Abstract
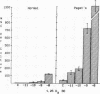
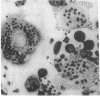
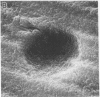
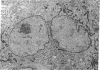
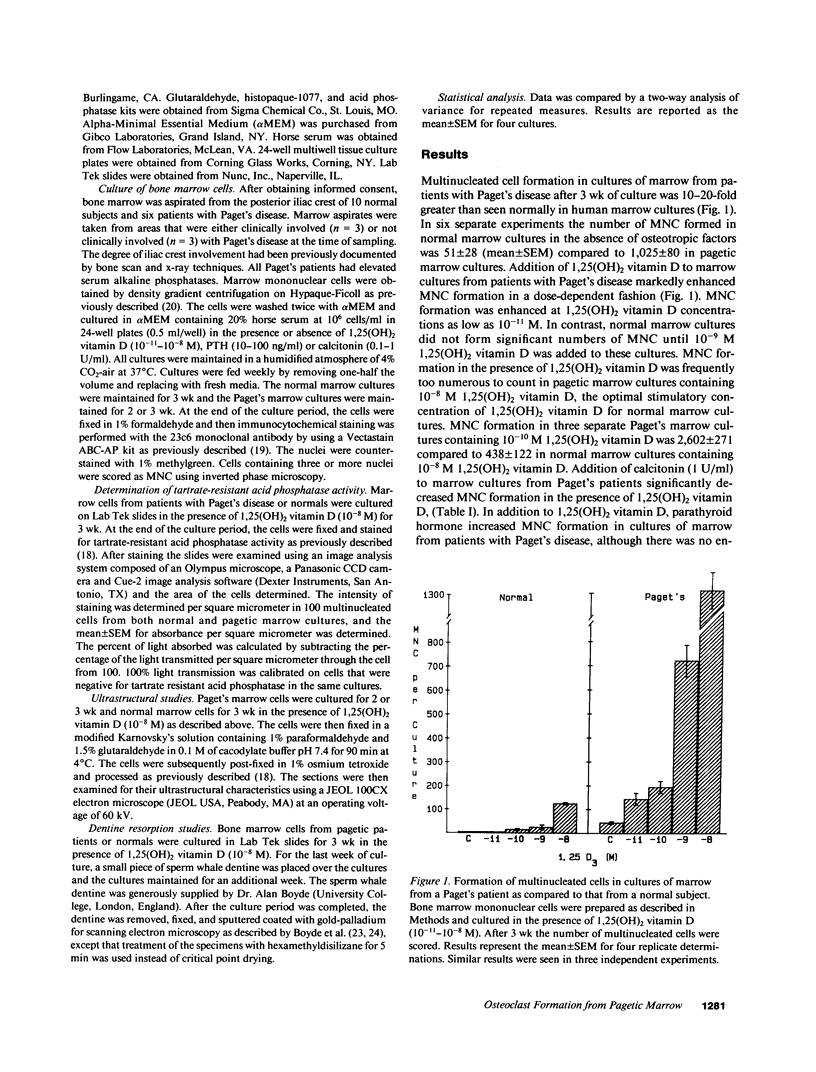
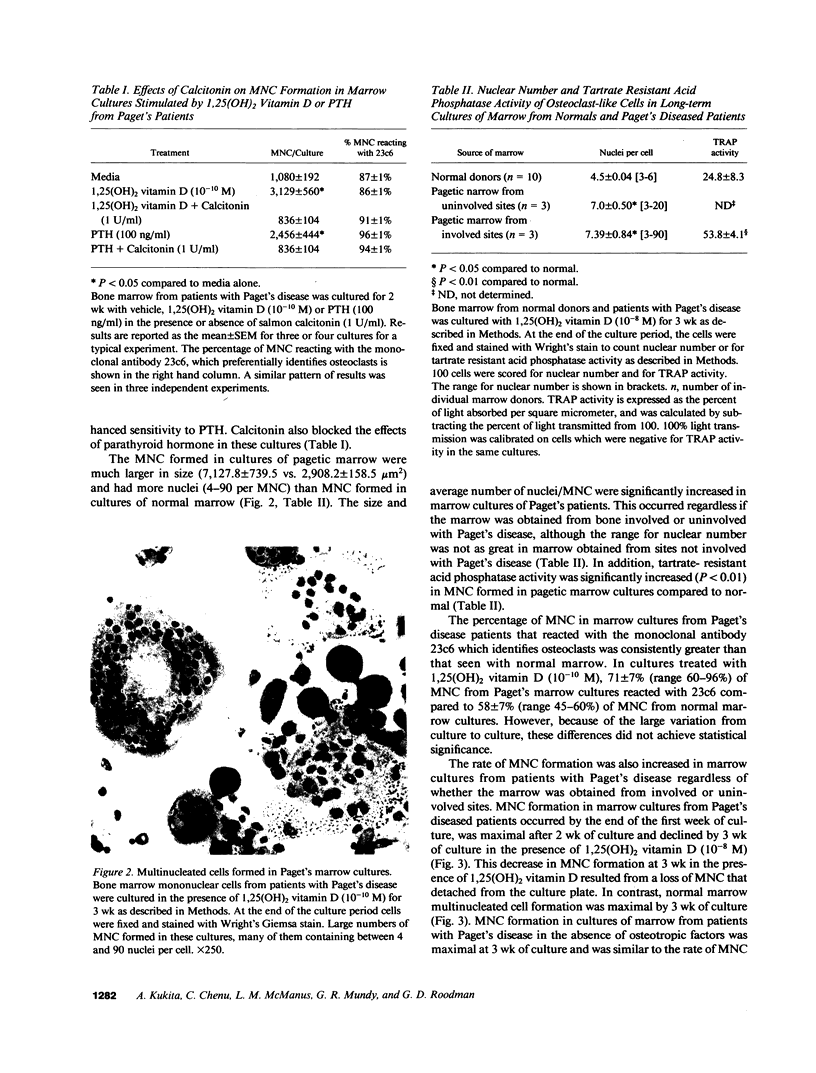
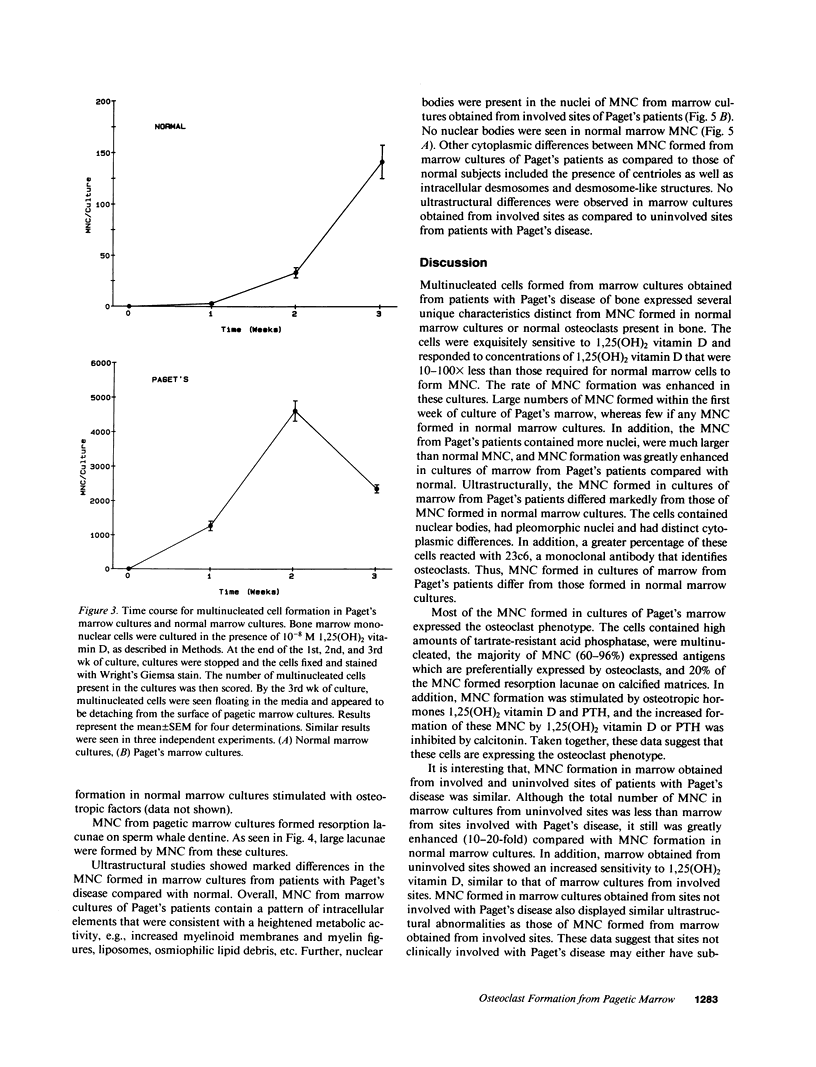
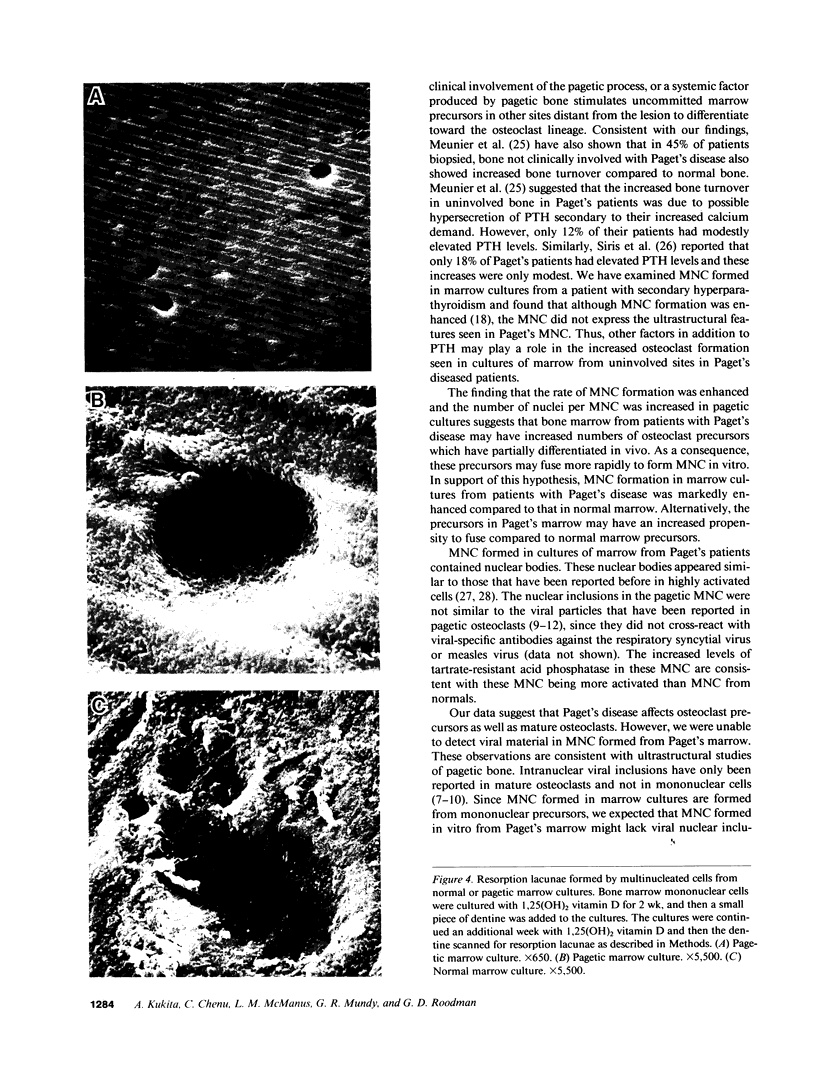
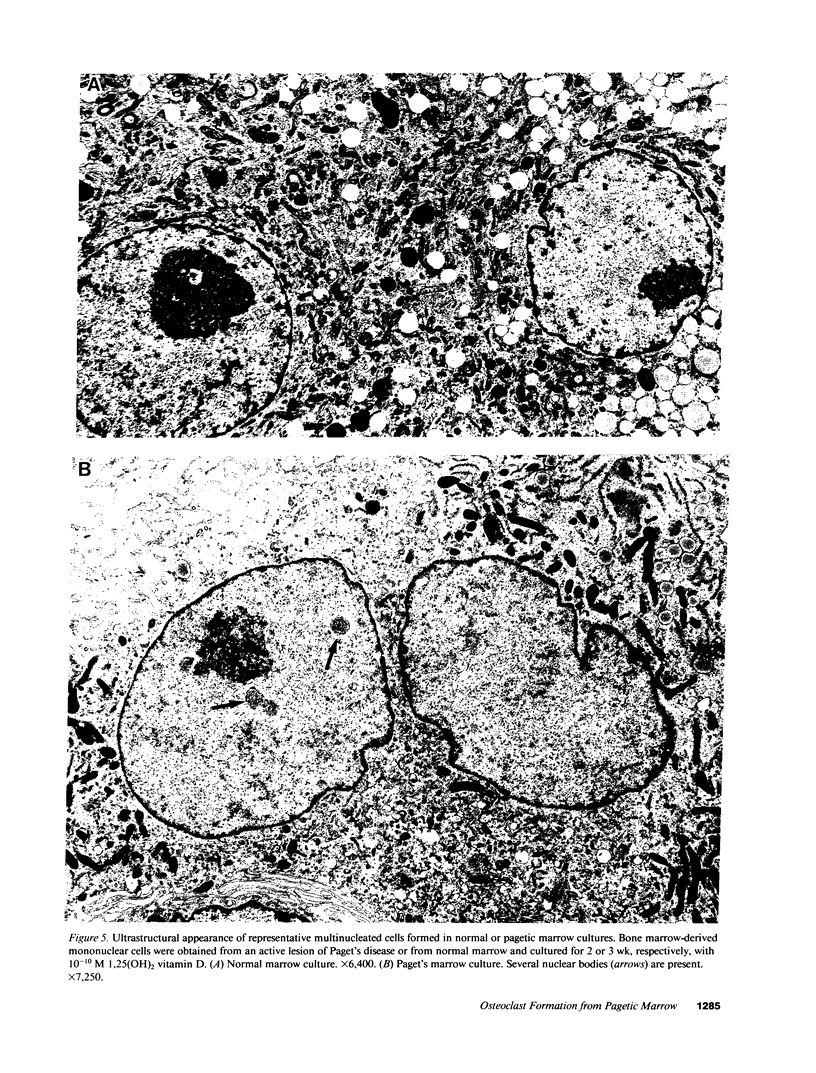
Although Paget's disease is the most flagrant example of a primary osteoclast disorder, little is known of osteoclast biology in this disease. In this report we have studied the formation of cells with the osteoclast phenotype in long-term cultures of marrow mononuclear cells derived from patients with Paget's disease, and compared these with similar cells formed in long-term marrow cultures from normal individuals, and with osteoclasts present in pagetic bone. Osteoclasts formed in pagetic marrow cultures resembled osteoclasts present in pagetic bone, but were distinctly different from osteoclasts formed in normal marrow cultures. Osteoclast formation was 10-20-fold greater in pagetic marrow cultures than in normal cultures. The multinucleated cells formed in cultures of pagetic marrow were much larger in size, were hyperresponsive to 1,25(OH)2 vitamin D, had more nuclei per cell, had increased levels of tartrate-resistant acid phosphatase activity and had ultrastructural features which were not seen in multinucleated cells formed from normal marrow mononuclear cells. These pagetic marrow-derived multinucleated cells formed large resorption lacunae on calcified matrices and cross-reacted with monoclonal antibodies which preferentially bind to osteoclasts. The multinucleated cells formed from marrow obtained from uninvolved sites in Paget's patients also displayed these abnormal features.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basle M. F., Rebel A., Renier J. C., Audran M., Filmon R., Malkani K. Bone tissue in Paget's disease treated by ethane-1, hydroxy-1, 1 diphosphonate (EHDP). Structure, ultrastructure, and immunocytology. Clin Orthop Relat Res. 1984 Apr;(184):281–288. [PubMed] [Google Scholar]
- Basle M. F., Russell W. C., Goswami K. K., Rebel A., Giraudon P., Wild F., Filmon R. Paramyxovirus antigens in osteoclasts from Paget's bone tissue detected by monoclonal antibodies. J Gen Virol. 1985 Oct;66(Pt 10):2103–2110. doi: 10.1099/0022-1317-66-10-2103. [DOI] [PubMed] [Google Scholar]
- Baslé M. F., Fournier J. G., Rozenblatt S., Rebel A., Bouteille M. Measles virus RNA detected in Paget's disease bone tissue by in situ hybridization. J Gen Virol. 1986 May;67(Pt 5):907–913. doi: 10.1099/0022-1317-67-5-907. [DOI] [PubMed] [Google Scholar]
- Baslé M. F., Rebel A., Fournier J. G., Russell W. C., Malkani K. On the trail of paramyxoviruses in Paget's disease of bone. Clin Orthop Relat Res. 1987 Apr;(217):9–15. [PubMed] [Google Scholar]
- Bouteille M., Kalifat S. R., Delarue J. Ultrastructural variations of nuclear bodies in human diseases. J Ultrastruct Res. 1967 Aug 30;19(5):474–486. doi: 10.1016/s0022-5320(67)80074-1. [DOI] [PubMed] [Google Scholar]
- Boyde A., Ali N. N., Jones S. J. Optical and scanning electron microscopy in the single osteoclast resorption assay. Scan Electron Microsc. 1985;(Pt 3):1259–1271. [PubMed] [Google Scholar]
- Cheung H. S., Singer F. R., Mills B., Nimni M. E. In vitro synthesis of normal bone (Type I) collagen by bones of Paget's disease patients. Proc Soc Exp Biol Med. 1980 Apr;163(4):547–552. doi: 10.3181/00379727-163-40812. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Experimental basis for the use of bisphosphonates in Paget's disease of bone. Clin Orthop Relat Res. 1987 Apr;(217):72–78. [PubMed] [Google Scholar]
- Gherardi G., Lo Cascio V., Bonucci E. Fine structure of nuclei and cytoplasm of osteoclasts in Paget's disease of bone. Histopathology. 1980 Jan;4(1):63–74. doi: 10.1111/j.1365-2559.1980.tb02898.x. [DOI] [PubMed] [Google Scholar]
- Harvey L., Gray T., Beneton M. N., Douglas D. L., Kanis J. A., Russell R. G. Ultrastructural features of the osteoclasts from Paget's disease of bone in relation to a viral aetiology. J Clin Pathol. 1982 Jul;35(7):771–779. doi: 10.1136/jcp.35.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Howatson A. F., Fornasier V. L. Microfilaments associated with Paget's disease of bone: comparison with nucleocapsids of measles virus and respiratory syncytial virus. Intervirology. 1982;18(3):150–159. doi: 10.1159/000149318. [DOI] [PubMed] [Google Scholar]
- Jones S. J., Boyde A., Ali N. N., Maconnachie E. Variation in the sizes of resorption lacunae made in vitro. Scan Electron Microsc. 1986;(Pt 4):1571–1580. [PubMed] [Google Scholar]
- Kanis J. A., Gray R. E. Long-term follow-up observations on treatment in Paget's disease of bone. Clin Orthop Relat Res. 1987 Apr;(217):99–125. [PubMed] [Google Scholar]
- Krane S. M. Paget's disease of bone. Clin Orthop Relat Res. 1977;(127):24–36. [PubMed] [Google Scholar]
- Krane S. M., Simon L. S. Metabolic consequences of bone turnover in Paget's disease of bone. Clin Orthop Relat Res. 1987 Apr;(217):26–36. [PubMed] [Google Scholar]
- Krane S. M. Skeletal metabolism in Paget's disease of bone. Arthritis Rheum. 1980 Oct;23(10):1087–1094. doi: 10.1002/art.1780231004. [DOI] [PubMed] [Google Scholar]
- Kukita T., McManus L. M., Miller M., Civin C., Roodman G. D. Osteoclast-like cells formed in long-term human bone marrow cultures express a similar surface phenotype as authentic osteoclasts. Lab Invest. 1989 Apr;60(4):532–538. [PubMed] [Google Scholar]
- MacDonald B. R., Takahashi N., McManus L. M., Holahan J., Mundy G. R., Roodman G. D. Formation of multinucleated cells that respond to osteotropic hormones in long term human bone marrow cultures. Endocrinology. 1987 Jun;120(6):2326–2333. doi: 10.1210/endo-120-6-2326. [DOI] [PubMed] [Google Scholar]
- Meunier P. J., Coindre J. M., Edouard C. M., Arlot M. E. Bone histomorphometry in Paget's disease. Quantitative and dynamic analysis of pagetic and nonpagetic bone tissue. Arthritis Rheum. 1980 Oct;23(10):1095–1103. doi: 10.1002/art.1780231005. [DOI] [PubMed] [Google Scholar]
- Mills B. G., Singer F. R. Nuclear inclusions in Paget's disease of bone. Science. 1976 Oct 8;194(4261):201–202. doi: 10.1126/science.959849. [DOI] [PubMed] [Google Scholar]
- Rebel A., Basle M., Pouplard A., Malkani K., Filmon R., Lepatezour A. Bone tissue in Paget's disease of bone. Ultrastructure and Immunocytology. Arthritis Rheum. 1980 Oct;23(10):1104–1114. doi: 10.1002/art.1780231006. [DOI] [PubMed] [Google Scholar]
- Rebel A., Malkani K., Baslé M., Bregeon C. Osteoclast ultrastructure in Paget's disease. Calcif Tissue Res. 1976 Apr 20;(2):187–199. doi: 10.1007/BF02546407. [DOI] [PubMed] [Google Scholar]
- Roodman G. D., Ibbotson K. J., MacDonald B. R., Kuehl T. J., Mundy G. R. 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8213–8217. doi: 10.1073/pnas.82.23.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer F. R., Mills B. G. Evidence for a viral etiology of Paget's disease of bone. Clin Orthop Relat Res. 1983 Sep;(178):245–251. [PubMed] [Google Scholar]
- Siris E. S., Clemens T. P., McMahon D., Gordon A., Jacobs T. P., Canfield R. E. Parathyroid function in Paget's disease of bone. J Bone Miner Res. 1989 Feb;4(1):75–79. doi: 10.1002/jbmr.5650040111. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kukita T., MacDonald B. R., Bird A., Mundy G. R., McManus L. M., Miller M., Boyde A., Jones S. J., Roodman G. D. Osteoclast-like cells form in long-term human bone marrow but not in peripheral blood cultures. J Clin Invest. 1989 Feb;83(2):543–550. doi: 10.1172/JCI113916. [DOI] [PMC free article] [PubMed] [Google Scholar]