triggering translocation of type III effectors into the host cell to derange the host cytoskeleton (Sperandio et al., 2003). Detection of noradrenaline by P. aeruginosa increases the expression of the bacterial PA-1 lectin; PA-1 lectin directly contributes to virulence by facilitating the action of the lethal cytotoxin, exotoxin A (Alverdy et al., 2000). Given that these pathogens can infect C. elegans, it might be possible to investigate how pathogens interact with host-derived neurotransmitters in this model. Additionally, effects of pharmacological agonists or antagonists of neurotransmitters or modulators of their signaling can be easily assayed in C. elegans by feeding with these small molecules (Petrascheck et al., 2007). Coupled with established methods of infecting worms with multiple enteric pathogens, it is possible to use the extensive C. elegans genetic tools to investigate the roles of neuropeptides and neurotransmitters in modulating host-pathogen interactions in vivo.
Additional factors influencing neuro-immune interactions
Diverse environmental and physiological factors influence neuro-immune interactions. However, the molecular mechanisms through which these factors influence nervous system regulation of immunity remains largely unknown. Here, we focus on the effects of aging, nicotine and alcohol on the neuro-immune axis, and discuss how studies of C. elegans could aid our understanding of the molecular mechanisms underlying these perturbations.
Aging
Aging is a programmed progressive decline in organismal function and physiology, and is often associated with increased susceptibility to diseases. Age-related decline in protective immune functions accounts for the increased susceptibility and severity of infections diseases in the elderly. Increased age is also associated with a gradual impairment of the nervous system. Alterations in the nervous and immune systems with advanced age consequently perturb neuro-immune interactions in the elderly (De la Fuente, 2008). Studies of C. elegans have contributed significantly to the field of aging research because this model organism shows several hallmarks of aging that are common to humans (Guarente and Kenyon, 2000; Tatar et al., 2003). Like humans, older worms show increased susceptibility to bacterial infections (Garigan et al., 2002) and display an age-associated decline in nervous system function (Collins et al., 2008). Genetic screens in C. elegans have led to the identification of mutants that have increased longevity as well as those with progeric phenotypes, and the molecular mechanisms underlying these phenotypes have been partly understood. Interestingly, the C. elegans long-lived insulin/IGF-1-receptor mutant is resistant to bacterial infection owing to a robust immune system (Garsin et al., 2003; Murphy et al., 2003; Evans et al., 2008). In addition to the well-characterized IIR-FOXO pathway, which determines lifespan, additional independent mechanisms that regulate longevity have been identified in worms as well as humans (Greer and Brunet, 2008). However, whether these mechanisms influence immune functions and neuro-immune regulation, and how, remains to be elucidated. C. elegans will probably continue to contribute to our understanding of the underlying molecular mechanisms of aging-associated deregulation of neuro-immune interaction.
Nicotine
Inhalation of cigarette smoke is associated with a significantly increased risk of microbial infections (Nuorti et al., 2000; Obeid and Bercy, 2000). Among other effects, cigarette smoke suppresses the antimicrobial activity of alveolar macrophages (King et al., 1988). Nicotine, the active addictive component of cigarette smoke, is an important contributor to the detrimental effects of smoking on immune function, because it influences the central and autonomic nervous systems (Sopori et al., 1998; Singh et al., 2000). Similarly to ACh, nicotine binds to the α7 nAChRs to downregulate immune function (Wang et al., 2003). C. elegans possesses a rich array of nAChRs (Mongan et al., 1998) and responds to nicotine in a manner similar to mammals (Feng et al., 2006). Worm responses to nicotine include an acute response upon exposure, a tolerance response to chronic exposure and a withdrawal response upon removal. Nicotine responses in C. elegans are also dependent on nAChRs (Feng et al., 2006). Recently, the immunosuppressive effect of cigarette smoke on C. elegans immunity was demonstrated (Green et al., 2009). Exposure to cigarette smoke decreases the ability to clear infections and is associated with reduced expression of lpb-7, which encodes a fatty-acid-binding protein. Interestingly, in human bronchial epithelial cells exposed to cigarette smoke, expression of FABP5, the homolog of lbp-7, is also downregulated (Green et al., 2009), suggesting that the effect of cigarette smoke on epithelial immunity is conserved. Whether these effects are mediated by nicotine and how a fatty-acid-binding protein mediates epithelial immunity remains to be determined. Interestingly, exposure to nicotine also increases the sensitivity of C. elegans to bacterial infections (Madhumitha Nandakumar and M.-W.T., unpublished). An association between smoking and downregulation of fatty acid biosynthetic enzymes (specifically delta-6 desaturases required for omega-3 and omega-6 fatty acid biosynthesis) has been noted. In C. elegans, omega-3 and omega-6 fatty acids, γ-linolenic, and stearidonic acids, are crucial for innate immune regulation. Reduction in γ-linolenic and stearidonic acids decreases p38 MAPK activation in intestinal epithelial cells and lowers the expression of antimicrobial genes (Nandakumar and Tan, 2008). Whether cigarette smoke influences p38 MAPK-mediated immunity through lbp-7 or through an effect on fatty acid levels remains an open question. The relative ease of exposing worms to environmental toxic agents suggests that C. elegans can serve as a facile model to uncover molecular mechanisms underlying the effects of nicotine as well as many other environmental factors that influence the neuroendocrine regulation of epithelial immunity in vivo.
Alcohol
Alcohol abuse results in various abnormalities of the immune system and is associated with compromised immunity (Szabo and Mandrekar, 2009). Among its many effects, alcohol influences immune function mainly through the HPA: acute alcohol exposure stimulates the HPA to release neural immunomodulators (Haddad, 2004). Studies in C. elegans have identified presynaptic proteins, including RAB-3 and UNC-18, as ethanol targets, suggesting that ethanol influences neuronal function by affecting crucial steps of vesicle priming (Kapfhamer et al., 2008; Graham et al., 2009). The mouse RAB-3 ortholog also functions in ethanol sensitivity (Kapfhamer et al., 2008), suggesting a conserved mechanism by which ethanol affects neuronal function. The extensive knowledge of the function and regulation of synaptic activity in C. elegans, combined with the ability to assess immune function, offers unexplored avenues for the investigation of the effects of alcohol and other neuroactive compounds on the neuro-immune axis.
Limitations of the C. elegans model for studying neuro-immune interactions
Despite its attractiveness as a model for the study of molecular, cellular and physiological mechanisms underlying the neural regulation of innate immunity, it must be noted that C. elegans, like any model system, has its inherent limitations in informing us about human neuro-immune mechanisms. The first and most crucial is the absence of a classical inflammatory response and of the vasoactive biogenic amine histamine that triggers this response. To induce and resolve inflammation, the CNS and peripheral nervous system induce the secretion of pro- or anti-inflammatory cytokines by immune cells through the secretion of hormones, neuropeptides and neurotransmitters. Studies addressing neuronal regulation of the inflammatory response must therefore be performed in other models, such as zebrafish and mammalian hosts, that have inflammatory responses similar to humans. However, the absence of an inflammatory response in C. elegans offers the opportunity to identify immune pathways or responses under neuroendocrine regulation that might otherwise be masked by the prominence of the inflammatory response. These pathways or responses in C. elegans could potentially provide testable hypotheses and targets for the identification of additional mechanisms of neuronal regulation of innate immune mechanisms in humans.
Second, the C. elegans nervous system lacks the complex structural organization of the mammalian nervous system. For example, in humans, the lymphoid organs and intestine are innervated (Elenkov et al., 2000; Sternberg, 2006). In C. elegans, neither the intestine nor the hypodermis (in which immune responses have been shown to be modulated by the nervous system) possesses any direct innervation or synapse (Sulston and Horvitz, 1977; Sulston, 1983; Kawli and Tan, 2008; Zugasti and Ewbank, 2009). Therefore, other models are necessary to unravel the structural organization and regulation of the neuro-immune axis.
Third, the C. elegans genome does not encode homologs of neuropeptides, such as substance P and NPY, that have been implicated in mammalian neuro-immune interactions (Elenkov et al., 2000; Sternberg, 2006). These differences change the landscape of signaling molecules involved in C. elegans neuro-immune regulation. This scenario could reveal new mediators and features of neuronal regulation of epithelial immunity that could be further tested for conservation in mammals. Alternatively, they might represent regulatory pathways that are unique to C. elegans owing to its evolutionary history. Regarding the latter point, although this information might not be directly relevant to human health, it could be useful for the control of parasitic nematodes, which share a high degree of similarity with C. elegans (De Ley, 2006). Finally, the neuroendocrine system also affects the function of B and T cells of the adaptive immune system, and molecular insights into the antigen-specific immune responses in which these cell types are involved can only be gained from studies of higher vertebrates.
Conclusions
Evidence for the impact of nervous system and immune system interaction on the fine balance between human health and disease states is compelling. Any therapy designed to intervene and sustain a robust immune function in the face of stressful life events must depend on an in-depth understanding of how the activity of the nervous system affects immunity. Such studies in humans have been very challenging owing to the complexity of the nervous system and the genetic heterogeneity of human populations. Studies of C. elegans, with its facile genetics and well-studied innate and nervous systems, are likely to contribute to our understanding of how the neuroendocrine system affects epithelial immunity at the level of the whole organism. Detailed insights into these mechanisms will encourage more targeted investigations in humans. By combining studies in C. elegans with other model systems, obtaining a comprehensive understanding of the complex triad interactions of the neuronal-endocrine-immune system in maintaining homeostasis might be an achievable goal.
Acknowledgments
We thank Madhumitha Nandakumar for critical reading of the manuscript. Research in the Tan lab is supported by grants from the National Institutes of Health. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Aballay A, Ausubel FM. (2001). Programmed cell death mediated by ced-3 and ced-4 protects Caenorhabditis elegans from Salmonella typhimurium-mediated killing. Proc Natl Acad Sci USA 98, 2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou AM, Higashiguchi S, Horie K, Kim M, Hatta H, Yokogoshi H. (2006). Relaxation and immunity enhancement effects of gamma-aminobutyric acid (GABA) administration in humans. Biofactors 26, 201–208 [DOI] [PubMed] [Google Scholar]
- Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, Kerbleski J, Gallo RL, Ganz T, Mauro T, et al. (2007). Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 117, 3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson DG, Thomson JN. (1976). The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 275, 299–325 [DOI] [PubMed] [Google Scholar]
- Alegado RA, Tan MW. (2008). Resistance to antimicrobial peptides contributes to persistence of Salmonella typhimurium in the C. elegans intestine. Cell Microbiol. 10, 1259–1273 [DOI] [PubMed] [Google Scholar]
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. (2005). Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46, 247–260 [DOI] [PubMed] [Google Scholar]
- Alper S, McBride SJ, Lackford B, Freedman JH, Schwartz DA. (2007). Specificity and complexity of the Caenorhabditis elegans innate immune response. Mol Cell Biol. 27, 5544–5553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverdy J, Holbrook C, Rocha F, Seiden L, Wu RL, Musch M, Chang E, Ohman D, Suh S. (2000). Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 232, 480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyanful A, Easley KA, Benian GM, Kalman D. (2009). Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5, 450–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. (1999). Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 2, 965–971 [DOI] [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. (2006). Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 126, 1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal-Sanz M, de Jong L, Tavernarakis N. (2006). Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol J. 1, 1405–1418 [DOI] [PubMed] [Google Scholar]
- Bargmann CI. (1998). Neurobiology of the Caenorhabditis elegans genome. Science 282, 2028–2033 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Kaplan JM. (1998). Signal transduction in the Caenorhabditis elegans nervous system. Annu Rev Neurosci. 21, 279–308 [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS. (2000). Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 102, 113–124 [DOI] [PubMed] [Google Scholar]
- Ben-Yakar A, Chronis N, Lu H. (2009). Microfluidics for the analysis of behavior, nerve regeneration, and neural cell biology in C elegans. Curr Opin Neurobiol. 19, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA, Guthmiller JM, Salzet M, Zasloff M. (2005). The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 6, 558–564 [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C. (2002). Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 115, 4399–4411 [DOI] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. (2007). Biogenic amine neurotransmitters in C. elegans. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Cohen S, Tyrrell DA, Smith AP. (1991). Psychological stress and susceptibility to the common cold. N Engl J Med. 325, 606–612 [DOI] [PubMed] [Google Scholar]
- Collins JJ, Huang C, Hughes S, Kornfeld K. (2008). The measurement and analysis of age-related changes in Caenorhabditis elegans. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Costa-Pinto FA, Palermo-Neto J. (2010). Neuroimmune interactions in stress. Neuroimmunomodulation 17, 196–199 [DOI] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. (2004). TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 5, 488–494 [DOI] [PubMed] [Google Scholar]
- Das AM, Flower RJ, Perretti M. (1997). Eotaxin-induced eosinophil migration in the peritoneal cavity of ovalbumin-sensitized mice: mechanism of action. J Immunol. 159, 1466–1473 [PubMed] [Google Scholar]
- Dawidson I, Blom M, Lundeberg T, Theodorsson E, Angmar-Mansson B. (1997). Neuropeptides in the saliva of healthy subjects. Life Sci. 60, 269–278 [DOI] [PubMed] [Google Scholar]
- De la Fuente M. (2008). Role of neuroimmunomodulation in aging. Neuroimmunomodulation 15, 213–223 [DOI] [PubMed] [Google Scholar]
- De Ley P. (2006). A quick tour of nematode diversity and the backbone of nematode phylogeny. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Dittman J. (2009). Worm watching: imaging nervous system structure and function in Caenorhabditis elegans. Adv Genet. 65, 39–78 [DOI] [PubMed] [Google Scholar]
- El Karim IA, Lamey PJ, Ardill J, Linden GJ, Lundy FT. (2006). Vasoactive intestinal polypeptide (VIP) and VPAC1 receptor in adult human dental pulp in relation to caries. Arch Oral Biol. 51, 849–855 [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Orr DF, Lundy FT. (2008). Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 200, 11–16 [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. (2000). The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 52, 595–638 [PubMed] [Google Scholar]
- Estes KA, Dunbar TL, Powell JR, Ausubel FM, Troemel ER. (2010). bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc Natl Acad Sci USA 107, 2153–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EA, Kawli T, Tan M-W. (2008). Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog. 4, e1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. (1997). Hypothalamic mechanisms mediating glutamate effects on the hypothalamo-pituitary-adrenocortical axis. J Neural Transm. 104, 633–642 [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. (2006). A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 127, 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. (2002). Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 161, 1101–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin DA, Villanueva JM, Begun J, Kim DH, Sifri CD, Calderwood SB, Ruvkun G, Ausubel FM. (2003). Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 300, 1921. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. (2005). Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 5, 243–251 [DOI] [PubMed] [Google Scholar]
- Graff J, Jemielity S, Parker JD, Parker KM, Keller L. (2007). Differential gene expression between adult queens and workers in the ant Lasius niger Mol Ecol. 16, 675–683 [DOI] [PubMed] [Google Scholar]
- Graham ME, Edwards MR, Holden-Dye L, Morgan A, Burgoyne RD, Barclay JW. (2009). UNC-18 modulates ethanol sensitivity in Caenorhabditis elegans. Mol Biol Cell 20, 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravato-Nobre MJ, Hodgkin J. (2005). Caenorhabditis elegans as a model for innate immunity to pathogens. Cell Microbiol. 7, 741–751 [DOI] [PubMed] [Google Scholar]
- Green RM, Gally F, Keeney JG, Alper S, Gao B, Han M, Martin RJ, Weinberger AR, Case SR, Minor MN, et al. (2009). Impact of cigarette smoke exposure on innate immunity: a Caenorhabditis elegans model. PLoS ONE 4, e6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. (2008). Signaling networks in aging. J Cell Sci. 121, 407–412 [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. (2000). Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262 [DOI] [PubMed] [Google Scholar]
- Haddad JJ. (2004). Alcoholism and neuro-immune-endocrine interactions: physiochemical aspects. Biochem Biophys Res Commun. 323, 361–371 [DOI] [PubMed] [Google Scholar]
- Hansen CJ, Burnell KK, Brogden KA. (2006). Antimicrobial activity of Substance P and Neuropeptide Y against laboratory strains of bacteria and oral microorganisms. J Neuroimmunol. 177, 215–218 [DOI] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. (1997). Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci USA 94, 13128–13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. (1999). Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 402, 181–184 [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E, Beutler B. (2004). The interface between innate and adaptive immunity. Nat Immunol. 5, 971–974 [DOI] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. (1982). Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216, 1012–1014 [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Urbach JM, Ausubel FM. (2010). Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol. 10, 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, He Z, Xu P, Fan J, Betz A, Brose N, Xu T. (2006). Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metab. 3, 463–468 [DOI] [PubMed] [Google Scholar]
- Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, McIntire SL. (2008). Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 7, 669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawli T, Tan M-W. (2008). Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol. 9, 1415–1424 [DOI] [PubMed] [Google Scholar]
- Kerry S, TeKippe M, Gaddis NC, Aballay A. (2006). GATA transcription factor required for immunity to bacterial and fungal pathogens. PLoS ONE 1, e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, et al. (2002). A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626 [DOI] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. (2007). Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55, 662–676 [DOI] [PubMed] [Google Scholar]
- King TE, Jr, Savici D, Campbell PA. (1988). Phagocytosis and killing of Listeria monocytogenes by alveolar macrophages: smokers versus nonsmokers. J Infect Dis. 158, 1309–1316 [DOI] [PubMed] [Google Scholar]
- Kioussis D, Pachnis V. (2009). Immune and nervous systems: more than just a superficial similarity? Immunity 31, 705–710 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. (2010). Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress 13, 1–14 [DOI] [PubMed] [Google Scholar]
- Kurz CL, Shapira M, Chen K, Baillie DL, Tan M-W. (2007). Caenorhabditis elegans pgp-5 is involved in resistance to bacterial infection and heavy metal and its regulation requires TIR-1 and a p38 map kinase cascade. Biochem Biophys Res Commun. 363, 438–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. (2005). The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology 131, S109–S127 [DOI] [PubMed] [Google Scholar]
- Li C, Kim K. (2008). Neuropeptides. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org
- Li C, Nelson LS, Kim K, Nathoo A, Hart AC. (1999). Neuropeptide gene families in the nematode Caenorhabditis elegans. Ann NY Acad Sci. 897, 239–25210676452 [Google Scholar]
- Linden GJ, Mullally BH, Burden DJ, Lamey PJ, Shaw C, Ardill J, Lundy FT. (2002). Changes in vasoactive intestinal peptide in gingival crevicular fluid in response to periodontal treatment. J Clin Periodontol. 29, 484–489 [DOI] [PubMed] [Google Scholar]
- Makara GB, Stark E. (1974). Effects of gamma-aminobutyric acid (GABA) and GABA antagonist drugs on ACTH release. Neuroendocrinology 16, 178–190 [DOI] [PubMed] [Google Scholar]
- Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, Kohara Y, Ewbank JJ. (2002). Inducible antibacterial defense system in C. elegans. Curr Biol. 12, 1209–1214 [DOI] [PubMed] [Google Scholar]
- Marutsuka K, Nawa Y, Asada Y, Hara S, Kitamura K, Eto T, Sumiyoshi A. (2001). Adrenomedullin and proadrenomudullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp Physiol. 86, 543–545 [DOI] [PubMed] [Google Scholar]
- McGhee JD. (2007). The C. elegans intestine. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org
- Mongan NP, Baylis HA, Adcock C, Smith GR, Sansom MS, Sattelle DB. (1998). An extensive and diverse gene family of nicotinic acetylcholine receptor alpha subunits in Caenorhabditis elegans. Recept Channels 6, 213–228 [PubMed] [Google Scholar]
- Mossner R, Lesch KP. (1998). Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immunol. 12, 249–271 [DOI] [PubMed] [Google Scholar]
- Moy TI, Conery AL, Larkins-Ford J, Wu G, Mazitschek R, Casadei G, Lewis K, Carpenter AE, Ausubel FM. (2009). High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem Biol. 4, 527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Ackenheil M, Hofschuster E, Mempel W, Eckstein R. (1993). Cellular immunity, HLA-class I antigens, and family history of psychiatric disorder in endogenous psychoses. Psychiatry Res. 48, 201–217 [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. (2003). Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283 [DOI] [PubMed] [Google Scholar]
- Nandakumar M, Tan M-W. (2008). Gamma-linolenic and stearidonic acids are required for basal immunity in Caenorhabditis elegans through their effects on p38 MAP kinase activity. PLoS Genet. 4, e1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, Hart AC. (2001). Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci USA 98, 14000–14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas HR, Hodgkin J. (2004). The ERK MAP kinase cascade mediates tail swelling and a protective response to rectal infection in C elegans. Curr Biol. 14, 1256–1261 [DOI] [PubMed] [Google Scholar]
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, Breiman RF, Active Bacterial Core Surveillance Team (2000). Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 342, 681–689 [DOI] [PubMed] [Google Scholar]
- O’Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. (1999). Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 163, 6718–6724 [PubMed] [Google Scholar]
- Obeid P, Bercy P. (2000). Effects of smoking on periodontal health: a review. Adv Ther. 17, 230–237 [DOI] [PubMed] [Google Scholar]
- Ooi JY, Yagi Y, Hu X, Ip YT. (2002). The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 3, 82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EM, Ericson ME, Hosoi J, Seiffert K, Hordinsky MK, Ansel JC, Paus R, Scholzen TE. (2006). Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 126, 1937–1947 [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB. (2007). An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556 [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, et al. (2001). Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 15, 672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Zugasti O, Wong D, Couillault C, Kurz CL, Schulenburg H, Ewbank JJ. (2008a). Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 4, e1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Cypowyj S, Ziegler K, Millet A, Astrain A, Goncharov A, Jin Y, Chisholm AD, Ewbank JJ. (2008b). Distinct innate immune responses to infection and wounding in the C. elegans epidermis. Curr Biol. 18, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen DM, Avery L. (1994). Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 12, 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB. (2007). Acetylcholine. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org
- Reddy KC, Andersen EC, Kruglyak L, Kim DH. (2009). A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 323, 382–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JD.(2005). Synaptic function. In WormBook (ed. The C. elegans Research Community ). http://www.wormbook.org
- Riddle DL, Blumenthal T, Meyer BJ, Preiss JR. (1997). C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- Roeder T, Seifert M, Kahler C, Gewecke M. (2003). Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch Insect Biochem Physiol. 54, 1–13 [DOI] [PubMed] [Google Scholar]
- Rogers C, Reale V, Kim K, Chatwin H, Li C, Evans P, de Bono M. (2003). Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci. 6, 1178–1185 [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Tracey KJ. (2009). Cholinergic control of inflammation. J Intern Med. 265, 663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631 [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, Muller MB. (2010). A novel chronic social stress paradigm in female mice. Horm Behav. 57, 415–420 [DOI] [PubMed] [Google Scholar]
- Schulenburg H, Kurz CL, Ewbank JJ. (2004). Evolution of the innate immune system: the worm perspective. Immunol Rev. 198, 36–58 [DOI] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. (2009). MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31, 965–973 [DOI] [PubMed] [Google Scholar]
- Shapira M, Tan MW. (2008). Genetic analysis of Caenorhabditis elegans innate immunity. Methods Mol Biol. 415, 429–442 [DOI] [PubMed] [Google Scholar]
- Shapira M, Hamlin BJ, Rong J, Chen K, Ronen M, Tan M-W. (2006). A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci USA 103, 14086–14091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Shigeri Y, Tatsu Y, Yoshikawa S, Yumoto N. (1998). Enhancement of antimicrobial activity of neuropeptide Y by N-terminal truncation. Antimicrob Agents Chemother. 42, 2745–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS, Schneider DS. (2007). Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol. 17, R353–R355 [DOI] [PubMed] [Google Scholar]
- Shivers RP, Kooistra T, Chu SW, Pagano DJ, Kim DH. (2009). Tissue-specific activities of an immune signaling module regulate physiological responses to pathogenic and nutritional bacteria in C. elegans. Cell Host Microbe 6, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri CD, Begun J, Ausubel FM. (2005). The worm has turned-microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13, 119–127 [DOI] [PubMed] [Google Scholar]
- Simon DJ, Madison JM, Conery AL, Thompson-Peer KL, Soskis M, Ruvkun GB, Kaplan JM, Kim JK. (2008). The microRNA miR-1 regulates a MEF-2-dependent retrograde signal at neuromuscular junctions. Cell 133, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Kalra R, Puttfarcken P, Kozak A, Tesfaigzi J, Sopori ML. (2000). Acute and chronic nicotine exposures modulate the immune system through different pathways. Toxicol Appl Pharmacol. 164, 65–72 [DOI] [PubMed] [Google Scholar]
- Snoeks L, Weber CR, Turner JR, Bhattacharyya M, Wasland K, Savkovic SD. (2008). Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells. Infect Immunol. 76, 4677–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, Perryman EK, Snow GE. (1998). Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology 23, 189–204 [DOI] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. (2003). Bacteria-host communication: the language of hormones. Proc Natl Acad Sci USA 100, 8951–8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. (2004). Elaborate interactions between the immune and nervous systems. Nat Immunol. 5, 575–581 [DOI] [PubMed] [Google Scholar]
- Sternberg EM. (2006). Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 6, 318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AA, Bovbjerg DH, Neale JM, Napoli A, Valdimarsdottir H, Cox D, Hayden FG, Gwaltney JM., Jr (1992). Development of common cold symptoms following experimental rhinovirus infection is related to prior stressful life events. Behav Med. 18, 115–120 [DOI] [PubMed] [Google Scholar]
- Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. (2008). Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322, 460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE. (1983). Neuronal cell lineages in the nematode Caenorhabditis elegans. Cold Spring Harbor Symp Quant Biol. 48, 443–452 [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. (1977). Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 56, 110–156 [DOI] [PubMed] [Google Scholar]
- Sulston JE, White JG. (1980). Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 78, 577–597 [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. (2009). A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 33, 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M-W. (2001). Genetic and genomic dissection of host-pathogen interactions using a P. aeruginosa-C. elegans pathogenesis model. Pediatr Pulmonol. 32, 96–97 [Google Scholar]
- Tan MW, Mahajan-Miklos S, Ausubel FM. (1999). Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci USA 96, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. (2003). The endocrine regulation of aging by insulin-like signals. Science 299, 1346–1351 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. (2006). p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2, 1725–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Felix MA, Whiteman NK, Barriere A, Ausubel FM. (2008). Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biol. 6, 2736–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM. (2007). Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 19, 55–62 [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. (2003). Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. (1986). The Structure of the Nervous System of the Nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 314, 1–340 [DOI] [PubMed] [Google Scholar]
- Wrona D. (2006). Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 172, 38–58 [DOI] [PubMed] [Google Scholar]
- Zakharova LA, Vasilenko AM. (2001). The opioidergic system in the combined regulation of pain and immunity. Biol Bull Russ Acad Sci. 28, 280–292 [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. (2005). Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438, 179–184 [DOI] [PubMed] [Google Scholar]
- Zhao C, Wang I, Lehrer RI. (1996). Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 396, 319–322 [DOI] [PubMed] [Google Scholar]
- Zugasti O, Ewbank JJ. (2009). Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol. 10, 249–256 [DOI] [PubMed] [Google Scholar]