Abstract
A Phormidium spp. collection from Key West, Florida afforded caylobolide B (1), an analogue of the known macrolactone caylobolide A, previously isolated from a Lyngbya majuscula collection from the Bahamas. The planar structure of 1 was determined using NMR and MS experiments. The relative configuration for subunits C7–C9 and C25–C29 was assigned using Kishi's Universal NMR database. Caylobolide B (1) displayed cytotoxic activity against HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells with IC50 values of 4.5 μM and 12.2 μM, respectively.
Marine cyanobacteria have been prolific sources of bioactive and structurally intriguing natural products. Secondary metabolites from these organisms mainly consist of nonribosomally derived peptides and peptide-polyketide hybrids.1,2 Pure polyketides, assembled only by polyketide synthases, represent a minor fraction of isolated compounds from the phylum Cyanobacteria. These usually polyhydroxylated compounds are reminiscent of secondary metabolites from dinoflagellates3 such as the cytotoxic amphidinolides, amphidinols and luteophanols as well as bacteria-derived antibiotics, desertomycins4 and oasomycins.5
Polyketides from marine and terrestrial cyanobacteria also possess interesting biological activities and may be decorated with unusual moieties. Tolytoxin and the related scytophycins, produced by terrestrial cyanobacteria, are potent cytotoxins.6 Tolytoxins are distinguished by an epoxide substituent in their backbone structure. Oscillariolide,7 a polyketide isolated from the genus Oscillatoria, inhibited the development of fertilized echinoderm eggs, suggestive of its effects on cell division. Phormidolide,8 a compound related to oscillariolide, was isolated from the genus Phormidium and is also a potent cytotoxin. Both oscillariolide and phormidolide macrocycles contain a tetrahydrofuran ring and a terminal vinyl bromide appended to their ring structure. In addition, one hydroxy group in phormidolide is esterified with a C-16 carboxylic acid. The well-studied marine cyanobacterium Lyngbya majuscula afforded the polyketide caylobolide A that is characterized by its contiguous pentad of 1,5 diols.9
The structure elucidation of polyketides is particularly challenging due to difficulty in establishing the relative and absolute configuration of the multiple stereocenters and substantial overlap in the methylene region. Their configurational assignment has greatly benefited from the development of Kishi's Universal NMR database10–12 as well as derivatization techniques, particularly Mosher's analysis13 and extensions of this method,14 although applications still have certain limitations, particularly for those bearing 1,n diol (n > 5) moieties. Assignment of the configuration of 1,n diols has so far been demonstrated on model systems using exciton coupling CD after derivatization with arylcarboxylate chromophores within liposomes.15
In this paper, we report the isolation, structure elucidation, and cytotoxic activity of a new macrolactone analogue of caylobolide A9 from a collection of Phormidium spp., termed caylobolide B (1). Compound 1 showed micromolar cytotoxic activity against several cancer cell lines.
A freeze-dried sample of an assemblage of P. cf. dimorphum and P. inundatum from Key West, Florida was extracted with EtOAc–MeOH (1:1). The resulting nonpolar extract was solvent partitioned to yield the hexanes-, n-BuOH- and H2O- soluble fractions. The n-BuOH fraction was cytotoxic to HT29 colorectal adenocarcinoma cells and subjected to bioactivity-guided isolation using silica gel chromatography and reversed-phase HPLC to yield caylobolide B (1) (Figure 1). The major cytotoxic activity was attributed to the known compound symplostatin 1, a microtubule depolymerizing agent, as evidenced from comparison of 1H NMR and LRESIMS data with reported values.16–18
Figure 1.
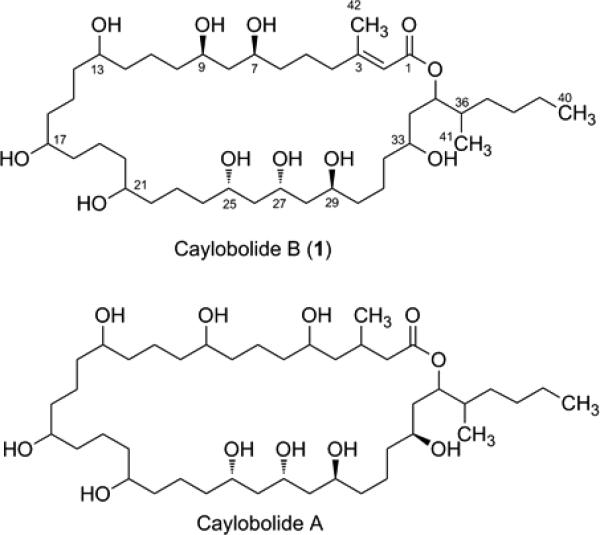
Caylobolide B (1) and closely related compound caylobolide A. The absolute configuration for C25, C27 and C29 is proposed by analogy to caylobolide A. Only the relative configuration is shown for C7 and C9, which could not be related to C25–C29.
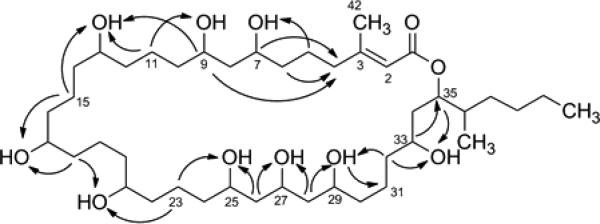
Caylobolide B (1) was isolated as a colorless, amorphous solid with molecular formula of C42H80O11 based on pseudomolecular ion peaks observed by HRESI/APCIMS at m/z 761.5767 [M + H]+ and m/z 783.5594 [M + Na]+. Fragmentation of the [M + H]+ peak using positive ionization showed repetitive loss of 18 amu, corresponding to elimination of H2O typical for alcohols. The structure of 1 was determined by NMR analysis in DMSO-d6. The presence of exchangeable hydroxy protons was evident from the lack of HSQC correlations for nine protons which resonate at δH 4.2 – 4.6 ppm. Detailed interpretation of HSQC, TOCSY, HSQC-TOCSY and HMBC experiments with 1 (Table 1, Figure 2) established that the hydroxy groups are part of methine carbinols that form a highly oxygenated backbone structure consisting of a 1,3-diol system (C-7, C-9), a 1,3,5-triol system (C-25, C-27, C-29) and repeating 1,5-diol moieties. Degenerate 1H and 13C NMR chemical shifts were observed for three oxygenated methines at δC 69.6 (C-13, C-17, C-21), seven methylenes at δC 37.3 (C-12, C-14, C-16, C-18, C-20, C-22, C-24), and two methylenes at δC 21.6 (C-15, C-19) that make up the contiguous chain of 1,5-diol. The 13C NMR chemical shifts are in good agreement with reported values for the 1,5-diol units of luteophanol.19 These degenerate signals together with HSQC-TOCSY correlations (Figure 2) between δC 37.3/δH 4.20 and δC 21.6/δH 4.20 supported the 1,5-diol substitution pattern. HSQC-TOCSY correlations (Figure 2) between C-15/9-OH, C-23/25-OH suggested that the contiguous chain of 1,5-diol is flanked by the 1,3-diol and 1,3,5-triol units. HSQC-TOCSY correlations between C-31/29-OH, C-32/29-OH, C-32/33-OH, C-33/H-35 enabled the extension of the polyhydroxylated chain which terminates to form an ester linkage with a carbonyl group at δC 165.4 (C-1). The low-field chemical shift of H-35 (δH 5.00) – due to anisotropy from an unsaturated system – and a HMBC correlation between C-1/H-35 confirmed the presence of the ester linkage. From HMBC and TOCSY correlations of C-35/H35 (Table 1), it was evident that C-35 was modified by an isohexyl side chain substitution. An additional unsaturation is present in 1 due to a carbon-carbon double bond between C-2 and C-3. HMBC correlations (C-1/H-2, C-3/H-2) and the characteristic chemical shifts for C-2 (δC 116.5) and C-3 (δC 159.4) were suggestive of a polarized carbon-carbon double bond, consistent with an α,β-unsaturated ester functionality. HMBC correlations between C-2/H3-42 and C-3/H3-42 indicated a methyl substitution at the β position.
Table 1.
NMR Data for Caylobolide B (1) in DMSO-d6
| Position | δ Ca | δH (J in Hz)b | HMBCb | TOCSYb |
|---|---|---|---|---|
| 1 | 165.4, C | |||
| 2 | 116.5, CH | 5.63, s | 1, 3, 42 | H-4a, H-4b, H3-42 |
| 3 | 159.4, C | |||
| 4a | 32.8, CH2 | 2.64, m | 2, 3, 5, 42 | H-2, H-4b, H-5a, H-7, 7-OH |
| 4b | 2.42, m | 2, 3, 5, 42 | H-2, H-4a, H-5a, H-7, 7-OH | |
| 5a | 23.6, CH2 | 1.52, m | H-4a, H-4b | |
| 5b | 1.40, m | 7 | H-4a, H-4b | |
| 6 | 37.1, CH2 | 1.37, m | ||
| 7 | 68.8, CH | 3.58, m | 9 | H-4a, H-4b, H-5a, 7-OH |
| 7-OH | 4.52, d (4.4) | 6, 7, 8 | H-4a, H-4b, H-5a, H-6, H-7 | |
| 8 | 44.15, CH2 | 1.39, m | ||
| 9 | 69.0, CH | 3.54, m | 10 | 13-OH, 9-OH |
| 9-OH | 4.47, d (4.8) | 8, 9, 10 | H-9, H-13 | |
| 10 | 37.6, CH2 | 1.28, m | ||
| 11 | 21.3, CH2 | 1.21, m | ||
| 12 | 37.3, CH2 | 1.28, m | ||
| 13 | 69.6, CH | 3.35, m | 9-OH, 13-OH | |
| 13-OH | 4.20,m | 12, 13, 14 | H-9, H-13 | |
| 14 | 37.3, CH2 | 1.28, m | ||
| 15 | 21.6, CH2 | 1.21, m | ||
| 16 | 37.3, CH2 | 1.28, m | ||
| 17 | 69.6, CH | 3.37, m | 17-OH | |
| 17-OH | 4.20,m | 16, 17, 18 | H-17 | |
| 18 | 37.3, CH2 | 1.28, m | ||
| 19 | 21.6, CH2 | 1.21, m | ||
| 20 | 37.3, CH2 | 1.28, m | ||
| 21 | 69.8, CH | 3.36, m | 21-OH, 25-OH | |
| 21-OH | 4.20,m | 20, 21, 22 | H-21, H-25 | |
| 22 | 37.3, CH2 | 1.28, m | ||
| 23 | 20.9, CH2 | 1.32, m | ||
| 24 | 37.3, CH2 | 1.29, m | ||
| 25 | 68.1, CH | 3.54, m | 23, 27 | 21-OH, 25-OH, H-27 |
| 25-OH | 4.37,d (4.4) | 24, 25, 26 | H-21, H-25 | |
| 26 | 44.4, CH2 | 1.42, m | ||
| 27 | 65.8, CH | 3.79, dq (13.5, 6.4) | 25, 26, 29 | H-25, 25-OH, 27-OH, H-29, 29-OH |
| 27-OH | 4.47,d (4.9) | 27 | H-27 | |
| 28 | 44.08, CH2 | 1.39, m | ||
| 29 | 66.6, CH | 3.61, m | 28, 31 | H-27, 29-OH |
| 29-OH | 4.28,d (5.2) | 28, 30 | H-29 | |
| 30 | 37.5, CH2 | 1.31, m | ||
| 31a | 21.0, CH2 | 1.29, m | ||
| 31b | 1.22, m | |||
| 32 | 38.1, CH2 | 1.29, m | ||
| 33 | 66.5, CH | 3.31, m | 32, 34 | 33-OH, H-35 |
| 33-OH | 4.29,d (6.03) | 33, 34 | H-33, H-35 | |
| 34 | 37.3, CH2 | 1.49, m | 35 | H-35 |
| 35 | 73.6, CH | 5.00, ddd (10.2, 4.3, 2.2) | 1, 33, 41 | H-33, OH-33, H-34, H-36, H-37b, H3-41 |
| 36 | 36.0, CH | 1.66, m | 35 | H-35, H-37b, H3-41 |
| 37a | 31.6, CH2 | 1.31, m | 41 | H-37a, H-38a |
| 37b | 1.06, dd (17.7, 8.9) | H-36a, H-37b | ||
| 38a | 28.8, CH2 | 1.31, m | H-37b | |
| 38b | 1.23, m | |||
| 39 | 22.3, CH2 | 1.26, m | 40 | H3-40 |
| 40 | 13.6, CH3 | 0.86, t (7.0) | 38, 39 | H-35, H-39 |
| 41 | 14.4, CH3 | 0.80, d (6.9) | 35, 36, 37 | H-36 |
| 42 | 24.5, CH3 | 1.85, s | 2, 3, 4 |
125 MHz.
600 MHz.
Figure 2.
Key HSQC-TOCSY correlations.
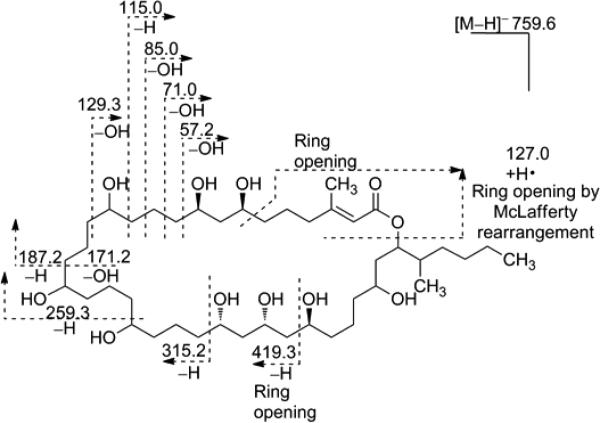
The structure of 1 bears a close resemblance to the 36-membered macrolactone ring present in the known compound caylobolide A (Figure 1) and was therefore termed caylobolide B. The C-1 to C-9 portion of these compounds presents a major difference, where an additional carbon-carbon double bond and a different hydroxylation pattern are present in 1. The isolated 1,3-diol system (C-7 to C-9) is a distinctive feature of 1, instead of a 1,5-diol unit from C-5 to C-9 in caylobolide A. The structure of caylobolide B (1) was confirmed using ESIMS fragmentation in the negative ionization mode (Figure 3). It was evident that fragmentation occurred mainly at positions α- and β- to the hydroxy groups, similar to fragmentation patterns observed for amphidinols.20
Figure 3.
ESI-MS/MS of caylobolide B (1).
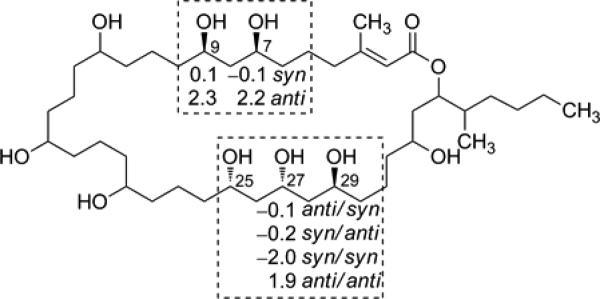
The relative configuration of selected stereogenic centers of 1 was assigned by independently considering the 1,3-diol and 1,3,5-triol moieties using Kishi's Universal NMR Database (Database 2).10–12 The 13C NMR chemical shifts of C-7/C-9 were in good agreement with syn arrangement of 1,3-diol model system (Figure 4). The 1,3,5- triol system was assigned as either syn/anti or anti/syn between C-25/C-27, C-27/C-29 based on comparison of δC at C-27 with the characteristic δC of the central carbon of the 1,3,5-triol model system (Figure 4). This method cannot differentiate between syn/anti or anti/syn orientation. Unfortunately, Mosher's analysis failed to give any conclusive result on the absolute configuration and was limited by the low yield of 1. The lack of chemical shift dispersion in the contiguous chain of 1,5 diol in caylobolide B limits the assignment of the absolute configuration in this moiety. The determination of the complete stereostructure may require chemical methods coupled with CD spectroscopy or new derivatization techniques.
Figure 4.
Assignment of the relative configuration of caylobolide B (1) based on Kishi's Universal NMR Database (Database 2). Δδ values between the model system and 1 are shown. The relative configuration shown is based on the best fit with the model system. The 1,3-diol is assigned as syn. The Δδ values for the characteristic central carbon of the 1,3,5-triol system suggest either anti/syn or syn/anti arrangement.
Caylobolide B (1) exhibited cytotoxic activity against HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells with IC50 of 4.5 μM and 12.2 μM, respectively. The cytotoxic activity of 1 is comparable to that of caylobolide A against HCT116 colon carcinoma cells (IC50 9.9 μM).9 The activity of the nonpolar extract was dominated by symplostatin 1 (HT29 IC50 ~1.5 nM), however, because our cyanobacterial collection was largely a binary mixture of two different Phormidium species, it is unclear if caylobolide B (1) and the co-isolated cytotoxin symplostatin 1 were produced by the same or both species.
Experimental Section
General Experimental Procedures
The optical rotation was measured on a Perkin-Elmer 341 polarimeter. The UV spectrum was recorded on SpectraMax M5 Molecular Devices. 1H and 2D NMR spectra were recorded in DMSO-d6 on a Bruker Avance II 600 MHz spectrometer equipped with a 5-mm triple resonance high-temperature superconducting (HTS) cryogenic probe using residual solvent signals (δH 2.50; δC 39.5) as internal standards. The 13C NMR spectrum was recorded in DMSO-d6 on a Bruker 500 MHz spectrometer, operating at 125 MHz. HSQC and HMBC experiments were optimized for 1JCH = 145 and nJCH = 7 Hz, respectively. TOCSY and HSQC-TOCSY experiments were done using a mixing time of 100 ms. HRMS data was obtained using an Agilent LC-TOF mass spectrometer equipped with an APCI/ESI multimode ion source detector. ESI-MS/MS data were obtained on a 3200 QTRAP (Applied Biosystems) by direct injection using a syringe driver.
Biological Material
The cyanobacteria, Phormidium spp., were hand collected on June 24, 2008 at the breakwater at Fort Zachary Taylor State Park (Key West), Florida by snorkeling in shallow waters. The collection was later identified to consist primarily of P. cf. dimorphum and P. inundatum. Voucher specimens (#VP_6_24_08_FZT1) are maintained at Smithsonian Marine Station, Fort Pierce, Florida.
Extraction and Isolation
The freeze-dried organism (54.16 g) was extracted with EtOAc–MeOH (1:1) to yield 5.7 g of the nonpolar extract. Subsequent extraction of the freeze-dried material with EtOH–H2O (1:1) gave 11.5 g of the polar extract. The nonpolar extract was further partitioned between hexanes and 20% aqueous MeOH. The latter was concentrated under reduced pressure and was further partitioned between n-BuOH and H2O. The n-BuOH (0.56 g) fraction was concentrated and subjected to Si gel column chromatography eluting first with CH2Cl2, followed by increasing concentrations of i-PrOH. After 100% i-PrOH, increasing gradients of MeOH were used until 100% MeOH. The fraction that eluted with 25% MeOH was subjected to reversed-phase HPLC (semipreparative, Phenomenex Synergi-Hydro RP, 4 μm) using a linear gradient of MeOH–H2O (40%–100% MeOH in 40 min and then 100% MeOH for 10 min) to yield caylobolide B (1) (tR 31.1 min, 2.1 mg). Purification of the fraction from 50% MeOH using the same condition yielded symplostatin 1 (tR 31.4 min, 1.5 mg).
Caylobolide B (1): colorless, amorphous solid; [α]20D –15 (c 0.15, MeOH); UV (MeOH); λmax (log ε) 215 (4.09); 1H NMR, 13C NMR, TOCSY, and HMBC data, see Table 1; HRESI/APCIMS m/z 761.5767 [M + H]+ (calcd for C42H81O11, 761.5779); m/z 783.5594 [M + Na]+ (calcd for C42H80O11Na, 783.5593).
Symplostatin 1: colorless, amorphous solid; [α]20D –98 (c 0.03, MeOH) {lit.16 [α]D –45 (c 1.6, MeOH)} ; UV (MeOH); λmax (log ε) 204 (3.52), 240 (2.94); 1H NMR spectrum is identical with that of an authentic sample,16 see Supporting Information; LRESIMS m/z 799.3 [M + H]+.
ESI-MS/MS Fragmentation
A solution of 1 in MeOH was directly infused into the mass spectrometer using a syringe driver. MS fragmentation was obtained by positive and negative ionization using the Enhanced Product Ion (EPI) and MS2 scan. The [M + H]+ (m/z 761.6) and [M–H]– ions (m/z 759.6) were fragmented by ramping the collision energy through the possible allowed range. Compound dependent and source gas parameters used were as follows: DP +/−65.0, EP +/−10.0, CUR 10.0, CAD High, IS +/−4500, TEM 0, GS1 10, GS2 0.
MTT Cell Viability Assay
HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells were cultured in Dulbecco's modified Eagle medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone) under a humidified environment with 5% CO2 at 37 °C. HeLa (3,000) and HT29 (12,500) cells were seeded in 96-well plates with varying concentrations of 1 added to each well 24 h later, with treatments done in duplicate. The cells were incubated for an additional 48 h before the addition of the MTT reagent. Cell viability was measured according to the manufacturer's instructions (Promega).
Supplementary Material
Acknowledgment
This research was supported by the National Institutes of Health, NIGMS grant P41GM086210. We thank Fort Zachary Taylor State Park for granting permission for sample collection. We are grateful to D. Littler for identifying the cyanobacteria, to J. Kwan and R. Ritson-Williams for collecting and extracting the cyanobacteria, and to J. Rocca for assistance in obtaining the NMR spectra. This is contribution #832 from the Smithsonian Marine Station.
Footnotes
Supporting Information Available: 1H, 13C, and 2D NMR spectra for compound 1. 1H NMR spectrum for symplostatin 1. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Tan LT. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Rein KS. Mar. Drugs. 2010;8:1817–1837. doi: 10.3390/md8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi J, Kubota T. J. Nat. Prod. 2007;70:451–460. doi: 10.1021/np0605844. [DOI] [PubMed] [Google Scholar]
- 4.Zerlin M, Thiericke R. J. Org. Chem. 1994;59:6986–6993. [Google Scholar]
- 5.Kretzschmar G, Krause M, Radics L. Tetrahedron. 1997;53:971–986. [Google Scholar]
- 6.Carmeli S, Moore RE, Patterson GML. J. Nat. Prod. 1990;53:1533–1542. doi: 10.1021/np50072a021. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M, Matsuda H, Makabe K, Yamaguchi K. Tetrahedron Lett. 1991;32:2391–2394. [Google Scholar]
- 8.Williamson RT, Boulanger A, Vulpanovici A, Roberts MA, Gerwick WH. J. Org. Chem. 2002;67:7927–7936. doi: 10.1021/jo020240s. [DOI] [PubMed] [Google Scholar]
- 9.MacMillan JB, Molinski TF. Org. Lett. 2002;4:1535–1538. doi: 10.1021/ol025759p. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y, Tan C-H, Kishi Y. Helv. Chim. Acta. 2000;83:2562–2571. [Google Scholar]
- 11.Kobayashi Y, Tan C-H, Kishi Y. Angew. Chem. Int. Ed. 2000;39:4279–4281. doi: 10.1002/1521-3773(20001201)39:23<4279::AID-ANIE4279>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Tan C-H, Kishi Y. J. Am. Chem. Soc. 2001;123:2076–2078. doi: 10.1021/ja004154q. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan GR, Dale JA, Mosher HS. J. Org. Chem. 1973;38:2143–2147. [Google Scholar]
- 14.Seco JM, Quinoá E, Riguera R. Tetrahedron: Asymmetry. 2001;12:2915–2925. [Google Scholar]
- 15.MacMillan JB, Molinski TF. J. Am. Chem. Soc. 2004;126:9944–9945. doi: 10.1021/ja047741a. [DOI] [PubMed] [Google Scholar]
- 16.Harrigan GG, Luesch H, Yoshida WY, Moore RE, Nagle DG, Paul VJ, Mooberry SL, Corbett TH, Valeriote FA. J. Nat. Prod. 1998;61:1075–1077. doi: 10.1021/np980321c. [DOI] [PubMed] [Google Scholar]
- 17.Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. J. Nat. Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 18.Mooberry SL, Leal RM, Tinley TL, Luesch H, Moore RE, Corbett TH. Int. J. Cancer. 2003;104:512–521. doi: 10.1002/ijc.10982. [DOI] [PubMed] [Google Scholar]
- 19.Kubota T, Tsuda M, Doi Y, Takahashi A, Nakamichi H, Ishibashi M, Fukushi E, Kawabata J, Kobayashi J. Tetrahedron. 1998;54:14455–14464. [Google Scholar]
- 20.Meng Y, Van Wagoner RM, Misner I, Tomas C, Wright JLC. J. Nat. Prod. 2010;73:409–415. doi: 10.1021/np900616q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.