Abstract
Obesity has generally been thought to increase the risk of operative mortality and postoperative complications in surgical patients. No data examining obesity as a factor in cadaveric renal transplantation were available. We therefore matched obese patients undergoing cadaveric renal transplantation with nonobese control patients and retrospectively analyzed mortality, morbidity, and graft survival in each group. Patients were matched for age, sex, diabetes mellitus, PRA, graft number, cardiovascular disease, date of transplantation, and posttransplant immunosuppression. There were significant differences found in mortality (11% in obese vs. 2% in nonobese patients, P≤0.01), immediate graft function (38% in obese vs. 64% in nonobese patients, P≤0.01), 1-year graft survival (66% in obese vs. 84% in nonobese patients, P≤0.05), and postoperative complications. Wound complications (20% vs. 2%, P≤0.01), intensive-care-unit admissions (10% vs. 2%, P≤0.01), reintubations (16% vs. 2%, P≤0.03), and new-onset diabetes (12% vs. 0%, P≤0.02) were all significantly more common in the obese group. These results suggest that an attempt at significant weight reduction is indicated in obese patients prior to renal transplantation.
Obesity is generally thought to be associated with an increased risk in surgical patients (1). Operative mortality tends to be higher (2-4), and postoperative complications, especially wound infections (1, 3-5), are more common. There are however little data describing the results after cadaveric renal transplantation in obese patients. The experience at the University of Pittsburgh was retrospectively analyzed to determine patient and graft survival as well as the incidence of various posttransplant complications. Obesity was defined as a body mass index of greater than 30, a commonly accepted definition (6, 7). A matched control group of nonobese renal transplant recipients was used for comparison.
MATERIALS AND METHODS
The medical records of obese patients with end-stage renal disease undergoing cadaveric renal transplantation between January 1986 and December 1988 at the University of Pittsburgh were reviewed. Living-related kidney and multiple-organ transplant recipients were excluded from the study. Obesity was defined as a body mass index (BMI = weight in Kg/height in m2) of ≥30 (7). It should be noted that morbid obesity is defined as a BMI >35. Thus, only 13 of our 46 obese patients could be described as morbidly obese. The parameters examined included: (1) patient survival; (2) graft survival; (3) initial graft function (defined as clearance of creatinine without dialysis by 1 week posttransplant); (4) duration of the operation; (5) need for reintubation postoperatively, after initial extubation; (6) admission to an intensive care unit; (7) colonic distension requiring either colonoscopic or surgical decompression; (8) wound complications including hematoma, infection, and dehiscence; (9) urinary-tract infections requiring antibiotic therapy; (10) duration of initial hospitalization; and (11) new-onset diabetes.
The records of control (nonobese, BMI ≤27 for men, ≤25 for women) patients were examined for the same parameters as the obese patients. Each obese patient was matched with a control patient for age, sex, transplant graft number, diabetes mellitus at the time of transplantation, pretransplant panel-reactive antibody within 30%, and pretransplant cardiovascular disease, defined by either a thallium stress test or coronary angiography and/or myocardial function by nuclear imaging (MUGA) or echocardiography. Control patients were also matched for date of transplantation (within 15 months) and posttransplant immunosuppression. From January 1986 through January 1987, CsA and prednisone immunosuppression was used in all patients. From January 1987 through January 1988, approximately 50% of the transplant patients followed a triple-drug (azathioprine, CsA, and prednisone) immunosuppression protocol; the remaining patients were maintained on a CsA and prednisone regimen. After February 1988, all patients received the triple-drug protocol. Steroid-resistant rejection episodes were treated with a 10–14 day course of monoclonal antibody (OKT3). Patients with a high PRA (>50%) and/or previous transplants were treated with induction OKT3 therapy.
Statistical methods
The statistical significance of differences between proportions was determined by chi-square analysis with Yates' correction. Data were also analyzed between the matched subjects for differences in proportions by the McNemar's test (8). Comparison among groups was performed by t test for independent samples. Survival analysis was done by Mantel-Cox test. Probability less than 0.05 was considered significant. Data are given as mean values.
RESULTS
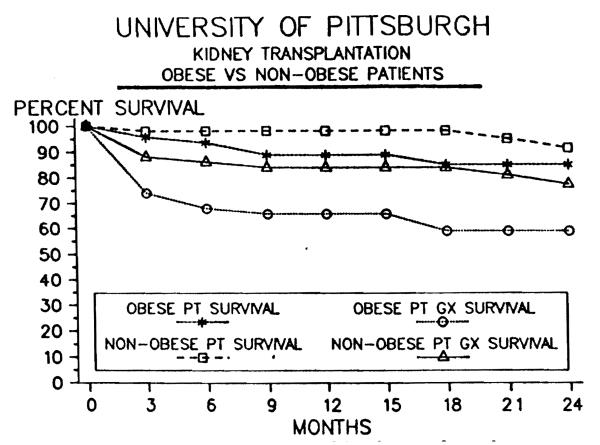
Table 1 demonstrates the postoperative morbidity and mortality observed in the 2 patient groups. Using the McNemar's test, significantly more patient deaths occurred during the initial hospital period in the obese group (5) than in the control group (1). Sepsis was the cause of all deaths in the obese patients: two of pulmonary etiology, two following cecal perforation, and one due to an abdominal abscess after a long and complicated hospital course. The patient death in the control group was secondary to sepsis caused by pyelonephritis. Figure 1 depicts patient survival over a 2-year follow-up period and shows no statistical difference between the poups but illustrates a trend toward poorer survival in the obese patients; 1-year patient survival 89% in obese patients vs. 98% in control patients (Fig. 1). The overall patient survival at the University of Pittsburgh during this period was 93% at 1 year and 90% at 2 years after cadaveric renal transplantation.
Table 1.
Postoperative morbidity and mortality
| Obese n(%) |
Control n(%) |
P value | |
|---|---|---|---|
| Deaths | 5 (11) | 1 (2) | 0.01a |
| Reintubation | 8 (16) | 1 (2) | 0.03 |
| ICU admission | 10 (20) | 1 (2) | 0.01 |
| Wound complications | 10 (20) | 1 (2) | 0.01 |
| New onset diabetes | 6 (12) | 0 | 0.02 |
| + Initial function | 19 (38) | 32 (64) | 0.01 |
| Duration of surgery (min) | 301±123 | 230±69b | |
| Days of hospitalization | 39±47 | 23±12c |
McNemar's test for matched subjects. All other data expressed as results of chi-square analysis.
P≤0.003.
P≤0.02.
Figure 1.
Patient and graft survival for obese and nonobese groups.
Initial allograft function and ultimate graft survival were both significantly worse in the obese group. Thirty-eight percent of the obese patients were clearing creatinine by 1 week posttransplant versus 64% of the control group (P≤0.01, Table 1). One-year acturial graft survival was 66% in the obese versus 84% in the nonobese group, P≤0.05 (Fig. 1).
Differences between the patient groups were noted in duration of surgery and hospitalization, both significantly longer in the obese patients (Table 1). The groups were matched, therefore no differences were appreciated in mean age, sex, presence of diabetes mellitus, PRA, transplantation date, or immunosuppressive protocol (Table 2).
Table 2.
Patient group characteristics
| Obese (n = 46) | Control (n = 50) | |
|---|---|---|
| No. transplants | 50 | 50 |
| Mean age (years) | 44.7 | 44.9 |
| Sex (F/M) | 21/25 | 23/27 |
| Mean BMI (range) | 33.5 (30–45) | 23.3 (18–27) |
Significantly increased morbidity occurred in the obese group including 10 intensive-care-unit admissions, 8 reintubations, and 10 wound complications (either hematoma, infection, or dehiscence, Table 1). There was no difference between the obese and control groups in the occurrence of urinary-tract infections or colonic distension requiring colonoscopic or surgical intervention. Two patients in the obese group however died because of colonic distension and subsequent sepsis. Significantly more obese patients who were not diabetic before transplantation required insulin therapy for glucose control after transplantation.
DISCUSSION
Obesity is known to increase the risk of surgical procedures (1), but little data are available regarding the risks of obesity associated with renal transplantation. In 1986 Pasulka et al. outlined areas in which obesity increased surgical risk. These1 included anesthetic problems of airway control and venous access problems; impaired lung function in approximately one third of obese patients (9); an increase in cardiac stroke work leading to circulatory problems (10), thromboembolic, and wound complications; and technical considerations, including exposure, hemostasia, anastomotic function, and wound apposition (1). The overall operative mortality has not been shown to be significantly increased in obese patients undergoing various surgical procedures. Trends however toward higher mortality rates in obese patients have been repeatedly noted (1-3, 11). Small numbers of patients in all these studies may compromise the statistical results. We have shown a significantly decreased patient survival during the initial hospital period in the obese patients.
Complications in the areas of anesthesia (1 technical complication of venous-access placement in an obese patient) and respiratory insufficiency (reintubation, required in 16%, Table 2), as suggested by Pasulka (1), were seen in our obese patients. Additionally, complications of increased stroke work were plausible in 2 control patients and 4 obese patients. Postoperative pulmonary edema occurred as a result of fluid administration in the setting of end-stage renal disease, most often in patients with some degree of myocardial dysfunction. Ultrafiltration therapy resolved the problem in all cases. Admission to an intensive care unit was more common in the obese group (20% vs. 2%) and was prompted by the need for reintubation (8 patients, 4 with associated sepsis) in most instances. Two obese patients required ICU admission for cardiovascular reasons. In 1 case this was due to a perioperative myocardial infarction and in another, a supraventricular arrthymia.
The most frequently reported postoperative complication in obese patients is wound infection (2-4) and wound disruption (2,5). Postlethwait has suggested that longer operations (due to technical difficulties), increased trauma to the abdominal wall from vigorous retraction, the low resistance of fat to infection, and an inability to obliterate dead space in the abdominal wall fat are all contributing factors (3). The increased wound area in obese patients has also been postulated as a major factor leading to higher rates of wound infection in these patients (12). We observed a significantly higher incidence of wound complications in our obese patients (20% vs. 2% in the nonobese patients, P≤0.01) and also noted longer operations in the obese group. Transplant nephrectomy, urine leak, and hematoma are associated with a higher incidence of wound infection in the transplant population (13, 14). The single wound complication in the control group was associated with a urine leak. Four of the 10 wound complications seen in the obese group occurred after transplant nephrectomy, and one followed a wound hematoma. The remaining five, however, may reflect the combined negative effects of prolonged surgery and the low resistance of fat to infection (3). The latter factor may be amplified in the immunosuppressed state that accompanies renal transplantation.
Acute colonic ileus is a recognized complication of renal transplantation aod is often a serious cause of morbidity and mortality from cecal perforation (15, 16). Two obese patients died of sepsis related to cecal perforation caused by Ogilvie's t syndrome, but no statistical differences in rates of colonic complications were noted between the obese and control groups.
Steroid-induced diabetes has long been recognized in the posttransplant population (17). The insulin resistance and impaired glucose tolerance seen with obesity (18,19), combined with steroid immunosuppression may exacerbate this metabolic iff derangement in obese transplant recipients. The additional hyperglycemic effects of CsA (20,21), presumably via impaired insulin secretion (22, 23), warrants further study in this group of patients.
Initial graft function and long-term graft survival (Table 2, Fig. 1) were both significantly worse in the obese patients. The reasons for these observations remain speculative and require prospective study. Factors such as longer graft warm ischemia time, due to the technical challenge the vascular anastomoses offer and, secondly, possible altered CsA pharmacokinetics in obese patients may both contribute to lower paft survival. Postoperative complications in the obese patients occurred significantly more often than in the control patients and no doubt contributed to poorer graft survival in the former group of patients.
Since each patient was matched for demographic characteristics, obesity was identified as an independent risk factor for successful renal transplantation. Mortality during the initial hospital period was increased in the obese population. More wound complications and an increased need for insulin therapy occurred in obese versus control patients. Serious complications requiring ICU admission and reintubation were also more frequent in this group of patients. In addition to higher rates of postoperative complications and longer hospital stays, graft function in these patients was significantly worse than in the control population. All these observations suggest that preoperative weight reduction should be stressed in obese patients prior to kidney transplantation. Furthermore, additional study is needed in order to elucidate the factors contributing to poorer graft survival in obese patients.
Acknowledgments
We thank Beth Piraino, M.D., for assistance in data analysis and for reviewing the manuscript.
REFERENCES
- 1.Pasulka PS, Bistrian BR, Benotti PN, et al. The risks of surgery in obese patients. Ann Int Med. 1986;104:540. doi: 10.7326/0003-4819-104-4-540. [DOI] [PubMed] [Google Scholar]
- 2.Pitkin RM. Abdominal hysterectomy in obese women. Surg Gynecol Obstet. 1976;142:532. [PubMed] [Google Scholar]
- 3.Postlethwait RW, Johnson WD. Complications following surgery for duodenal ulcer in obese patients. Arch Surg. 1972;105:438. doi: 10.1001/archsurg.1972.04180090043011. [DOI] [PubMed] [Google Scholar]
- 4.Pemberton LB, Manax WG. Relationship of obesity to postoperative complications after cholecystectomy. Am J Surg. 1971;121:87. doi: 10.1016/0002-9610(71)90081-x. [DOI] [PubMed] [Google Scholar]
- 5.Strauss RJ, Wise L. Operative risks of obesity. Surg Gynecol Obstet. 1978;146:286. [PubMed] [Google Scholar]
- 6.Krai JG, Heymsfield S. Morbid obesity: definitions, epidemiology, and methodological problems. Gastroent Clin North Am. 1987;16:197. [PubMed] [Google Scholar]
- 7.Obesity in America. Dept Health, Education and Welfare, Public Health Service . PHS; Nov, 1979. (National Institute of Health publication No. 79-359). [Google Scholar]
- 8.Schefler WC. Statistics for health professionals. Vol. 234. Addison-Wesley; Reading, MA: 1984. [Google Scholar]
- 9.Ray CS, Sue DY, Bray G, et al. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 10.Argarwal N, Shibutani K, SanFilippo JA, et al. Hemodynamic and respiratory changes in surgery of the morbidly obese. Surgery. 1982;92:226. [PubMed] [Google Scholar]
- 11.Prem KA, Mensheha NM, McKelvey JL. Operative treatment of adenocarcinoma of the endometrium in obese women. Am J Obstet Gynecol. 1965;92:16. doi: 10.1016/0002-9378(65)90100-6. [DOI] [PubMed] [Google Scholar]
- 12.Nystrom P-O, Jonstam A, Hojer H, et al. Incisional infection after colorectal surgery in obese patients. Acta Chir Scand. 1987;153:225. [PubMed] [Google Scholar]
- 13.Lee HM, Madge GE, Mendez-Picon G, et al. Surgical complications in renal transplant recipients. Surg Clin North Am. 1978;58:285. doi: 10.1016/s0039-6109(16)41484-2. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakides GK, Simmons RL, Najarian JS. Wound infections in renal transplant wounds: pathogenetic and prognostic factors. Ann Surg. 1975;182:770. doi: 10.1097/00000658-197512000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powis SJA, Barns AD, Dawson-Edwards P, et al. Ileocolonic problems after cadaveric renal transplantation. Br Med J. 1982;1:99. doi: 10.1136/bmj.1.5792.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratta RJ, Starling JR, D'Alessandro AM, et al. Acute colonic ileus (pseudoobstruction) in renal transplant recipients. Surgery. 1988;104:616. [PubMed] [Google Scholar]
- 17.Ruiz JO, Simmons RL, Callender CO, et al. Steroid diabetes in renal transplant recipients: pathogenetic factors and prognosis. Surgery. 1973;73:759. [PubMed] [Google Scholar]
- 18.Rabinowitz D, Zierler KL. Forearm metabolism in obesity and its response to intra-arterial insulin: characterization of insulin resistance and evidence for adaptive hyperinsulinemia. J Clin Invest. 1962;41:2173. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toeller M, Gries FA, Dannehl K. Natural history of glucose intolerance in obesity: a ten year observation. Int J Obes. 1982;6(suppl 1):145. [PubMed] [Google Scholar]
- 20.Yoshimura N, Nakai I, Ohmori Y, et al. Effect of cyclosporine on the endocrine and exocrine pancreas in kidney transplant recipients. Am J Kidney Dis. 1988;12:11. doi: 10.1016/s0272-6386(88)80065-9. [DOI] [PubMed] [Google Scholar]
- 21.Roth D, Milogrom M, Esquenazi V, et al. Post transplant hyperglycemia. Transplantation. 1989;47:278. [PubMed] [Google Scholar]
- 22.Nielson JH, Mandrup-Paulsen T, Nerup J. Direct effects of cyclosporin A on human pancreatic B-cells. Diabetes. 1986;35:1049. doi: 10.2337/diab.35.9.1049. [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP. Cyclosporin-induced inhibition of insulin secretion in isolated rat islets and HIT cells. Diabetes. 1986;35:1016. doi: 10.2337/diab.35.9.1016. [DOI] [PubMed] [Google Scholar]