Abstract
Timing of the mammalian circadian clock of the suprachiasmatic nucleus (SCN) is regulated by photic input from the retina. Retinorecipient units entrain rhythmicity of SCN pacemaker cells in part through their release of vasoactive intestinal polypeptide (VIP). The underlying nature of this process is conjectural however, as in vivo SCN VIP release has never been measured. Here, SCN microdialysis was used to investigate mechanisms regulating VIP. Hamsters under LD 14:10 exhibited a daily peak in synaptic VIP release near midday. Under constant darkness, this output was arrhythmic. Light and the glutamatergic agonist NMDA stimulated VIP release at night, while the 5-HT1A,7 agonist, 8-OH-DPAT, suppressed release at midday. Thus, SCN VIP activity is stimulated by photic input and inhibited by serotonin.
Keywords: circadian, SCN, microdialysis, VIP, hamster, light, serotonin
INTRODUCTION
The suprachiasmatic nucleus (SCN), located in the anterior hypothalamus, receives direct entraining photic input from the retina via glutamate release from retinohypothalamic tract (RHT) terminals [1, 2]. Retinorecipient cells stimulated by RHT signaling are believed to release vasoactive intestinal polypeptide (VIP) to help synchronize SCN pacemaker cell activity to the light-dark cycle (LD) [3]. Evidence supporting such an action of VIP action consists of findings that intra-SCN administration of VIP at night induces per1 and per2 clock gene expression and causes phase-shifts in clock timing in a manner similar to that produced by light exposure [4, 5]. Also, mice lacking VIP display circadian activity similar to wild-types under a normal light:dark cycle (LD), but are arrhythmic under constant darkness (DD), and those lacking functional VIP (VPAC2) receptors do not maintain circadian rhythmicity under LD and lack rhythmic expression of per1 and per2 [6].
Despite this information, little is known concerning the nature of in vivo VIP regulation. This knowledge gap stems largely from the lack of information on synaptic VIP release in the SCN. The aim of this study, therefore, was to undertake brain microdialysis assessments of VIP in the hamster SCN to characterize its 24 hr release profile under LD and under constant darkness (DD). We also assessed the effects of stimulations with photic phase-resetting stimuli (light pulse and intra-SCN NMDA treatment). Further, given the modulatory role of serotonin (5-HT) in SCN photic signaling, the effect of intra-SCN treatment with 8-OH-DPAT, a 5-HT1A,7 receptor agonist, on VIP release was also explored.
METHODS
Animals
Adult male Syrian hamsters (Mesocricetus auratus) were maintained in a climate-controlled environmental chamber (20–22°C) under a 14L:10D photoperiod (LD; 200–250 lux illuminance). Rodent chow (Prolab 3000; PMI Feeds, Inc.; St. Louis, MO) and water were provided ad libitum. Prior to experimentation animals were acclimated to a circular polycarbonate cage (Raturn; Bioanalytical Systems Inc.; West Lafayette, IN). The experiments were approved by the Kent State Institutional Animal Care and Use Committee and were conducted using the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
SCN microdialysis
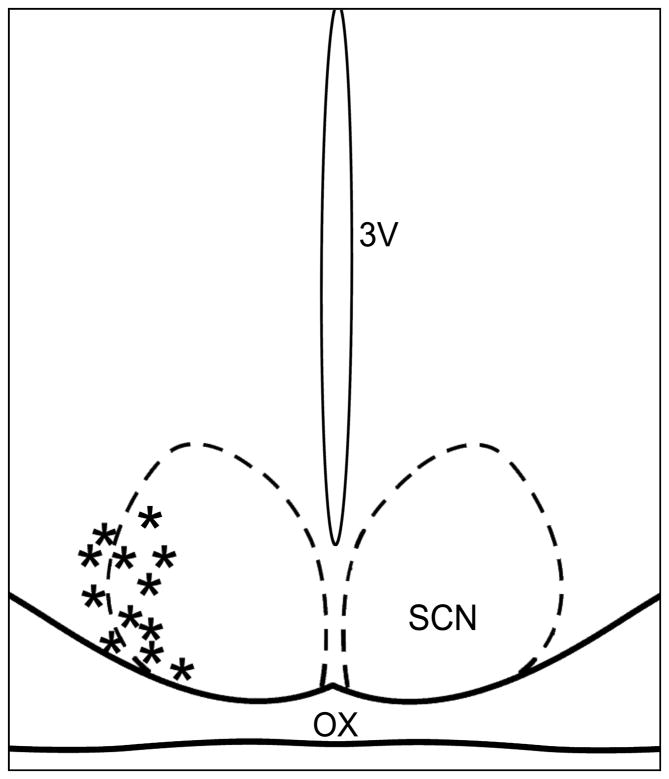
The microdialysis procedures used are similar to those described in previous studies on SCN neuropeptide release [7, 8]. Animals received a probe implant (CMA/12; 20kDa cutoff; CMA Microdialysis, Inc.; North Chelmsford, MA) with the tip aimed at the lateral margin of the SCN (coordinates: AP: +0.03 from bregma, L: +0.04 from midline, H: -0.80 from dura; head level). Following 48 hr of recovery, artificial cerebral spinal fluid (ACSF) was perfused through the probe at a rate of 1.0 μL/min. The microdialysate samples were frozen at −70°C until analysis. Probe tip placement was verified histologically at the end of the experiment (probe tip placement diagrammed in Figure 1).
Figure 1.
Diagrammatic representation of the SCN demonstrating microdialysis probe tip locations verified histologically (*). OX, optic chiasm; 3V, third ventricle.
SCN microdialysate neuropeptide measurements
The content of VIP in SCN microdialysate was measured by radioimmunoassay (RIA; Phoenix Pharmaceuticals, Inc.; Burlingame, CA). The assay is highly specific for VIP, with low cross-reactivity of the primary antibody with other peptides (data supplied with the kit, RK-064-16). The assay sensitivity was ~0.2 pg, with an IC50 of 159 pg/mL. The intra-assay coefficient of variability was 11.7% and the typical peptide yield was ~2.0–60.0 pg/sample.
Circadian locomotor activity measurements
In experiments measuring 24 hr profiles of VIP release under LD (n=7) and DD (n=5), general locomotor activity rhythms were monitored 4–5 days prior to, during and 2 wks after microdialysis sampling to verify a normal pattern of circadian behavioral activity, and to establish subjective circadian time (CT) under DD.
Experimental protocols
Validation of neuronal VIP release
Two separate intra-SCN reverse-dialysis experiments were performed to verify that the VIP collected in microdialysate samples was from neuronal release. First, SCN VIP release was measured following a 1 hr pulse of depolarizing medium consisting of ACSF with high [K+] (150 mM) and veratridine, a sodium channel-activator (100 μM; Sigma; n=4). Second, a calcium channel-blocking cocktail (diltiazem, 200μM; verapamil, 200μM; cinnarizine, 15μM; flunarizine, 12μM in Ca2+-free ACSF with EDTA [10 mM], n=4) was similarly administered. For both trials, microdialysis was initiated two days after probe implantation starting with a 2 hr equilibration period, followed sequentially by a 1 hr baseline collection with normal ACSF, a 1 hr pulse of depolarizing or Ca2+-blocking medium and a 2 hr post-drug sample collection with normal ACSF. Controls using normal ACSF were run in parallel (n=7).
Daily profile of SCN VIP release under LD and DD
Two days after probe implantation, the probe lining was connected to a syringe pump at ZT 4 (LD; n=7) or CT 4 (DD; n=5) and perfusion with ACSF was initiated. After a 2 hr equilibration period, sample collection was initiated at ZT 6 or CT 6 and continued over a 24 hr period. The automated fraction collector was set with a time delay to account for the lag-time of effluent flow from the probe to reach the collector. Microdialysis probe implantation under DD was undertaken by anesthetizing animals in the darkness and masking the eyes with black tape to prevent photic stimulation during surgery. The microdialysis procedures, including probe connection, were undertaken under dim red light (0.5 lux).
Photic and nonphotic treatment effects on SCN peptide release
Here photic (light and glutamatergic) and nonphotic (serotonergic) mechanisms regulating VIP release were evaluated. Two days after probe implantation microdialysis was initiated and hamsters underwent 2 hr of equilibration with normal ACSF. This was followed by a 1 hr baseline collection that preceded a 1 hr light pulse (~280 lux; ZT 13-14; n=4), or 1 hr reverse dialysis perfusion with NMDA (200μM; ZT 13–14; n=3) or 8-OH-DPAT (1.2 mM; ZT 6–7; n=4) in ACSF followed by 2–3 hr of post-treatment sampling.
Statistics
Drug effects were normalized as a percentage of the pretreatment baseline collections and were analyzed by repeated measures ANOVA. Treatment effects were determined using a post-hoc Student Newman-Keuls test. Individual 24 hr profiles of VIP release were normalized by expressing values as a percentage of the daily mean. Daily variations in this release were analyzed as in previous experiments [9] using a repeated measures ANOVA followed by Dunnet’s test procedure for comparing multiple group means [10]. For all procedures the level of significance was set at p<0.05.
RESULTS
Neuronal release of VIP from the SCN
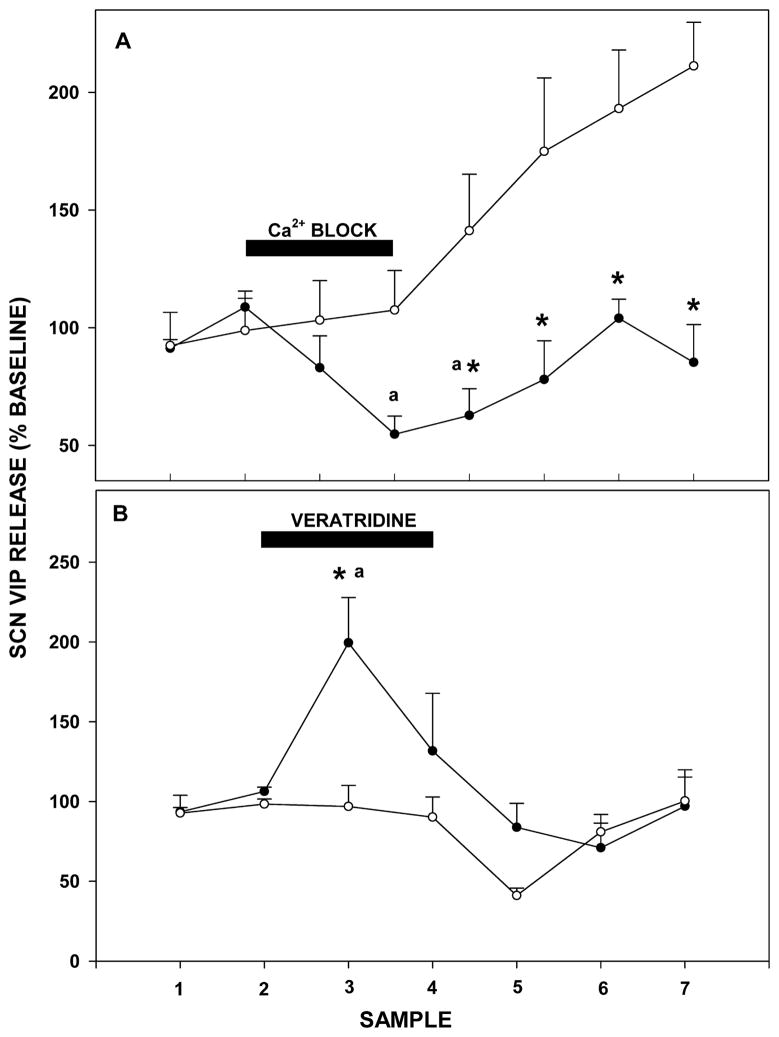
Synaptic VIP release was confirmed by local suppression of SCN neurons with reverse-dialysis perfusion of ACSF containing Ca2+ blockers. This treatment acutely decreased VIP release over pretreatment baseline levels (55±8% of baseline; F4,19=3.846; p=0.019 vs. baseline; Figure 2A). Conversely, similar perfusion with a depolarizing medium of high [K+] ACSF with veratridine stimulated VIP release (199±28%; F7,11=5.111; p<0.001 vs. baseline; Figure 2B).
Figure 2.
A: Suppressive effect of a 1 hr intra-SCN reverse dialysis of Ca2+-free ACSF with EDTA (closed circles) vs. ACSF control (open circles) on SCN VIP release. B: Stimulatory effect of a 1 hr reverse dialysis of ACSF containing veratridine (closed circles) vs. ACSF controls (open circles) on VIP release from the SCN. For each treatment, the sampling interval was 30 min and black bars indicate the time of drug treatment. Values are the mean±S.E.M. * p<0.05 vs. baseline; a p<0.05 vs. vehicle control.
Daily profiles of VIP release from the SCN
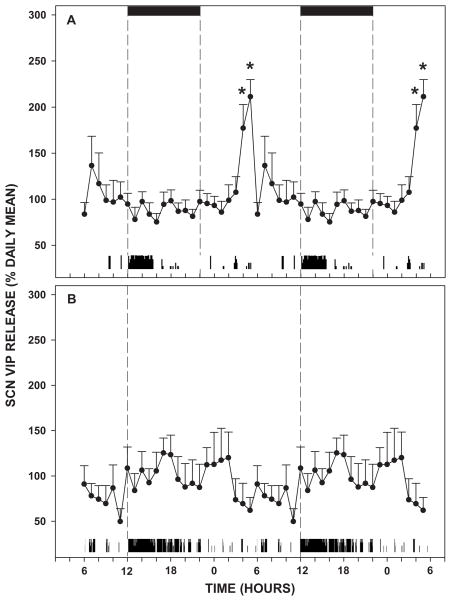
Release under LD
Output of VIP from the SCN was rhythmic under LD, with maximal release near midday (211±19% of the daily mean; F6,23=2.291; p=0.002; Figure 3A). Output was low at lights-on but rose sharply to an acrophase at ZT 5. Thereafter, output quickly dropped and remained low through the remainder of the day and most of the night to a nadir at ZT 16 (75±9% of the daily mean).
Figure 3.
A: Daily profile of SCN VIP release under LD. The 24 hr profiles are double-plotted to highlight the rhythmic diurnal release pattern. Black bars represent the dark-phase. Below each graph is a representative actogram displaying locomotor activity of an animal undergoing microdialysis sampling. B: Average daily profile of VIP release from the SCN under DD. Dotted lines indicate CT 12. The sampling interval was 1 hr and data points are the mean±S.E.M. * p<0.05 vs other time-points.
Release under DD
Under DD, VIP release was arrhythmic. No discernable rhythmic pattern of release was evident (F2,23=1.581; p=0.092; Figure 3B).
Photic and nonphotic (serotonergic) effects on SCN VIP release
Light pulse
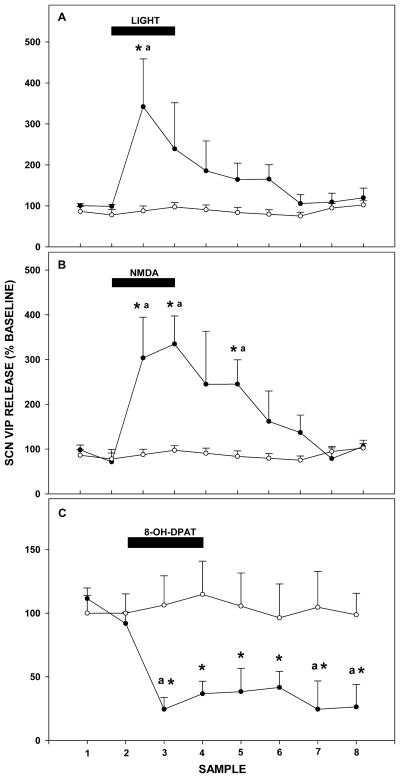
A 1 hr light pulse delivered in the early night (ZT 13–14) caused an acute increase in VIP release from the SCN lasting the duration of the stimulation (342±117% of baseline levels; F6,14=3.539; p<0.001; Figure 4A).
Figure 4.
A: A 1 hr light pulse (closed circles) stimulates VIP release from the SCN vs. ACSF controls (open circles). B: A 1 hr reverse dialysis pulse of the glutamate receptor agonist, NMDA (closed circles), stimulates VIP release from the SCN vs. ACSF controls (open circles). C: A 1 hr reverse dialysis pulse of the 5-HT1A,7 agonist, 8-OH-DPAT (closed circles), suppresses VIP release from the SCN vs. ACSF controls (open circles). For each treatment, the sampling interval was 30 min and black bars represent the time of drug or light treatment. Values are the mean±S.E.M. * p<0.05 vs. baseline; a p<0.05 vs. control.
NMDA
A 1 hr reverse microdialysis perfusion with the glutamate agonist, NMDA, in the early night (ZT 13–14) caused an increase in VIP release from the SCN spanning the duration of drug perfusion, and lasting ~1 hr post-treatment (335±62% of baseline; F6,13=6.265; p<0.001; Figure 4B).
8-OH-DPAT
A 1 hr reverse microdialysis perfusion with the 5-HT1A,7 agonist, 8-OH-DPAT, in midday caused a decrease in VIP release throughout the duration of the drug perfusion, and extending at least 2 hr post-treatment (24±12% of pretreatment baseline; F7,14=3.317; p=0.001; Figure 4C).
DISCUSSION
Photic input to the SCN is essential for synchronizing circadian clock time to the external LD cycle. Based on several lines of evidence, VIP is implicated as a mediator of this process, as light induces c-Fos expression in SCN cells containing VIP and VIP microinjection into the SCN can reset the clock in a manner similar to that of light [11, 12]. However, to date little is known about the regulatory nature of VIP activity in the SCN, as its release from the SCN has never been measured in vivo. Our results show that under LD VIP is rhythmically released from the hamster SCN, with peak release at midday. This is temporally consistent with observations that VIP mRNA in the rat SCN is highest 4 hr prior to lights on, followed by peak levels of VIP-like immunoreactivity approximately 2–6 hr later [13]. Although it is uncertain how daily changes in neuropeptide immunoreactivity may reflect a diurnal pattern of release, the present microdialysis assessments of VIP in the extracellular fluid compartment offer a clear picture of the rhythmic nature of synaptically-released peptide.
The concept that SCN VIP neuronal activity is driven by photic (RHT) input is supported by several observations from the present study. First, the daily fluctuation in VIP release seen under LD is lost under DD. This fits with previous studies where VIP-like immunoreactivity did not vary in animals in DD [14]. Second, light exposure at night increases SCN VIP release. This is consistent with the finding that light also increases per gene expression in VIP-containing neurons [15]. Third, localized perfusion of the SCN with NMDA at night stimulates VIP release with maximal levels (~300% of baseline) similar in magnitude to those induced by photic stimulation (the more prolonged rise induced by the NMDA possibly was due to longer time of clearance of the drug vs. endogenous glutamate). Notably, NMDA also increases VIP release in the SCN slice during the early subjective night [16]. The function of the photically-induced increase in VIP release could be to provide photic input to oscillator cells, as VIP neurons project to AVP-expressing pacemaker neurons of the dorsal SCN shell region [17]. Possibly the midday peak in VIP is a critical cue for pacemaker neuron phase control.
Serotonergic actions in the SCN are also well-documented, with 5-HT particularly implicated in the negative modulation of photic phase-shifting responses. For example, increasing 5-HT by administering selective reuptake inhibitors attenuates light-induced phase-shifts [18] and systemic administration of the 5-HT1A,7 agonist 8-OH-DPAT abolishes light-induced Fos expression in the SCN and attenuates light-induced phase shifts in activity rhythms [19]. Conversely, inhibiting serotonergic signaling with 5-HT receptor antagonists enhances photic shifting [20]. As VIP neurons likely participate in the SCN photic signaling cascade as retinorecipient units [21], and receive innervation from serotonergic fibers [22], it is feasible that their activity could be attenuated by 5-HT. This idea consistent with the present finding that SCN VIP release is strongly suppressed by 8-OH-DPAT. This effect implicates 5-HT1A and/or 5-HT7 receptors in mediating this response, which is supported by reports that postsynaptic 5-HT7 receptors inhibit SCN photic signaling [23, 24]. It is also important to note that a pre-synaptic inhibitory photic effect mediated by SCN 5-HT1B receptors has also been reported [25], but this does not preclude the aforementioned post-synaptic action(s) of 5-HT.
CONCLUSIONS
The in vivo circadian pattern of VIP release from the SCN of hamsters was characterized for the first time using microdialysis. A circadian rhythm of release was expressed under LD, but was abolished under DD. Photic regulation of VIP release was also verified by findings that neuropeptide release was stimulated by nocturnal light and NMDA treatments. Finally, VIP is inhibited by serotonergic activation, suggesting a possible mode for the attenuating action of 5-HT in SCN photic signaling. Collectively, these results indicate a role for VIP in the maintenance of circadian rhythms with respect to environmental lighting, and point to a mechanism for 5-HT in the processing of SCN photic input.
Acknowledgments
Funding received from National Institutes of Health #NS35229 / JDG
ABBREVIATIONS
- 5-HT
serotonin
- 8-OH-DPAT
(±)8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide
- ACSF
artificial cerebro-spinal fluid
- DD
constant darkness
- LD
light-dark cycle
- NMDA
N-methyl-D-aspartate
- RIA
radioimmunoassay
- SCN
suprachiasmatic nucleus
- VIP
vasoactive intestinal polypeptide
References
- 1.Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 2.Ebling FJP. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 3.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur J Neurosci. 2002;15:570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- 5.Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- 6.Harmar AJ, Martson HM, Shen S, Spratt C, West KM, Sheward WJ, et al. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nucleus. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 7.Glass JD, Guinn J, Kaur G, Francl JM. Intrinsic regulation of in vivo neuropeptide Y release in the SCN circadian clock. Eur J Neurosci. 2010;31:1117–1126. doi: 10.1111/j.1460-9568.2010.07139.x. [DOI] [PubMed] [Google Scholar]
- 8.Francl JM, Kaur G, Glass JD. Roles of light and serotonin in the regulation of gastrin-releasing peptide and arginine vasopressin output in the SCN circadian clock. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07374.x. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley TE, DiNardo LA, Glass JD. Endogenous regulation of serotonin release in the hamster suprachiasmatic nucleus. J Neurosci. 1998;18:5045–5052. doi: 10.1523/JNEUROSCI.18-13-05045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zar JH. Biostatistical analysis. Englewood Cliffs: Prentice Hall; 1983. [Google Scholar]
- 11.Castel M, Belenky M, Cohen S, Wagne S, Schwartz W. Light-induced c-Fos expression in the mouse suprachiasmatic nucleus: immunoelectron microscopy reveals colocalization in multiple cell types. Eur J Neurosci. 1997;9:1950–1960. doi: 10.1111/j.1460-9568.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 12.Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto S, Okamura H, Miyake M, Takahashi Y, Takagi S, Akagi Y, et al. A diurnal variation of vasoactive intestinal peptide (VIP) mRNA under a daily light-dark cycle in the rat suprachiasmatic nucleus. Histochemistry. 1991;95:525–528. doi: 10.1007/BF00315750. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara K, Tominaga K, Isobe Y, Inouye SIT. Photic regulation of peptides located in the ventrolateral subdivision of the rat suprachiasmatic nucleus: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res. 2002;958:261–271. doi: 10.1016/s0006-8993(02)03563-1. [DOI] [PubMed] [Google Scholar]
- 16.Shibata S, Ono M, Tominaga K, Hamada T, Watanabe A, Watanabe S. Involvement of vasoactive intestinal polypeptide in NMDA-induced phase delay of firing activity rhythm in the suprachiasmatic nucleus in vitro. Neurosci Biobehav Rev. 1994;18 :591–595. doi: 10.1016/0149-7634(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervations, intrinsic organization, and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 18.Gannon RL, Millan MJ. Evaluation of serotonin, noradrenaline, and dopamine reuptake inhibitors on light-induced phase advances in hamster circadian activity rhythms. Psychopharmacol. 2007;195:325–332. doi: 10.1007/s00213-007-0903-z. [DOI] [PubMed] [Google Scholar]
- 19.Rea MA, Glass JD, Colwell CS. Serotonin modulates photic responses in the hamster suprachiasmatic nuclei. J Neurosci. 1994;14:3635–3642. doi: 10.1523/JNEUROSCI.14-06-03635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smart CM, Biello SM. WAY-100635, a specific 5HT1A receptor antagonist, can increase the responsiveness of the mammalian circadian pacemaker to photic stimuli. Neurosci Lett. 2001;305:33–36. doi: 10.1016/s0304-3940(01)01797-9. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y. The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res Bull. 1993;31:637–640. doi: 10.1016/0361-9230(93)90134-w. [DOI] [PubMed] [Google Scholar]
- 22.Bosler O, Beaudet A. VIP neurons as prime synaptic targets for serotonin afferents in rat suprachiasmatic nucleus: a combined radioautographic and immunocytochemical study. J Neurocytol. 1985;14:749–763. doi: 10.1007/BF01170826. [DOI] [PubMed] [Google Scholar]
- 23.Ying S-W, Rusak B. Effects of serotonergic agonists on firing rates of photically responsive cells in the hamster suprachiasmatic nucleus. Brain Res. 1994;651:37–46. doi: 10.1016/0006-8993(94)90678-5. [DOI] [PubMed] [Google Scholar]
- 24.Quintero JE, McMahon DG. Serotonin modulates glutamate responses in isolated suprachiasmatic nucleus neurons. J Neurophysiol. 1999;82:533–539. doi: 10.1152/jn.1999.82.2.533. [DOI] [PubMed] [Google Scholar]
- 25.Pickard GE, Rea MA. TFMPP, a 5HT1B receptor agonist, inhibits light-induced phase shifts of the circadian activity rhythm and c-Fos expression in the mouse suprachiasmatic nucleus. Neurosci Lett. 1997;231:95–98. doi: 10.1016/s0304-3940(97)00534-x. [DOI] [PubMed] [Google Scholar]