Abstract
Objectives/Hypothesis
Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine and apoptotic molecule that appears to be a mediator in inflammation and fibrosis. The objective of this investigation was to examine the effects of TNF-α on three-dimensional (3D) Carbylan-GSX in vitro cultured human vocal fold fibroblasts (hVFF), to provide insight into the mechanism responsible for improved vocal fold wound healing that has been previous reported with Carbylan-GSX treatment.
Study Design
In vitro cell culture.
Methods
hVFF were cultured in three dimensional (3D) Carbylan-GSX and on polystyrene with different dosages of TNF-α (0, 0.1, 1, 10 and 100 ng/ml) with and without 10% fetal bovine serum (FBS). hVFF response to TNF-α was characterized by morphology, proliferation rates and gene transcript levels for MMP1, MMP2, TIMP3, collagen I, collagen III, fibronectin and TNF-α receptor.
Results
In 3D Carbylan-GSX, TNF-α inhibited hVFF proliferation in a dose-dependent manner, TNF-α (0.1–100ng/ml) was shown to significantly down-regulate TIMP3 and ECM related mRNA transcript levels for collagen III and fibronectin, and to up-regulate MMP1 and MMP2 expression, resulting in increased MMP/TIMP3 ratios. TNF-α receptor expression was significant up-regulated in Carbylan-GSX compared to control polystyrene. Responses were more marked in 10%FBS culture.
Conclusions
After vocal fold injury, locally injected Carbylan-GSX can enhanced the role of TNF-α in remodeling the lamina propria layer of vocal fold, accelerating wound healing, suggesting Carbylan-GSX as a potential new therapeutic approach that may lead to better treatment of vocal fold wound healing.
Keywords: human vocal fold fibroblasts, Carbylan-GXS, three-dimensional (3D) cell culture, TNF-α, wound healing, fibrosis
INTRODUCTION
Wound healing is a complex process composed of several overlapping phases --inflammation, proliferation and remodeling. Cell types and the order in which they appear in the wound bed have been previously established, conversely for growth factors - their presence and timing in healing has yet to be fully elucidated 1. Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine produced by macrophages/monocytes during the acute inflammation phase. It has been shown to exert a wide variety of biological effects and responses for a diverse range of signaling events within cells, leading to necrosis and apoptosis. It has been reported to be a mediator of several disease processes, such as cancer cachexia 2, endotoxic shock 3, hematopoiesis 4. TNF-α has been shown to stimulate fibroblasts proliferation 5, induce prostaglandin production and collagenase gene expression 6 7 8, release growth factors (including platelet-derived growth factor), modulate macrophage function and local TNF-α in collagen based biomaterials can improve wound disruption strength 9, The role of TNF-α in vocal fold wound healing as yet to be ascertained.
Recent evidence suggests that altered matrix metalloproteinases (MMPs) may play an important role in extracellular matrix (ECM) remodeling in other parts of the body10. MMPs are a family of functionally related enzymes that cleave matrix components, resulting in fibrillar collagen denaturation and degradation, and the synthesis of new fibrotic tissue 11. TNF-α has been shown to induce MMP1 expression in human lung fibroblasts 8 and rat skin fibroblasts 7. The relationship between these two players and their concerted efforts in ECM synthesis and degradation are not completely understood, in other parts of the body and the human vocal fold. Moreover, alterations in this relationship in early wound healing with the addition of a biomaterial have yet to be elucidated.
Recent work using an engineered synthetic extracellular matrix (sECM) injectable hydrogel --Carbylan-GSX in an in vivo vocal fold injury model, suggests that Carbylan-GSX enhances tissue regeneration restoring the tissue’s biomechanical properties and improving overall vocal fold wound healing 12. We hypothesize that in Carbylan-GSX treated vocal folds, TNF-α through its actions with MMPs play a role in the improved wound healing that was measured in the literature. The goal of this in vitro investigation is to examine the effects of TNF-α on three-dimensional (3D) Carbylan-GSX cultured hVFF as a model for understanding how Carbylan-GSX improves vocal fold wound healing.
MATERIALS AND METHODS
Preparation of sECM hydrogel
Injectable chemically-modified HA-gelatin hydrogel–Carbylan-GSX was developed in conjunction with the Center for Therapeutic Biomaterials at The University of Utah 13. It is composed of polyethylene glycol diacrylate (PEGDA)-crosslinked di(thiopropionyl) bishydrazide-modified gelatin (Gtn-DTPH). 5% Carbylan-GSX was prepared as described previously 13,14. The final Carbylan-GSX was cast and allowed to gel for 10 minutes in 37 C incubator before adding medium.
Human vocal fold fibroblasts and Cell Line
Primary cultures of human vocal fold fibroblasts were obtained from outgrowths of normal human donor vocal fold tissue as described previously 15. Donor tissue was received in compliance with the University of Wisconsin Madison Institutional Review Board. Vocal fold lamina propria tissue was cut into small prices and re-suspended in DMEM supplemented with 10% FBS (Sigma Inc. St. Louis, MO), 100U/ml penicillin (Sigma), 0.01 mg/ml streptomycin (Sigma) and 1X non-essential amino acid (NEAA, Sigma). Cells were cultured at 37°C in a humidified 5% CO2 atmosphere. These cells were considered fibroblasts using standard morphological and immunohistochemical criteria 16. Subcultures revealed stable morphological and functional features. The immortalized hVFF line was established by transduction of the primary hVFF with a defective retrovirus expressing hTERT, which was generated from pBABE-hTERT-neo vector 17. The immortalized hVFF were grown in DMEM cell culture medium with 10% Fetal Bovine Serum (FBS), 1X NEAA. Media was changed every three days. These cells showed similar morphological features and ECM gene expression past passage 25. Full immortalization details and characterization of this cell line is described elsewhere 17. For all experiments hVFF between the 3th and 12th passage were used.
Cell proliferation assay for 2D and 3D culture
To determine proliferation of immortalized hVFF in Carbylan-GSX, single cells were mixed into Carbylan-GSX at a final concentration of 2 × 103 cells/ml gel and 75 μl of the cell-seeded gel was cast per well of 96-well plates. Cells growing in untreated wells (polystyrene) served as control. For each condition, cells were treated with 0, 0.1, 1, 10 or 100 ng/ml TNF-α (Roche, Indianapolis, IN). Media with TNF-α was changed every 3 days. On day 0, 3, 6 and 9 of culture, cell numbers were monitored in quadruplicate for each condition using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) as described previously 14. Detection is based on use of a bioluminescent reaction to measure the amount of ATP in viable cells. The luminescent signal (relative light unit, RLU), reflects ATP levels, and is proportional to the number of viable cells 18. RLU levels were plotted against the time course of the assay to yield the growth profile of the cells seeded on polystyrene and in Carbylan-GSX.
Gene transcription Assay for 2D and 3D culture
In order to investigate hVFF transcriptional responses to TNF-α two conditions were utilized -- Carbylan-GSX (3D) and polystyrene (2d) as control. For 3D culture, cell suspensions were mixed with Carbylan-GSX solutions at a final concentration of 2X106 cells/ml by gently vortex, and then 500μl of this mixture was placed in each well of a 6-well plate with the inserts. After gelation, cell culture medium was added above and under gel. For polystyrene control, the cells were directly seeded on standard polystyrene 6-well-culture plate at a 2X106 cells/ml concentration in DMEM-10%FBS. hVFF in both conditions were treated with 0, 0.1, 1, 10 or 100 ng/ml of TNF-α in either DMEM-10%FBS or 0.1%BSA for 24 hours after 24-hour cell starvation.
Total cellular RNA was isolated from hVFF in Carbylan-GSX or on polystyrene using an RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. First strand cDNA was synthesized from 0.7μg of total RNA by using a QuantiTect Reverse Transcription Kit (Qiagen, CA). mRNA levels were quantified by real-time PCR -- standard curve method -- using LightCycler System (Roche, IN), with amplification of β-actin as control as described previously 14. mRNA from the cDNA sample was amplified with specific primer pairs for matrix metalloproteinase 1 (MMP1), matrix metalloproteinase 2 (MMP2), tissue inhibitor of metalloproteinase 3 (TIMP3), collagen I α-2 (Col I), Collagen III α-1(Col III), fibronectin (FN), TNF receptor (TNFR) and β-actin. The primer sequences, gene bank access number and expected PCR product sizes are listed in Table 1. The specificity of every pair of PCR primers was confirmed by melting curves and PCR reactions, which shows the single peak and the predicted sized DNA band of each gene product. Results were shown by target gene mRNA concentration (ng/μl) normalized by housekeeping gene β-actin mRNA (ng/μl). Each sample was tested in triplicate.
Table 1.
Primer Sequences and Products of Real-Time Polymerase chain reaction (RT-PCR)
| Gene | GenBank# | Forward Primer | Reverse Primer | Size of PCR product |
|---|---|---|---|---|
| MMP1 | NM_002421 | 5′-TGCAACTCTGACGTTGATCCCAGA-3′ | 5′-ACTGCACATGTGTTCTTGAGCTGC-3′ | 122bp |
| MMP2 | NM_004530 | 5′-AGAAGGATGGCAAGTACGGCT-3′ | 5′-AGTGGTGCAGCTGTCATAGGATGT-3′ | 128bp |
| TIMP3 | NM_000362 | 5′-TGATGCAGCACACACACAATTCCC-3′ | 5′-AAGCTCTGTTATTCTGGCCTGGGT-3′ | 102bp |
| Collagen I α-2 | NM_000089 | 5′-AACAAATAAGCCATCACGCCTGCC-3′ | 5′-TGAAACAGACTGGGCCAATGTCCA- 3′ | 101bp |
| Collagen III α-1 | NM_000090 | 5′-CCATTGCTGGGATTGGAGGTGAAA-3′ | 5′-TTCAGGTCTCTGCAGTTTCTAGCGG- 3′ | 187bp |
| Fibronectin | NM_002026 | 5′-ACCTACGGATGACTCGTGCTTTGA-3′ | 5′-CAAAGCCTAAGCACTGGCACAACA-3′ | 116bp |
| TNFR | NM_003842 | 5′-TGGAGTGCAATGGTGCAATCTTGG-3′ | 5′-ACCTGTGGTCCCAGCTATTTGGAT-3′ | 98bp |
| β-Actin | NM_001101 | 5′-ACGTTGCTATCCAGGCTGTGCTAT-3′ | 5′-CTCGGTGAGGATCTTCATGAGGTAGT-3′ | 188bp |
Statistical analyses
Multiple comparisons in ANOVA was performed to compare the group conditions for cell proliferation at culture day 9, gene expression for each condition and at each the experimental time points. A p<0.05 was considered statistically significant difference. Statistical interpretations were made using PROC mixed in SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
hVFF morphology responds to TNF-α
hVFF cultured on polystyrene demonstrated the typical spindle-like fibroblast shape (Figure 1A), while in Carbylan-GSX (3D condition) fibroblasts were of a single rounded appearance (Figure 1B). There was no change in appearance after treatment with 10ng/ml TNF-α for 48 hours (Figure 1C and D). This experiment was repeated more than three times with consistent results.
Figure 1.
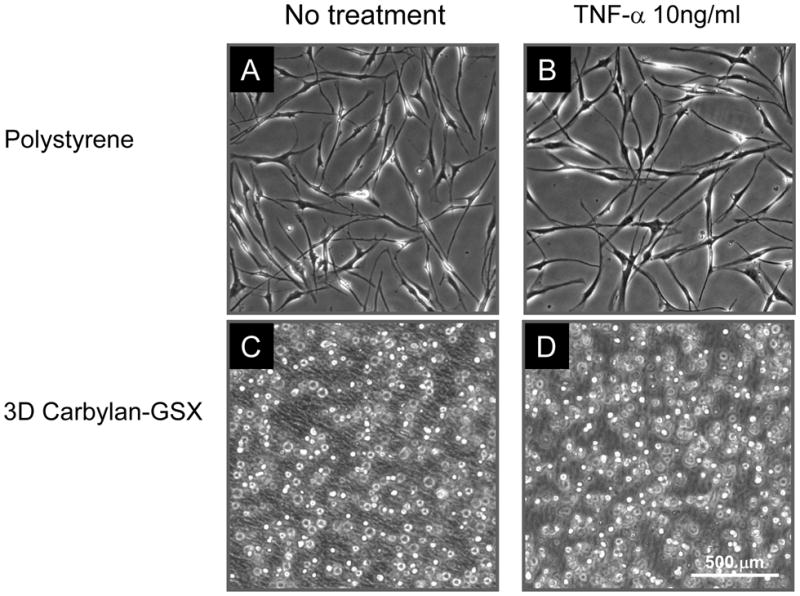
hVFF morphology in response to TNF-α on polystyrene (A and B) and in 3D Carbylan-GSX (C and D). There was no change in hVFF phenotype after 48 hours of TNF-α (10ng/ml) treatment (B and D) compared to baseline (A and C).
hVFF proliferation responds to TNF-α in Carbylan-GSX
hVFF proliferation rates in response to TNF-α were assessed by CellTiter-Glo Luminescent Cell Viability Assay. The results of the cell proliferation assay are expressed as mean ± standard deviations (SD) of quadruplicated samples per condition at each experimental time interval in Figure 2. The cells seeded in Carbylan-GSX and on polystyrene were treated with different dosages of TNF-α (0.1 to 100 ng/ml). At Day 0, 3, 6 and 9 of culture, the cell number for each condition was quantified. For the control condition, TNF-α (0.1 to 100 ng/ml) caused significant inhibition from basal proliferation (without TNF-α) in hVFF (Figure 2B, p<0.0001). The proliferation of hVFF in 3D Carbylan-GSX was significantly inhibited by TNF-α (1 to 100ng/ml) in dose-dependent manner (Figure 2A, p<0.0002).
Figure 2.
hVFF proliferation rates in polystyrene and 3D Carbylan-GSX conditions. The cells were initially seeded in 96-well plate at 2 × 103 cells/well with DMEM-10%FBS, then treated with different dose of TNF-α (0.1 to 100ng/ml), and cell number was determined in quadruplicate by the CellTiter-Glo Luminescence cell viability assay at Day 0, 3, 6 and 9. Values represent the mean SD of four RLU (relative luminescence unit) for each TNF-α concentration. (A) Cell proliferation rates in 3D Carbylan-GSX: at day 9, TNF-α (1 to 100ng/ml) caused significant inhibition (p<0.0002) compared to control without TNF-α; (B) Cell proliferation rates on polystyrene. TNF-α caused significant inhibition compared to control without TNF-α (p<0.0001).
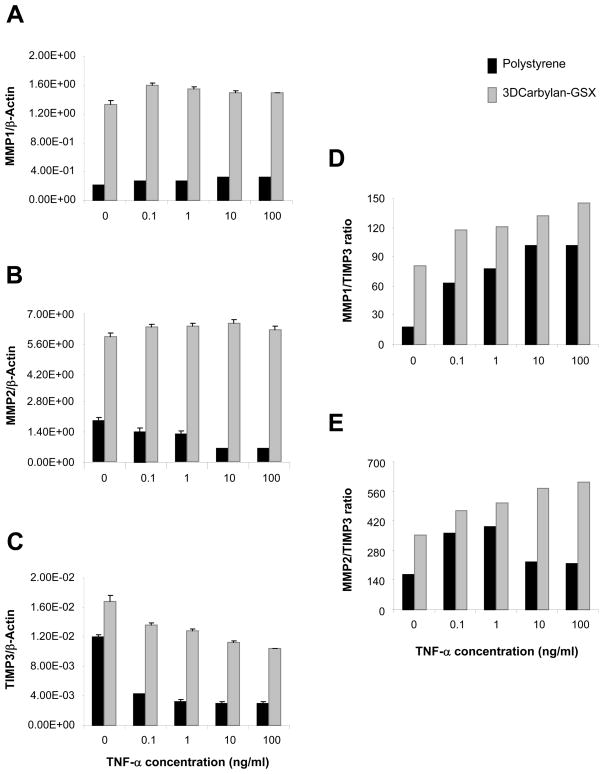
MMPs/TIMP3 balance responds to TNF-α in sECM
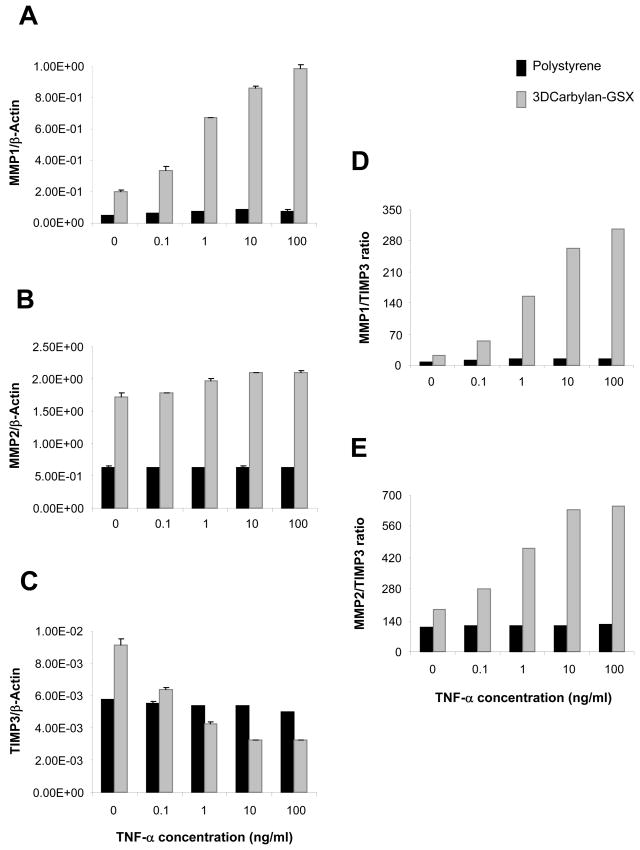
For serum-free cultured hVFF, MMP1 and MMP2 genes (Figures 3A and B) were significant up-regulated in Carbylan-GSX comparing to polystyrene (p<0.0001, respectively). A TNF-α dose-dependent response was not measured for either 2D or 3D condition. In contrast, TIMP3 a significant down-regulated dose response was measured when hVFF were treated with TNF-α for both Carbylan-GSX and polystyrene conditions (Figure 3C, p<0.0001 respectively). Collectively, these responses produced a TNF-α dose-dependent increased MMP1/TIMP3 and MMP2/TIMP3 ratio (Figure 3D and E). For the same culture conditions -- polystyrene and Carbylan -GSX, cells growing in the medium containing 10%FBS (Figure 4), increasing TNF-α dosage (from 0.1 to 100 ng/ml) induced a significant increase in MMP1 induction (Figure 4A, p<0.0001). Increasing TNF-α dosage (from 1 to 100 ng/ml) induced a significant increase in MMP2 (Figure 4B, p<0.0001). Increasing TNF-α dosage (from 0.1 to 100 ng/ml) induced a down regulation of TIMP3 for the 3D condition only. (Figure 4C, p<0.0001). Combining TNF-α induced MMPs and TIMP3 outcomes instigated a dose related increase in MMP1/TIMP3 (Figure 4D) and MMP2/TIMP3(Figure 4E) ratios of hVFF cultured in Carbylan-GSX.
Figure 3.
hVFF mRNA levels for MMP1, MMP2, TIMP3 and MMPs/TIMP3 ratio on polystyrene and in Carbylan-GSX after treated with TNF-α (0.1 to 100ng/ml) in serum-free medium (DMEM-0.1BSA). Y axis is target gene (mRNA concentration ng/μl) normalized by the housekeeping gene, β-Actin (ng/μl); x axis is TNF-α concentration. (A) MMP1 mRNA level; (B) MMP2 mRNA level; (C) TIMP3 mRNA level; (D) MMP1 to TIMP3 ratio; (E) MMP2 to TIMP3 ratio.
Figure 4.
hVFF mRNA levels for MMP1, MMP2, TIMP3 and MMPs/TIMP3 ratio on polystyrene and in Carbylan-GSX after treated with TNF-α (0.1 to 100ng/ml) in DMEM-10%FBS medium. Y axis is target gene (mRNA concentration ng/μl) normalized by the housekeeping gene, β-Actin (ng/μl); x axis is the TNF-α concentration. (A) MMP1 mRNA level; (B) MMP2 mRNA level; (C) TIMP3 mRNA level; (D) MMP1 to TIMP3 ratio; (E) MMP2 to TIMP3 ratio.
hVFF ECM-related genes respond TNF-α in Carbylan-GSX
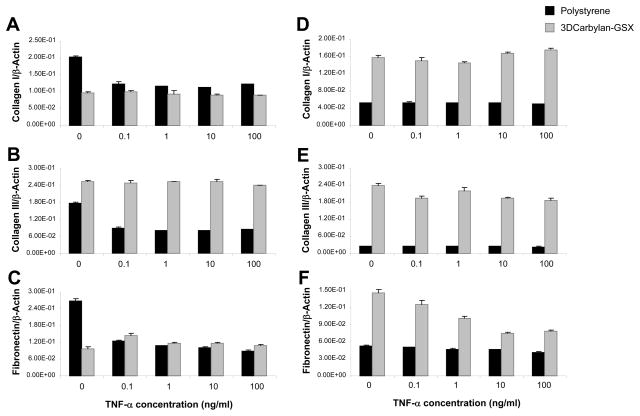
In serum-free conditions (Figure 5A, B and C), collagen I, III and fibronectin expression of hVFF cultured on polystyrene were down-regulated by TNF-α for each dosage compared to no treatment (p<0.0001). hVFF cultured in Carbylan-GSX did not alter expression of collagen I, III and fibronectin, when treated with TNF-α compared to no treatment. For hVFF cultured with 10%FBS medium in Carbylan-GSX, fibronectin and collagen III were down-regulated by TNF-α in dose-dependent manner (Figure 5E and F; p<0.0001). Those cells cultured in 10% FBS and on polystyrene did not have any significant differences in response to TNF-α at any dosage for collagen I, III and fibronectin (Figure 5D, E and F; p>0.05).
Figure 5.
hVFF ECM-related genes (mRNA) responses to TNF-α (0.1 to 100ng/ml) on polystyrene and in Carbylan-GSX after treated with DMEM-0.1%BSA (A, B and C) and DMEM-10%FBS (D, E and F). Y axis is target gene (mRNA concentration ng/μl) normalized by the housekeeping gene, β-Actin (ng/μl); x axis is TNF-α concentration. A and D - Collagen I mRNA level; B and E - Collagen III mRNA level; C and F - fibronectin mRNA level.
hVFF TNF receptor expression in Carbylan-GSX
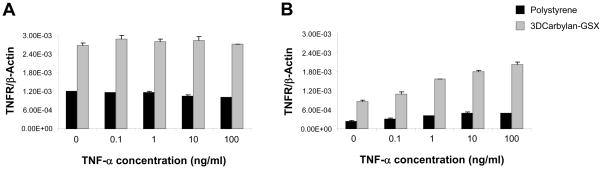
In 3D Carbylan-GSX, hVFF showed significant higher expression (p<0.0001) for all genes in response to TNF-α, implying a difference in the quantity of TNF-α receptors on the cells cultured in 3D versus 2D. For serum-free conditions, TNF-α receptor expression for hVFF cultured in Carbylan-GSX was significant higher compared to controls (p<0.0001, Figure 6A). Cells grown in 10%FBS and Carbylan – GSX demonstrated a TNF-α in dose-dependent response for TNF-α receptor expression (p<0.0001, Figure 6B).
Figure 6.
TNF-α receptor expression (mRNA) response to TNF-α (0.1 to 100ng/ml) on polystyrene and in Carbylan-GSX after treated with DMEM-0.1%BSA (A) and DMEM-10%FBS (B). Y–axis is TNF-α receptor (mRNA concentration ng/μl) normalized by the housekeeping gene, β-Actin (ng/μl); x-axis is TNF-α concentration.
DISCUSSION
TNF-α, an important cytokine is secreted by invading macrophages/monocytes after injury 2–5 days 19. In 1985, it was reported that TNF-α induces the release of collagenase by synovial cells 6. In 1998, Mariani and his colleagues demonstrated that TNF-α did not induce interstitial collagenase expression in rat lung fibroblast but did in rat skin fibroblast, revealing tissue specificity in the regulation of this gene 7. It was also determined that TNF-α can significant increase MMP1 activity and decrease the MMP2 and MMP9 activity in lung fibroblasts 8. The regulation of the MMP and TIMP gene families is complex. These genes are regulated across development, in adult physiology and in disease. In many situations, a number of genes within these families can be transcribed simultaneously, though such subsets will differ depending on cell type and stimulus 20. Hence, the combinatorial regulation of MMP and TIMP gene expression is important for controlling ECM remodel. However, little is known concerning the interaction between TNF-α, MMPs and TIMP in hVFF.
MMP1 and MMP2 are known to degrade a wide range of vocal fold ECM molecules, including type I and III collagen, fibronectin, elastin and decorin 21, while TIMP3 is the only TIMP in ECM 22. Consequently, in this investigation we focused on the regulation of MMP1, MMP2 and TIMP3. MMP1 was significantly up-regulated by TNF-α when hVFF were cultured in Carbylan-GSX, with a concomitant down regulation of TIMP3. Taken together, these results demonstrate that TNF-α dose-dependent increasing of MMP1/TIMP3 and MMP2/TIMP3 ratios could cause collagen denaturation and degradation, inducing ECM remodel 23. Translationally ECM remodeling promotes wound healing and minimizes scar formation after vocal fold injury.
It is known that the classic wound healing is divided into three phases: inflammatory, proliferative and remodeling. The inflammation plays roles in fighting infection, clearing debris and inducing the proliferation phase, it is a necessary part of healing. During this phase, there are various cytokines produced and secreted by infiltrating platelets, macrophages, fibroblasts and residential cells such as IL-1, PDGF, EGF, FGF, TGF-β, TGF-α, HGF and TNF-α, which accumulate and interact each other at the wound site to involve a variety of events including cell migration, proliferation, extracellular matrix protein synthesis and degradation to cause remodeling, wound healing or fibrosis formation 19. However, inflammation also can lead to tissue damage and larger scar formation if it is too marked or lasts too long, in which much more cell accumulation and migration, growth factors and cytokines release. Therefore, the degree of inflammatory and levels of wound local levels of growth factors and cytokines are important for wound healing. TNF-α is one of growth factor secreted from macrophages, which combines with other growth factors, PDGF or IL-1β enhance and inhibit respective collagen production, fibroblast proliferation 8. In this experiment, we have shown that MMP1, TIMP3, fibronectin and TNF-α receptor expression induced by TNF-α were more marked in presence of 10% FBS (FBS contains various of growth factors) than in medium with 0.1%BSA (does not contain growth factors). This phenomenon is similar to an earlier report 5. In human neonatal foreskin fibroblasts (FS-4), growth stimulation by TNF-α was marked and more sustained in the presence 10% FBS than in medium with less than or equal to 5% FBS, and the stimulation of confluent cultures by TNF-α in serum-free medium was enhanced by insulin. Our results indicate that in Carbylan-GSX, TNF-α in combination with other growth factors from fresh serum can regulate MMP and its inhibitor (TIMP) production in hVFF, enhancing ECM remodeling and promoting wound healing process.
In our study, it was shown that TNF-α inhibited the hVFFs proliferation both in 3D Carbylan-GSX and on polystyrene, and it was more markedly in Carbylan-GSX environment. But it did stimulate the cell growth for human neonatal foreskin fibroblasts5. These revealed that TNF-α has tissue or cell specificity in regulation of cell growth.
Three-dimensional ECM has been used to model normal cell behavior 24. Culturing cells in a three-dimensional (3D) context produces distinct cellular morphology and signaling events compared with two-dimensional (2D) culture systems 25. Expression of cell receptors is necessary for cells to respond to the extracellular signal. Growth factors though binding with their receptors regulate downstream signaling molecules and change cell function. The hVFF grown in 3D Carbylan-GSX demonstrated a marked response to TNF-α, which corresponded to an increase in TNF-α receptor transcript levels. This finding is analogous to a report by Petridou and his colleagues, who established that the binding of TGF-β and TGF-β receptor was increased at low cell density 26. hVFF morphology in 3D was single and rounded (Figure 1), as previously reported by our group 14. This morphology may be responsible for increased of growth factor receptor expression. Whether or not the Carbylan –GSX was responsible solely cannot be determined without a 3D control.
CONCLUSIONS
TNF-α appears to promote vocal fold ECM degradation and remodeling and this response appears to be amplified in the presence of Carbylan-GSX. This study suggests that after vocal fold injury, locally injected Carbylan-GSX can enhanced TNF-α role in remodeling the lamina propria of the vocal fold, accelerating wound healing.
Acknowledgments
The authors would like to acknowledge the National Institute of Deafness and other Communication Disorders R01 DC4336 for supporting this research project. This paper will be presented at the 130th American Laryngological Association Meeting in Las Vegas, Nevada, 2010.
Contributor Information
Xia Chen, Email: chenx@surgery.wisc.edu, Division of Otolaryngology – Head and Neck Surgery, Department of Surgery, University of Wisconsin Madison, 5136 WIMR, 1111 Highland Ave, Madison, WI 53705-2275, Phone: 6082650488.
Susan L Thibeault, Email: thibeault@surgery.wisc.edu, Division of Otolaryngology- Head and Neck Surgery, Department of Surgery, University of Wisconsin Madison, 5107 WIMR, 1111 Highland Ave, Madison, WI 53705-2275, Phone: 6082636751, Fax: 6082520929.
BIBLIOGRAPHY
- 1.Ueno C, Hunt TK, Hopf HW. Using physiology to improve surgical wound outcomes. Plast Reconstr Surg. 2006;117:59S–71S. doi: 10.1097/01.prs.0000225438.86758.21. [DOI] [PubMed] [Google Scholar]
- 2.Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985;229:867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 4.Murphy M, Perussia B, Trinchieri G. Effects of recombinant tumor necrosis factor, lymphotoxin, and immune interferon on proliferation and differentiation of enriched hematopoietic precursor cells. Exp Hematol. 1988;16:131–138. [PubMed] [Google Scholar]
- 5.Vilcek J, Palombella VJ, Henriksen-DeStefano D, et al. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986;163:632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayer JM, Beutler B, Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariani TJ, Sandefur S, Roby JD, Pierce RA. Collagenase-3 induction in rat lung fibroblasts requires the combined effects of tumor necrosis factor-alpha and 12-lipoxygenase metabolites: a model of macrophage-induced, fibroblast-driven extracellular matrix remodeling during inflammatory lung injury. Mol Biol Cell. 1998;9:1411–1424. doi: 10.1091/mbc.9.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki M, Kashima M, Ito T, et al. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1beta and TNF-alpha. Mediators Inflamm. 2000;9:155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooney DP, O’Reilly M, Gamelli RL. Tumor necrosis factor and wound healing. Ann Surg. 1990;211:124–129. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 11.Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 12.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 13.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Thibeault SL. Characteristics of age-related changes in cultured human vocal fold fibroblasts. Laryngoscope. 2008;118:1700–1704. doi: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thibeault SL, Li W, Bartley S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol Head Neck Surg. 2008;139:816–822. doi: 10.1016/j.otohns.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Thibeault S. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C Methods. 2009;15:201–212. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 19.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 20.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 21.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 22.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 23.Nap AW, Dunselman GA, de Goeij AF, Evers JL, Groothuis PG. Inhibiting MMP activity prevents the development of endometriosis in the chicken chorioallantoic membrane model. Hum Reprod. 2004;19:2180–2187. doi: 10.1093/humrep/deh408. [DOI] [PubMed] [Google Scholar]
- 24.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 26.Petridou S, Maltseva O, Spanakis S, Masur SK. TGF-beta receptor expression and smad2 localization are cell density dependent in fibroblasts. Invest Ophthalmol Vis Sci. 2000;41:89–95. [PubMed] [Google Scholar]