Abstract
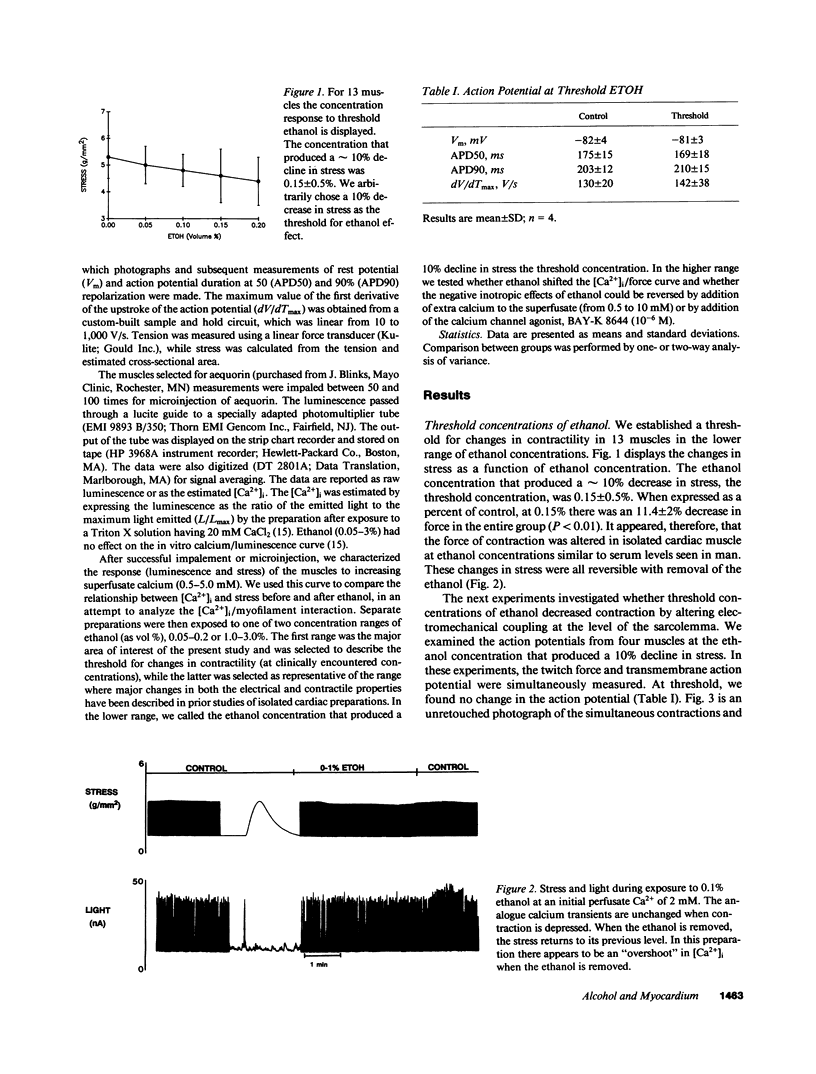
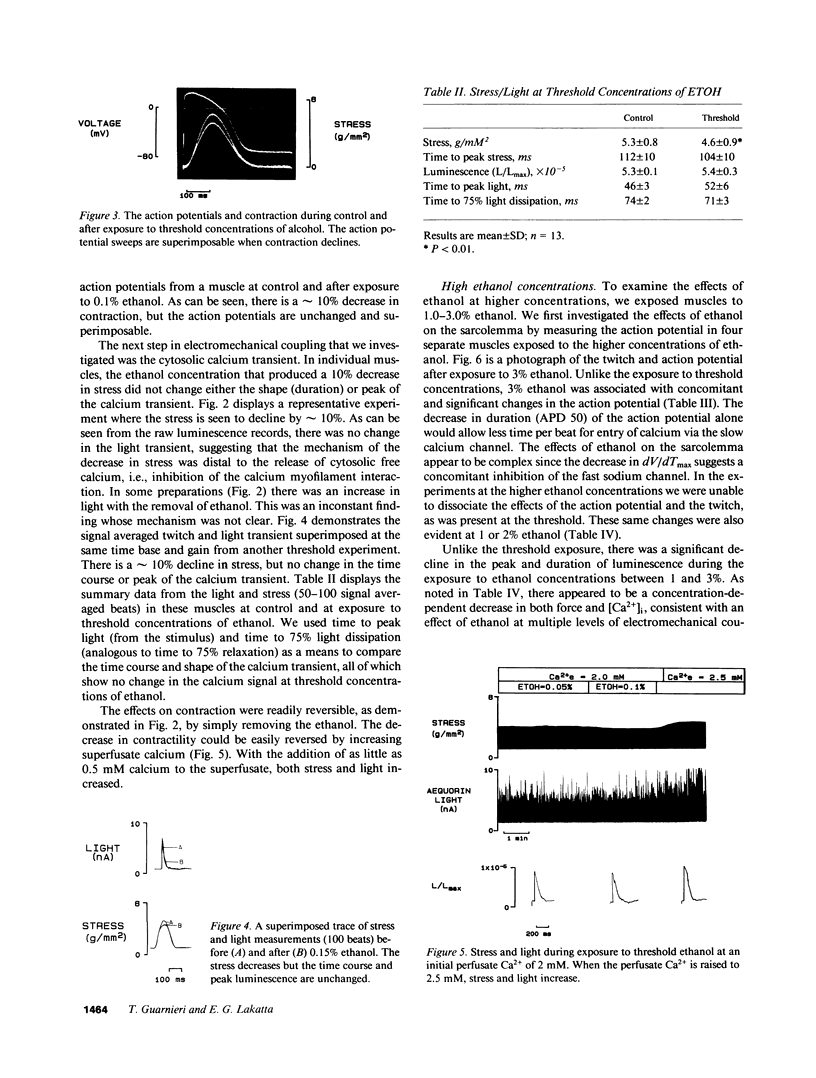
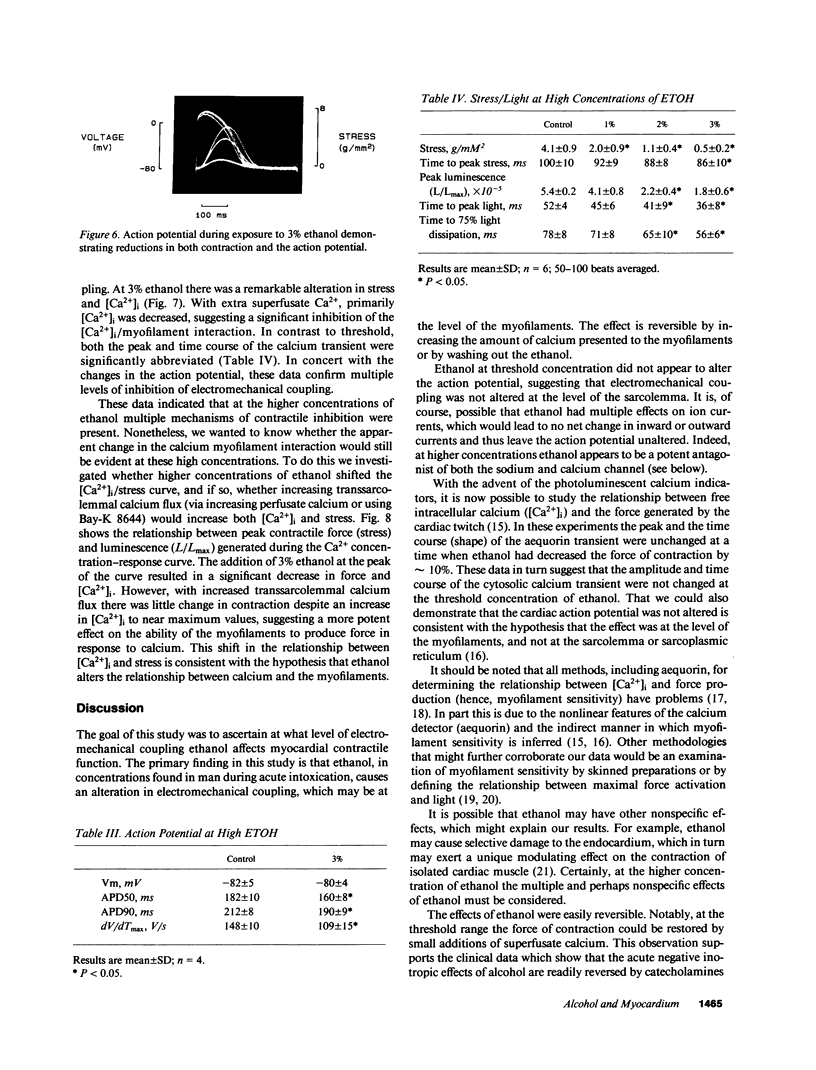
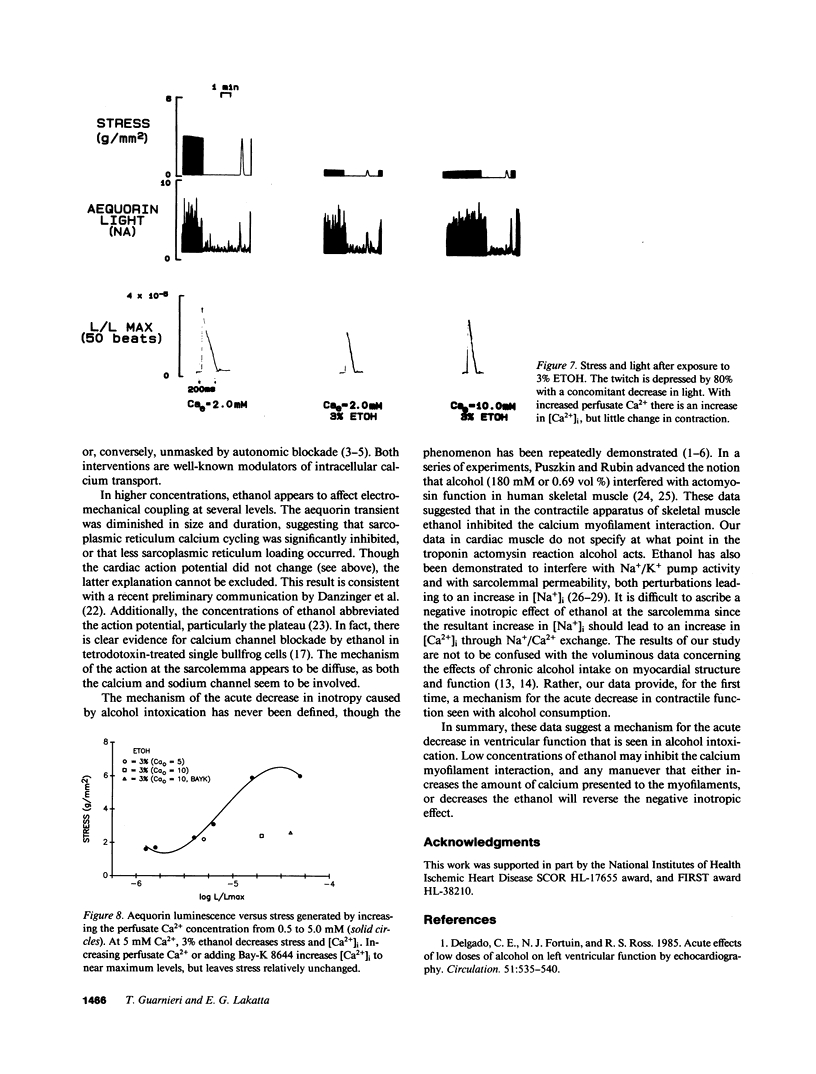
Moderate alcohol intoxication in man, a ubiqitious social event, causes acute but reversible myocardial depression, the mechanism of which is unknown. We investigated whether this depression could be due to a direct effect of ethanol on the process of electromechanical coupling by simultaneously measuring the transmembrane action potential and contraction, or the cytosolic calcium transient (via aequorin photoluminescence) and contraction in isolated ferret right ventricular papillary muscle. Ethanol, in concentrations that are similar to plasma levels in man during intoxication (0.15 vol %), depressed the force of contraction approximately 10%. The step in the electromechanical process that was affected appeared to be the calcium-myofilament interaction, as there was no change in the transmembrane action potential or cytosolic calcium transient. This inhibition was quickly reversed by removal of the ethanol from the perfusate. On the other hand, higher concentrations of ethanol produced changes in contraction, the calcium transient, and the action potential, suggesting multiple levels of inhibition of electromechanical coupling. Increasing the perfusate calcium or use of the calcium channel agonist, BAY-K 8644, increased cytosolic calcium to near maximum but had little effect on contractility, confirming that the relationship between calcium and the myofilaments had been altered. These data suggest that the acute depression in ventricular function seen with alcohol consumption may be due to a direct effect on electromechanical coupling through inhibition of the calcium myofilament interaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S. S., Levinson G. E., Regan T. J. Depression of myocardial contractility with low doses of ethanol in normal man. Circulation. 1973 Aug;48(2):378–385. doi: 10.1161/01.cir.48.2.378. [DOI] [PubMed] [Google Scholar]
- Bing R. J., Tillmanns H., Fauvel J. M., Seeler K., Mao J. C. Effect of prolonged alcohol administration on calcium transport in heart muscle of the dog. Circ Res. 1974 Jul;35(1):33–38. doi: 10.1161/01.res.35.1.33. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Meulemans A. L., Sipido K. R., Sys S. U. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circ Res. 1988 Feb;62(2):358–366. doi: 10.1161/01.res.62.2.358. [DOI] [PubMed] [Google Scholar]
- Child J. S., Kovick R. B., Levisman J. A., Pearce M. L. Cardiac effects of acute ethanol ingestion unmasked by autonomic blockade. Circulation. 1979 Jan;59(1):120–125. doi: 10.1161/01.cir.59.1.120. [DOI] [PubMed] [Google Scholar]
- Delgado C. E., Gortuin N. J., Ross R. S. Acute effects of low doses of alcohol on left ventricular function by echocardiography. Circulation. 1975 Mar;51(3):535–540. doi: 10.1161/01.cir.51.3.535. [DOI] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- GIMENO A. L., GIMENO M. F., WEBB J. L. Effects of ethanol on cellular membrane potentials and contractility of isolated rat atrium. Am J Physiol. 1962 Jul;203:194–196. doi: 10.1152/ajplegacy.1962.203.1.194. [DOI] [PubMed] [Google Scholar]
- Israel Y., Kalant H., Laufer I. Effects of ethanol on na, K, mg-stimulated microsomal ATPase activity. Biochem Pharmacol. 1965 Dec;14(12):1803–1814. doi: 10.1016/0006-2952(65)90270-4. [DOI] [PubMed] [Google Scholar]
- Kelbaek H., Gjørup T., Brynjolf I., Christensen N. J., Godtfredsen J. Acute effects of alcohol on left ventricular function in healthy subjects at rest and during upright exercise. Am J Cardiol. 1985 Jan 1;55(1):164–167. doi: 10.1016/0002-9149(85)90320-0. [DOI] [PubMed] [Google Scholar]
- Kino M., Thorp K. A., Bing O. H., Abelmann W. H. Impaired myocardial performance and response to calcium in experimental alcoholic cardiomyopathy. J Mol Cell Cardiol. 1981 Nov;13(11):981–989. doi: 10.1016/0022-2828(81)90473-9. [DOI] [PubMed] [Google Scholar]
- Kischuk R. P., Otten M. D., Polimeni P. I. Effect of acute alcoholic intoxication on myocardial electrolyte and water distributions. J Mol Cell Cardiol. 1986 Feb;18(2):197–205. doi: 10.1016/s0022-2828(86)80472-2. [DOI] [PubMed] [Google Scholar]
- Knochel J. P. Cardiovascular effects of alcohol. Ann Intern Med. 1983 May;98(5 Pt 2):849–854. doi: 10.7326/0003-4819-98-5-849. [DOI] [PubMed] [Google Scholar]
- Noren G. R., Staley N. A., Einzig S., Mikell F. L., Asinger R. W. Alcohol-induced congestive cardiomyopathy: an animal model. Cardiovasc Res. 1983 Feb;17(2):81–87. doi: 10.1093/cvr/17.2.81. [DOI] [PubMed] [Google Scholar]
- Puskin S., Rubin E. Effects of ADP, ethanol and acetaldehyde on the relaxing complex of human muscle and its adsorption by polystyrene particles. Arch Biochem Biophys. 1976 Dec;177(2):574–584. doi: 10.1016/0003-9861(76)90469-0. [DOI] [PubMed] [Google Scholar]
- Puszkin S., Rubin E. Adenosine diphosphate effect on contractility of human muscle actomyosin: inhibition by ethanol and acetaldehyde. Science. 1975 Jun 27;188(4195):1319–1320. doi: 10.1126/science.124949. [DOI] [PubMed] [Google Scholar]
- Regan R. J., Koroxenidis G., Moschos C. B., Oldewurtel H. A., Lehan P. H., Hellems H. K. The acute metabolic and hemodynamic responses of the left ventricle to ethanol. J Clin Invest. 1966 Feb;45(2):270–280. doi: 10.1172/JCI105340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retig J. N., Kirchberger M. A., Rubin E., Katz A. M. Effects of ethanol on calcium transport by microsomes phosphorylated by cyclic AMP-dependent protein kinase. Biochem Pharmacol. 1977 Mar 1;26(5):393–396. doi: 10.1016/0006-2952(77)90197-6. [DOI] [PubMed] [Google Scholar]
- Rubin E. Alcoholic myopathy in heart and skeletal muscle. N Engl J Med. 1979 Jul 5;301(1):28–33. doi: 10.1056/NEJM197907053010107. [DOI] [PubMed] [Google Scholar]
- Sarma J. S., Ikeda S., Fischer R., Maruyama Y., Weishaar R., Bing R. J. Biochemical and contractile properties of heart muscle after prolonged alcohol administration. J Mol Cell Cardiol. 1976 Dec;8(12):951–972. doi: 10.1016/0022-2828(76)90077-8. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Bounds on the estimation of calcium by aequorin luminescence in the presence of inhomogeneity. Biophys J. 1985 Nov;48(5):853–857. doi: 10.1016/S0006-3495(85)83845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper D., Capasso J. M., Sonnenblick E. H. Excitation-contraction coupling in rat myocardium: alterations with long term ethanol consumption. Cardiovasc Res. 1986 May;20(5):369–374. doi: 10.1093/cvr/20.5.369. [DOI] [PubMed] [Google Scholar]
- Timmis G. C., Ramos R. C., Gordon S., Gangadharan V. The basis for differences in ethanol-induced myocardial depression in normal subjects. Circulation. 1975 Jun;51(6):1144–1148. doi: 10.1161/01.cir.51.6.1144. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Tada M., Katz A. M., Rubin E. Effect of ethanol and acetaldehyde on the (Na+ +K+)-activated adenosine triphosphatase activity of cardiac plasma membranes. Biochem Pharmacol. 1975 Jan 1;24(1):27–32. doi: 10.1016/0006-2952(75)90308-1. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Marban E., Wier W. G. Relationship between force and intracellular [Ca2+] in tetanized mammalian heart muscle. J Gen Physiol. 1986 Feb;87(2):223–242. doi: 10.1085/jgp.87.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Wier W. G. Estimation of intracellular [Ca2+] by nonlinear indicators. A quantitative analysis. Biophys J. 1985 Sep;48(3):533–537. doi: 10.1016/S0006-3495(85)83810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



