Abstract
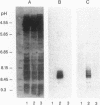
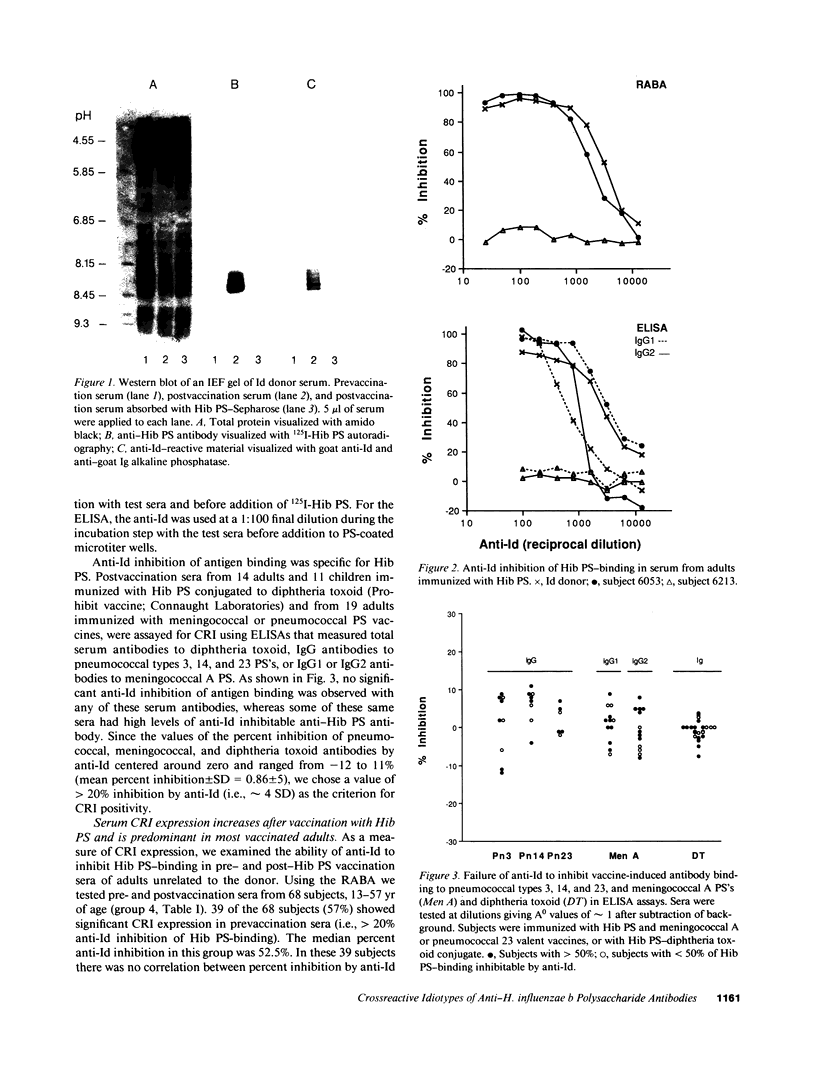
Using idiotypic analysis, we examined the variable (V) region diversity of human antibodies specific for the capsular polysaccharide of Haemophilus influenzae b (Hib PS). A goat anti-idiotypic serum (anti-Id) was prepared against anti-Hib PS antibodies isolated from the serum of an adult immunized with Hib PS. The anti-Id bound donor anti-Hib PS antibodies and inhibited Hib PS binding of donor anti-Hib PS. In contrast, the anti-Id did not bind donor or pooled Ig depleted of Hib PS antibodies, nor did it inhibit antigen binding of human antibodies to pneumococcal PS's, meningococcal A PS or diphtheria toxoid. Crossreactive idiotype (CRI), as measured by anti-Id inhibition of Hib PS binding, was found in 74 of 98 subjects (76%) vaccinated with Hib PS at 1.7-57 yr of age. 60 of these 74 subjects had greater than 50% of their serum Hib PS-binding activity inhibited by anti-Id. No correlation was found between age and CRI expression. In subjects showing both IgG1 and IgG2 antibody responses, CRI was most frequently detected in both subclasses (71% of subjects). CRI was limited to either IgG1 or IgG2 in 19% of subjects, a finding suggestive of independent B cell lineages. 13 of 15 infants less than 17 mo of age, who responded to Hib PS-outer membrane protein conjugate vaccine, had greater than 50% of their serum anti-Hib PS antibody activity inhibited by anti-Id. The ability of native Hib PS and Hib PS oligomer to partially inhibit (60 and 35%, respectively) the binding between anti-Id and heterologous anti-Hib PS, indicated that some CRI determinants are in or near the combining site. In summary, our findings demonstrate a highly penetrant and frequently predominant CRI, which is expressed in both infants and adults. The results underscore the limited V region diversity of anti-Hib PS antibodies and indicate that CRI predominance is manifest early in ontogeny and is induced by both TI and TD forms of the Hib PS antigen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Seiden M. V., Greenspan N. S., Lutz C. T., Bartholow T. L., Clevinger B. L. Structural correlates of idiotopes. Annu Rev Immunol. 1986;4:147–165. doi: 10.1146/annurev.iy.04.040186.001051. [DOI] [PubMed] [Google Scholar]
- Einhorn M. S., Weinberg G. A., Anderson E. L., Granoff P. D., Granoff D. M. Immunogenicity in infants of Haemophilus influenzae type B polysaccharide in a conjugate vaccine with Neisseria meningitidis outer-membrane protein. Lancet. 1986 Aug 9;2(8502):299–302. doi: 10.1016/s0140-6736(86)90001-2. [DOI] [PubMed] [Google Scholar]
- Ely S., Tippett J., Kroll J. S., Moxon E. R. Mutations affecting expression and maintenance of genes encoding the serotype b capsule of Haemophilus influenzae. J Bacteriol. 1986 Jul;167(1):44–48. doi: 10.1128/jb.167.1.44-48.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Cates K. L. Haemophilus influenzae type b polysaccharide vaccines. J Pediatr. 1985 Sep;107(3):330–336. doi: 10.1016/s0022-3476(85)80502-3. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., McKinney T., Boies E. G., Steele N. P., Oldfather J., Pandey J. P., Suarez B. K. Haemophilus influenzae type b disease in an Amish population: studies of the effects of genetic factors, immunization, and rifampin prophylaxis on the course of an outbreak. Pediatrics. 1986 Mar;77(3):289–295. [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Pandey J. P., Boies E. G. Antibody responses to Haemophilus influenzae type b polysaccharide vaccine in relation to Km(1) and G2m(23) immunoglobulin allotypes. J Infect Dis. 1986 Aug;154(2):257–264. doi: 10.1093/infdis/154.2.257. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Suarez B. K., Pandey J. P., Shackelford P. G. Genes associated with the G2m(23) immunoglobulin allotype regulate the IgG subclass responses to Haemophilus influenzae type b polysaccharide vaccine. J Infect Dis. 1988 Jun;157(6):1142–1149. doi: 10.1093/infdis/157.6.1142. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Weinberg G. A., Shackelford P. G. IgG subclass response to immunization with Haemophilus influenzae type b polysaccharide-outer membrane protein conjugate vaccine. Pediatr Res. 1988 Aug;24(2):180–185. doi: 10.1203/00006450-198808000-00008. [DOI] [PubMed] [Google Scholar]
- Griswold W. R., Lucas A. H., Bastian J. F., Garcia G. Functional affinity of antibody to the Haemophilus influenzae type b polysaccharide. J Infect Dis. 1989 Jun;159(6):1083–1087. doi: 10.1093/infdis/159.6.1083. [DOI] [PubMed] [Google Scholar]
- Hetherington S. V. The intrinsic affinity constant (K) of anticapsular antibody to oligosaccharides of Haemophilus influenzae type b. J Immunol. 1988 Jun 1;140(11):3966–3970. [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Kearney J. F., Vakil M. Idiotype-directed interactions during ontogeny play a major role in the establishment of the adult B cell repertoire. Immunol Rev. 1986 Dec;94:39–50. doi: 10.1111/j.1600-065x.1986.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1987 Jun;6(6):519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- Lucas A. H. Expression of crossreactive idiotypes by human antibodies specific for the capsular polysaccharide of Hemophilus influenzae B. J Clin Invest. 1988 Feb;81(2):480–486. doi: 10.1172/JCI113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Deich R. A., Connelly C. Cloning of chromosomal DNA from Haemophilus influenzae. Its use for studying the expression of type b capsule and virulence. J Clin Invest. 1984 Feb;73(2):298–306. doi: 10.1172/JCI111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä O., Mattila P., Rautonen N., Seppälä I., Eskola J., Käyhty H. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J Immunol. 1987 Sep 15;139(6):1999–2004. [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Rao P., Pattabiraman T. N. Reevaluation of the phenol-sulfuric acid reaction for the estimation of hexoses and pentoses. Anal Biochem. 1989 Aug 15;181(1):18–22. doi: 10.1016/0003-2697(89)90387-4. [DOI] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Ward J. I., Band J. D., Hightower A., Fraser D. W., Broome C. V. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985 Mar 22;253(12):1749–1754. [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Scott M. G., Briles D. E., Shackelford P. G., Smith D. S., Nahm M. H. Human antibodies to phosphocholine. IgG anti-PC antibodies express restricted numbers of V and C regions. J Immunol. 1987 May 15;138(10):3325–3331. [PubMed] [Google Scholar]
- Scott M. G., Tarrand J. J., Crimmins D. L., McCourt D. W., Siegel N. R., Smith C. E., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. II. IgG antibodies contain VH genes from a single VH family and VL genes from at least four VL families. J Immunol. 1989 Jul 1;143(1):293–298. [PubMed] [Google Scholar]
- Shackelford P. G., Granoff D. M., Nelson S. J., Scott M. G., Smith D. S., Nahm M. H. Subclass distribution of human antibodies to Haemophilus influenzae type b capsular polysaccharide. J Immunol. 1987 Jan 15;138(2):587–592. [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Stein K. E. Network regulation of the immune response to bacterial polysaccharide antigens. Curr Top Microbiol Immunol. 1985;119:57–74. doi: 10.1007/978-3-642-70675-2_5. [DOI] [PubMed] [Google Scholar]
- Tarrand J. J., Scott M. G., Takes P. A., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type B polysaccharide. Demonstration of three types of V regions and their association with H and L chain isotypes. J Immunol. 1989 Apr 1;142(7):2519–2526. [PubMed] [Google Scholar]
- Wang A. C., Wang I. Y., Fudenberg H. H. Immunoglobulin structure and genetics. Identity between variable regions of a mu and a gamma2 chain. J Biol Chem. 1977 Oct 25;252(20):7192–7199. [PubMed] [Google Scholar]