Abstract
The influence of the lipid environment on docking and fusion of synaptobrevin 2 (Syb2) vesicles with target SNARE complex membranes was examined in a planar supported membrane fusion assay with high time-resolution. Previously, we showed that approximately eight SNARE complexes are required to fuse phosphatidylcholine (PC) and cholesterol model membranes in ∼20 ms. Here we present experiments, in which phosphatidylserine (PS) and phosphatidylethanolamine (PE) were added to mixtures of PC/cholesterol in different proportions in the Syb2 vesicle membranes only or in both the supported bilayers and the Syb2 vesicles. We found that PS and PE both reduce the probability of fusion and that this reduction is fully accounted for by the lipid composition in the vesicle membrane. However, the docking efficiency increases when the PE content in the vesicle (and target membrane) is increased from 0 to 30%. The fraction of fast-activating SNARE complexes decreases with increasing PE content. As few as three SNARE complexes are sufficient to support membrane fusion when at least 5% PS and 10% PE are present in both membranes or 5% and 30% PE are present in the vesicle membrane only. Despite the smaller number of required SNAREs, the SNARE activation and fusion rates are almost as fast as previously reported in reconstituted PC/cholesterol bilayers, i.e., of 10 and ∼20 ms, respectively.
Introduction
Synaptic transmission between neurons occurs via Ca2+-regulated exocytosis during which presynaptic vesicles fuse at the active zone with the neuronal plasma membrane. Membrane fusion of synaptic vesicles is mediated by three SNARE proteins: syntaxin 1a (Syx1a), SNAP25, and synaptobrevin 2 (Syb2). SNARE proteins share as a common feature 60–70 amino-acid-long highly conserved sequences called SNARE motifs that fold into a coiled-coil helical bundle during fusion (1). Both Syx1a and Syb2 possess a single SNARE motif, whereas SNAP25 contains two such motifs connected by a flexible linker sequence. In the monomeric state, these regions are mostly unstructured, but upon interaction with each other, they assemble in a zipperlike fashion into the parallel α-helical coiled-coil bundle (2–5). It has been shown by reconstitution from purified components that the pairing of appropriate SNAREs is sufficient to drive membrane fusion (6–8).
Previous studies on SNARE-mediated membrane fusion focused mostly on the roles of the SNAREs and several regulatory proteins in this process. However, increasing evidence in vivo and in vitro indicates that the lipid environment including the membrane geometry, lipid charge, and lipid shape also regulate SNARE equilibria as well as SNARE-mediated fusion. Studies have shown that cholesterol and perhaps lipid rafts play important roles in regulated exocytosis (9). For example, Lang et al. (10) and Sieber et al. (11) showed that Syx1a forms cholesterol-dependent clusters of ∼75 molecules in plasma membranes of neuroendocrine phosphatidylcholine (PC)12 cells. These clusters partially overlap with SNAP25 nanodomains that are also observed in the same membranes. However, although dispersed by cholesterol extraction, the clustered proteins do not fractionate in detergent-insoluble lipid raft fractions, suggesting that the SNAREs are localized in cholesterol-induced domains that are distinct from lipid rafts. This has been confirmed in lipid model membranes where Syx1a clusters in a cholesterol-dependent fashion that does not involve lipid rafts. Moreover, the cholesterol-induced Syx1a clusters are dispersed by acidic lipids such as phosphatidylserine (PS) or low concentrations of the regulatory lipid PI-4,5-P2 (12).
It has been shown that increased PS levels in PC12 cells produce an increase in Ca2+-triggered exocytosis. However, PS also slows down the rate of fusion pore dilation in this system (13). PIP2 has been shown to be required for Ca2+-triggered exocytosis and its impaired synthesis in neuronal cells causes defects in synaptic vesicle trafficking at multiple steps of the vesicle fusion and retrieval cycle (14,15). Moreover, PIP2 levels determine the size of the readily releasable vesicle pool and rates of vesicle priming as well as rates of vesicle fusion (16–19). Interactions between Syx1a and phosphatidic acid as well as multiple phosphoinositides (PIPs) have a direct influence on secretion in PC12 cells (20) and the juxtamembrane polybasic region of Syx1a has been recognized as a lipid-binding domain (D. H. Murray and L. K. Tamm, unpublished).
Although SNARE-mediated membrane fusion has been extensively studied in reconstituted systems and although lipids exert diverse physiological effects on exocytosis in cells, the influence of the lipid environment has received only limited attention in reconstituted fusion systems. The standard lipid compositions used in most liposome fusion assays included 75–85 mol % POPC and 15–25 mol % DOPS (or porcine brain PC and PS) or a mixture that was intended to mimic the cytoplasmic leaflets of the plasma and vesicle membranes consisting of PC/PE/PS/PI/Chol at a 5:2:1:1:1 molar ratio (5,6). Similarly, in planar bilayer fusion systems, membranes were composed of POPC and 20% cholesterol, or POPC and DOPS (15–35% PS), or POPC/DOPS/DOPE (15% PS and 0–60% phosphatidylethanolamine (PE)) (21–24). In single vesicle fusion experiments, vesicles composed of POPC/DOPS (65% PC and 35% PS) or POPC/DOPE/DOPS/Chol/PIP2 (25% PE, 3–15% PS, 20% Chol, and 6% PIP2) were used (25,26).
Because bulk fusion assays cannot distinguish between the docking and membrane merger steps of fusion, several laboratories have developed single vesicle fusion assays either with partner vesicles or with planar supported membranes. In the latter, acceptor SNAREs are typically reconstituted into a planar membrane and Syb2 proteoliposomes are observed by total internal reflection fluorescence (TIRF) microscopy to fuse with the planar bilayer. A distinct advantage of this approach is that docking and membrane merger can be easily separated, that the geometry, including the lipid asymmetry of the target membrane, roughly approximates that of the synaptic vesicle and plasma membranes, and that fusion rates can be measured with millisecond time-resolution. The reconstitution method that our group has developed to reproduce cell physiological fusion as closely as possible allows us to reconstitute SNAREs in planar membranes with a defined right-side-out topology and in a laterally mobile form (27,28). Fusion is SNAP25-dependent, specifically blocked by well-established fusion blockers, and occurs in ∼20 ms, i.e., close to the rate of sustained, Ca2+-independent, physiological fusion. Analysis of the fusion kinetics further revealed that ∼6–9 SNARE complexes are required to form an active fusion pore (28).
Since that work was published, another study using the standard bulk liposome fusion assay reported that one SNARE complex was sufficient to mediate fusion between appropriately reconstituted SNARE proteoliposomes (29). Although the same SNAREs and SNARE acceptor complexes were used in those and our studies, there are three fundamental differences between them:
-
1.
The single vesicle fusion assay with its high time-resolution detects fusion that is approximately four orders-of-magnitude faster than that observed in the liposome fusion assay. Fast fusion may require a greater number of SNAREs to proceed efficiently than slow fusion.
-
2.
The membrane curvature is vastly different in the two experiments. Fewer SNARE complexes may be required to fuse 40-nm vesicles with high curvature strain than fusion with a planar target membrane.
-
3.
The lipid compositions and SNARE concentrations were different in the two experiments. The planar membrane study used POPC/Chol (4:1), and the liposome assay used PC/PE/PS/Chol (5:2:1:1). Fewer SNAREs may be required to promote fusion in the presence of PE and PS than in their absence.
To resolve whether the lipid composition could contribute to the number of SNAREs required for fusion and to better understand whether lipid composition affects mostly the docking or membrane merger steps of fusion, we have systematically investigated the roles of PS and PE on SNARE-mediated membrane fusion. We tested the influence of increasing DOPS and DOPE concentrations in a background of POPC/Chol (4:1) on the fusion of single vesicles to planar bilayers. This was done by modulating the lipid composition of only the vesicles or both the vesicles and planar bilayers. In the latter experiments, the planar bilayers were further constructed with asymmetric lipid distributions, i.e., with the DOPS and DOPE additives only present in the vesicle-exposed leaflet of the planar membrane, as is also the case in plasma membranes of cells. We find that the docking efficiency increases, but the fusion probability decreases with increasing PS and PE concentrations. We also find that with some of these PS- and PE-enriched lipid compositions, 3–5 SNARE complexes are sufficient to support membrane fusion.
Materials and Methods
Materials
All lipids were from Avanti Polar Lipids (Alabaster, AL) and used without further purification. CHAPS was from Anatrace (Maumee, OH) and other chemicals from Sigma (St. Louis, MO) or Fisher Scientific (Fair Lawn, NJ). Water purified with deionizing and organic-free filters (Virginia Water Systems, Richmond, VA) and a NANOpure system from Barnstead (Dubuque, IA) had a resistivity of 18.2 MΩ/cm.
Protein expression and purification
SNARE proteins cloned in pET28a vector were expressed in BL21(DE3) Escherichia coli and purified as described previously (30,31). To produce the ΔN complex, Syx1a (183–288) and Syb2 peptide (49–96) were coexpressed in the pETDuet-1 vector expression system (32). The cysteine-free variant of SNAP25A (1–206) and Syb2 (1–117, Cys117) were used. The acceptor SNARE complex containing Syx1a, SNAP25, and Syb49–96 was either assembled overnight at 4°C from individual subunits or purified from BL21(DE3) E. coli expressing all three proteins. All proteins were purified by Ni2+-NTA affinity chromatography followed by ion exchange chromatography. Proteins with transmembrane domains were purified in the presence of 15 mM CHAPS (32).
SNARE reconstitution into proteoliposomes and planar supported bilayers
Single SNAREs and ternary acceptor SNARE complexes were reconstituted into vesicles by rapid dilution of micellar protein/lipid/detergent mixtures followed by dialysis as described previously (27,28). Planar supported bilayers with reconstituted SNAREs were prepared by a combined Langmuir-Blodgett/vesicle fusion technique as previously described (27,33,34).
TIRF microscopy
All experiments were carried out on a prism-based TIRF microscope (Axiovert 35; Zeiss, Jena, Germany) that has been previously described (27). Illumination was with the 514 line of an argon ion laser and images were captured with an electron-multiplying charge-coupled device (model No. DU-860E; Andor Technology, South Windsor, CT).
Single vesicle docking and fusion assay
Supported bilayers containing acceptor complex were perfused on the microscope stage with 1 mol % Rh-DOPE-labeled Syb2 vesicles in fusion buffer (20 mM HEPES, 120 mM potassium glutamate, 20 mM potassium acetate, pH7.4) at room temperature as described (28). Movies were acquired with frame exposure times of 4 ms starting ∼1 min after the beginning of vesicle injection. The initial fast image acquisition period lasting 5–10 min was followed by ∼30 min of single image acquisitions every 30 s with 20-ms exposure times to measure additional vesicle docking in bulk mode. For further details, see Domanska et al. (28).
Analysis of single vesicle fusion and docking data
Images were analyzed using a homemade program written in LabView (National Instruments, Austin, TX) as described in Domanska et al. (28). Central pixel fluorescence intensity traces of recognized vesicles were used to classify observed events as docking or docking followed by fusion. Experimentally obtained docking curves were fitted with first-order kinetics according to
| (1) |
where D∞ is the final concentration of occupied docking sites and kon is the docking rate.
Fusion kinetic analysis
Fusion kinetic data were analyzed using the previously described multiparticle parallel activation model (Eq. 2) (28). In this model, we assume that a fusion site consists of particles (e.g., SNARE complexes) that activate in parallel. Two types of particles that activate at different rates are randomly distributed within one fusion site according to the probability of their occurrence by a binomial distribution,
| (2) |
where N is the total number of fusion events, m the total number of particles in one fusion site, l the number of particles of slow fraction, and p the probability of slow fraction. The values k1 and k2 are the activation rates of the fast- and slow-activating particles, respectively.
Results
Our previous results showed that docking and fusion, which are dependent on the presence of all three neuronal SNAREs, can be separated into two distinct kinetic steps in a fast single vesicle fusion assay (28). In this assay, Syx1a, SNAP25, and a short peptide corresponding to the C-terminal SNARE motif of Syb2 forms the acceptor SNARE complex in a planar target membrane that is supported on a quartz microscope slide. The acceptor complex is laterally mobile and oriented right-side-out for maximal physiological engagement with reconstituted Syb2 vesicles that are superfused and allowed to dock and fuse with the target membrane. The use of this so-called ΔN-acceptor SNARE complex is important because it prevents the nonproductive formation of a 2:1 Syx1a:SNAP25 complex. It has been shown previously that the Syb2 peptide leaves the complex quickly when full-length Syb2 binds in the N-terminal Syb2-binding grove of the acceptor SNARE complex; therefore, this peptide does not interfere with docking but it may slow down fusion to some extent (5). These earlier experiments were conducted with the ΔN-acceptor SNARE complex and Syb2 reconstituted into model membranes composed of POPC/Chol (4:1). However, because mammalian plasma and synaptic vesicle membranes also contain ∼15 mol % PS and 30 mol % PE in the cytoplasmic leaflet, we asked whether these lipids affect the docking, membrane merger, or both steps of SNARE-mediated membrane fusion.
In all experiments that will be described here we have included various amounts of DOPS in POPC/Chol (4:1) membranes or various amounts of DOPE in POPC/DOPS/Chol (75:5:20) membranes. We have recorded Syb2 vesicle docking and fusion in systems, in which these lipids were added symmetrically to the vesicle and target membranes or asymmetrically to only the vesicle membranes. Because we record the spread of lipid dye from the docked vesicles, we actually monitor lipid mixing and not fusion pore formation in our fusion assay. Because the lipids of both leaflets mix and there is no evidence for hemifusion in our assay (see below), we still call the lipid mixing events “fusion events” throughout this study.
Effect of DOPS in Syb2 vesicles and supported target membranes on docking and fusion
In preliminary experiments, we found that the inclusion of 15 mol % DOPS in the vesicle membrane, in the target membrane, or in both membranes produced many nonspecific docking and some nonspecific fusion events. These are defined as docking of Syb2 vesicles to pure lipid bilayers without acceptor SNARE complex or to reconstituted acceptor SNARE complex that has been blocked with the full-length soluble fragment of Syb2 (28). Nonspecific docking did not happen when the 15 mol % DOPS membranes also contained 30 mol % DOPE. However, although efficient specific docking occurred, to our surprise very few fusion events were observed under these conditions (Table S1 in the Supporting Material). Therefore, we systematically and separately increased DOPS and DOPE concentrations in the vesicle and planar target membranes to assess each lipid's influence on docking and fusion.
The quantities of 5 and 10 mol % DOPS were included in the vesicle and target membranes or in the Syb2 vesicles only. The system was more robust with 5 than with 10 mol % DOPS in both configurations. Inclusion of 5 mol % DOPS did not significantly change docking or fusion compared to our previous results with bilayers containing only POPC/Chol (Table S1) (28). The first four entries of Fig. 1, A and B, show in graphical format the docking and fusion results, respectively. The insensitivity of docking and fusion to 5 mol % DOPS was not dependent on whether this lipid was present in both membranes (shaded bars) or in the vesicle membrane only (solid bars).
Figure 1.
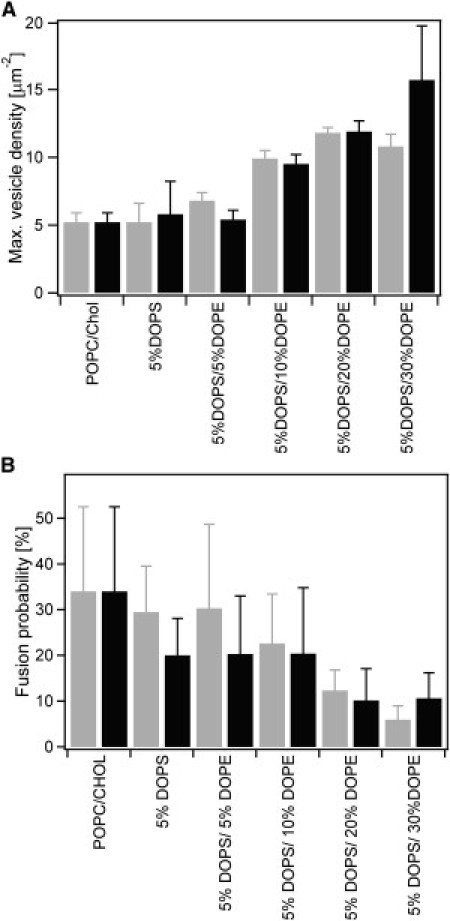
Lipid composition of the vesicle and target membrane affects docking and fusion of Syb2 vesicles in single vesicle to planar supported membrane fusion assay. (A) Mean final docking after saturation of reconstituted acceptor SNARE complex-containing target membranes with Syb2 vesicles. Numbers of docked vesicles per μm2 are average values of 16–18 independent experiments (different bilayers), in which the final docking densities were obtained by fitting Eq. 1. Error bars represent standard deviations of the mean final docking density between different bilayers. (B) Fusion probability calculated as the percentage of observed total fusion events over all recorded docking events. Error bars represent standard deviations from all measurements in multiple membranes of the same lipid composition. The “extracellular” leaflets in all supported target membranes were composed of POPC/Chol (4:1), whereas the “cytoplasmic” leaflets contained in addition different concentrations of DOPS and DOPE as indicated. These lipids were added to vesicle and target membranes (gray bars) or to vesicle membranes only (black bars).
Effect of DOPE in symmetric and asymmetric membranes on v-SNARE vesicle docking
To examine the effect of DOPE on vesicle docking and fusion, we progressively added 5, 10, 20, and 30 mol % DOPE to POPC/DOPS/Chol bilayers containing 5 mol % DOPS and 20 mol % Chol. The final density of docked Syb2 vesicles per unit area of target membrane, increased 1.5- to 3-fold over this concentration range of DOPE (Fig. 1 A). No statistically significant difference was found when both or only the Syb2 vesicles contained the increased PE concentrations, suggesting that the effect of PE on docking is more dominant in the vesicle than in the target membrane. The statistics of these docking as well as the subsequent fusion experiments are summarized in Table 1.
Table 1.
Statistics of single Syb2 vesicle docking and fusion events recorded with model membranes of different lipid compositions with up to 5 mol % PS
| Second “cytoplasmic” leaflet lipid composition of supported membrane∗ | Syb2 vesicle lipid composition | Total number of supported bilayers | Total number of docking events | Total number of fusion events |
|---|---|---|---|---|
| POPC/Chol | POPC/Chol | 30 (28)† | 3205 | 1363 |
| POPC/DOPS/Chol | POPC/DOPS/Chol (75:5:20) | 18 (11) | 483 | 161 |
| POPC/Chol | 3 (2) | 112 | 28 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (70:5:5:20) | 18 (16) | 3255 | 1135 |
| POPC /Chol | 16 (15) | 2205 | 674 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (65:5:10:20) | 18 (15) | 2824 | 781 |
| POPC/Chol | 16 (13) | 2595 | 754 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (55:5:20:20) | 18 (9) | 1661 | 223 |
| POPC/Chol | 17 (15) | 2823 | 328 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (45:5:30:20) | 18 (13) | 1218 | 77 |
| POPC/Chol | 16 (16) | 3142 | 416 |
First “extracellular” leaflet of all supported membranes consisted of POPC/Chol (4:1).
Numbers in parentheses represents total number of bilayers in which fusion and docking of Syb2 vesicles was observed.
Effect of DOPE in symmetric and asymmetric membranes on v-SNARE vesicle fusion probability
Having established that docking increases with increasing PE concentration in the vesicle membrane, we next examined the probability that a docked vesicle would fuse with the planar target membrane as a function of DOPE in the vesicle and target membranes. Here, we define fusion as the probability of a vesicle to fuse within 1 s after docking. Analysis of 1000–3000 docking events under each condition shows that the probability of fusing with the target membrane in a SNARE-specific fashion decreases ∼3–6-fold when DOPE is increased from 0 to 30 mol % (Fig. 1 B). Again, this effect is achieved when PE is present only in the vesicle membrane and little or no further change is observed when PE is present in both membranes. Therefore, as was the case for docking, PE in the vesicle membrane, but not PE in the target membrane is primarily responsible for the decrease in the fusion probability.
Effect of DOPE in POPC/DOPS/Chol membranes on fusion kinetics
Four typical traces of single Syb vesicle fusions after docking on acceptor SNARE complex-containing planar membranes are shown in Fig. S1 in the Supporting Material. A statistical analysis of the single vesicle fusion data furnishes three different types of information:
-
1.
The mean fusion delay time after docking may be determined from histograms of the number of fusion events occurring within a specified time interval after docking.
-
2.
The activation time for individual particles (SNARE complexes) transitioning into a fusion-competent state extracted from kinetic modeling of the data.
-
3.
The number of particles (SNARE complexes) participating in a fusion pore obtained from parameter fitting of the kinetic data (28).
Therefore, we analyzed 4388 fusion delay times, Δtfus, that were obtained under eight different conditions with different concentrations of DOPE in both membranes or only the vesicle membrane. These data are organized in eight fusion delay-time histograms shown in Fig. 2.
Figure 2.
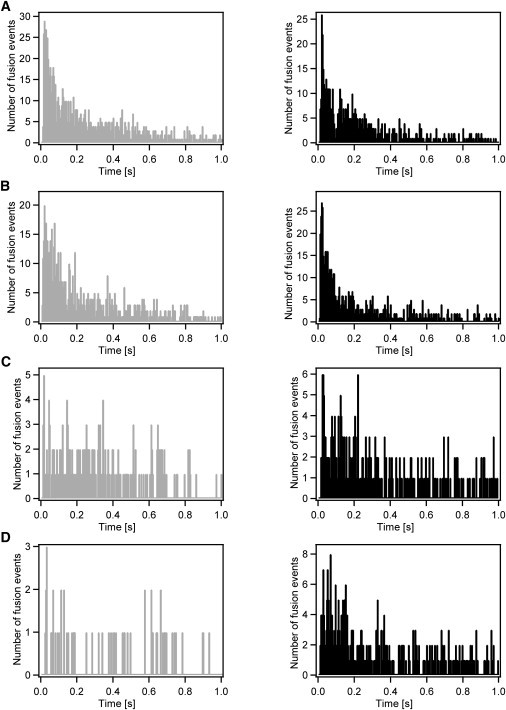
Histograms of fusion delay times obtained in membranes of different lipid composition. The “extracellular” leaflets in all supported target membranes were composed of POPC/Chol (4:1), whereas the “cytoplasmic” leaflets contained in addition: (A) 5 mol % DOPS and 5 mol % DOPE, (B) 5 mol % DOPS and 10 mol % DOPE, (C) 5 mol % DOPS and 20 mol % DOPE, and (D) 5 mol % DOPS and 30 mol % DOPE. (Gray histograms on the left) Data obtained with DOPS and DOPE present in the Syb2 vesicle and planar target membrane. (Black histograms on the right) Data collected with DOPS and DOPE that is present only in Syb2 vesicle membranes.
The histograms on the left of Fig. 2 show the data obtained with DOPE present in both membranes and those on the right show comparable data with DOPE present only in the vesicle membrane. All histograms show a wide distribution of Δtfus between 0 and 1 s, with those at 5 and 10 mol % PE exhibiting a clear preference for short fusion delays in the first 100 ms. In the presence of 20 or 30 mol % PE, the fusion delays are more widely distributed, but also fewer fusion events were observed under these conditions (Table 1). At 5 and 10 mol % PE, a main peak was observed at 22 and 18 ms, respectively, when the lipids were symmetrically included in both membranes and at 23 ms and 17 ms, respectively, when the DOPE was present only in the vesicle membrane. These results are in good general agreement with our previously published results in the POPC/Chol system, in which case the Δtfus distribution histogram showed a main peak at ∼18 ms.
Fusion kinetic analysis: effect of DOPE in symmetric and asymmetric membranes on SNARE activation rates and number of SNAREs per fusion site
We previously showed that fast fusion kinetic data such as those presented in Fig. 2 are best analyzed using a binomial multiparticle activation model for fusion pore formation (28) that is inspired by Hodgkin and Huxley's multisubunit activation model for the opening of voltage-gated ion channels (35). Because of the broad distribution of Δtfus values observed under all conditions of Fig. 2, we have to take into account a fast and a slow activating population of particles. According to the model, we assume that fast and slow particles (SNARE complexes) can distribute randomly within a fusion site and that particles activate in a parallel fashion. Fig. 3 shows the cumulative distribution functions that were generated by integrating the experimental Δtfus distributions reported in Fig. 2. Because the data have been obtained one-by-one from single vesicle fusion events, these kinetics are synchronized such that the docking time always represents time zero in these kinetic traces of the membrane merger.
Figure 3.
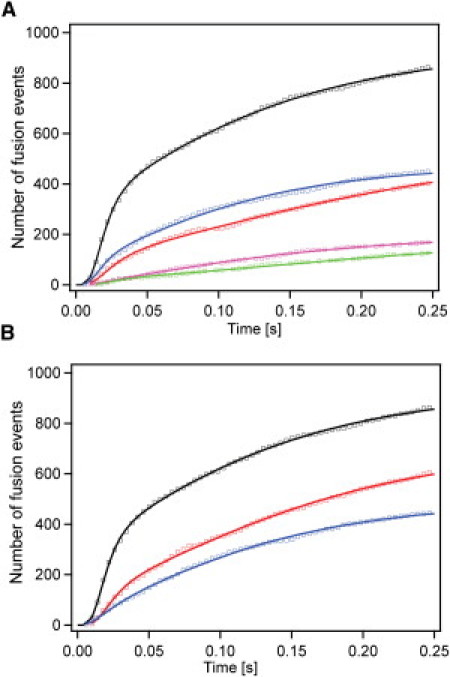
Cumulative distribution functions of single Syb2 vesicle fusion events to acceptor SNARE-complex containing membranes of different lipid compositions. Cumulative distribution functions representing the synchronized kinetics of membrane merger were fitted with the multiparticle parallel activation model as described in the text. (Solid lines) Best-fit curves. (Squares) Experimental data. Fusion kinetics obtained in asymmetric membranes, in which DOPS and DOPE were present in Syb2 vesicle membranes only (A) and in both vesicle and target membranes (B). (Black, POPC/Chol (4:1); red, plus 5 mol % DOPS and DOPE; blue, plus 5 mol % DOPS and 10 mol % DOPE; green, plus 5 mol % DOPS and 20 mol % DOPE; magenta, plus 5 mol % DOPS and 30 mol % DOPE.)
We fitted Eq. 2 that describes the multiparticle parallel activation model as explained above and in more detail in Materials and Methods to the data of Fig. 3. To compare our results on the influence of the different lipids on fusion with our previously published results obtained with POPC/Chol membranes, we fitted only the first 250 ms of the synchronized fusion kinetics. The best-fit curves are shown along with the data in Fig. 3 and the resulting best-fit parameters are listed in Table 2. Generally, excellent fits were obtained for four lipid conditions with PS and PE only included in the vesicle membrane (Fig. 3 A), and for the lower PE concentration conditions when PS and PE were included in both membranes (Fig. 3 B). We considered several other models to fit the data (28), but the modified Hodgkin-Huxley model clearly fits the data best (28). All models returned populations of fast and slow particles as required by the tails to long fusion delay times in all data sets.
Table 2.
Analysis of single vesicle fusion kinetics in model membranes of different lipid compositions
| Second “cytoplasmic” leaflet lipid composition of supported membrane∗ | Syb2 vesicle lipid composition | Number of particles per fusion site (m) | Fast fraction activation rate (s−1) | Fast fraction (%) | Slow fraction activation rate (s−1) |
|---|---|---|---|---|---|
| POPC/Chol | POPC/Chol | 8 ± 2 | 136 ± 15 | 87 ± 2 | 10.3 ± 1.2 |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (70:5:5:20) | 8 ± 2 | 112 ± 16 | 68 ± 4 | 9.2 ± 1.1 |
| POPC/Chol | 5 ± 1 | 89 ± 17 | 70 ± 6 | 5.2 ± 1.4 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (65:5:10:20) | 3 ± 1 | 86 ± 25 | 48 ± 9 | 11.2 ± 1.7 |
| POPC/Chol | 7 ± 2 | 138 ± 22 | 67 ± 4 | 12.7 ± 1.4 | |
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (55:5:20:20) | N/A | |||
| POPC/Chol | N/A | ||||
| POPC/DOPS/DOPE/Chol | POPC/DOPS/DOPE/Chol (45:5:30:20) | N/A | |||
| POPC/Chol | 3 ± 2 | 74 ± 49 | 41 ± 23 | 10 ± 3 | |
First “extracellular” leaflet of all supported membranes consisted of POPC/Chol (4:1).
In the case of asymmetric membranes (Fig. 3 A), we found that with 5%/5% PS/PE in the Syb2 vesicles, 5 ± 1 SNARE complexes assemble to form an active fusion site (Table 2). Seventy-percent of the subunits (complexes) in these sites are activated with a rate of 89 ± 17 s−1 with the remainder activated at 5.2 ± 1.4 s−1. With 5%/10% PS/PE in the Syb2 vesicles, the fusion sites comprise 7 ± 2 SNARE complexes. Sixty-seven-percent of the complexes are activated with a rate of 138 ± 22 s−1 and 33% with a rate of 12.7 ± 1.4 s−1. The numbers of SNARE complexes per fusion site are not significantly different from each other, between 0, 5, and 10 mol % PE plus 5 mol % PS in the vesicles. They are in the 5–7 range for each of these conditions. However, when the Syb2 vesicles contained 30 mol % PE (in addition to 5 mol % PS), the fusion sites were composed of only 3 ± 2 SNARE complexes. A quantity of 41% of these activated with a rate of 74 ± 49 s−1 and 59% with a rate of 10 ± 3 s−1. Although the data with 5%/20% PS/PE in the Syb2 vesicles could be fitted to our model, the fits did not yield useful information because they resulted in large errors of the fit parameters. We therefore exclude this condition from further discussion.
Similar results were obtained when symmetric PS/PE distributions between vesicles and target membranes were used at low PE concentrations (Fig. 3 B). However, a reduction of the number of SNARE complexes required for fusion was observed at lower PE concentrations than in the corresponding asymmetric cases. For example, only 10 mol % PE plus 5 mol % PS in vesicle and target membranes were required to bring down the number of SNARE complexes per fusion site to 3 ± 1 subunits, where 30 mol % PE was required when these lipids were included in the vesicle membranes only. Similar to the asymmetric 30 mol % PE case, 48% of the subunits activated at a rate of 86 ± 25 s−1 and 52% at a rate of 11.2 ± 1.7 s−1 in the 10 mol % symmetric case (Table 2). Thus, the effects that inclusion of PE exerts on the fast fusion kinetics appear to be qualitatively additive between the two membranes. Even though docking was reasonably efficient, relatively few fusion events were observed when 20 and 30 mol % PE were included symmetrically in both membranes. Therefore, statistically relevant fits of the fusion kinetic traces could not be obtained for these cases.
Discussion
Although SNARE-mediated membrane fusion has been widely studied in vitro by bulk liposome fusion (5–8) and single vesicle fusion with liposomes (26,36) or with planar supported membranes (21–24,28), the control of lipid composition in these assays has, with very few exceptions, received relatively little attention. Diverging results and conclusions that have been reached on the reconstitution of SNARE-mediated fusion and its regulatory factors may depend not only on the proteins used, on reconstitution methods, on protein and lipid concentrations, on membrane size and curvature, but also on significantly different lipid compositions that have been used in the different fusion assays and by the different groups working in this field.
In this work, we have applied a systematic approach to study the influence of PS and PE on SNARE-mediated membrane fusion using a previously established single-vesicle membrane fusion assay. Our assay uses protein/lipid ratios and membrane geometries that are approximately physiological: 1:200 in 40–50-nm reconstituted Syb2 vesicles and 1:3000 in reconstituted acceptor SNARE complex planar supported membranes (28). Our assay depends on the presence of SNAP25 in the acceptor complex, which is blocked specifically by competitive binding of the cytosolic fragment of Syb2 to the acceptor complex. Five parameters describing the overall fusion reaction are obtained from this assay:
-
1.
The docking efficiency is directly measured as the number of total vesicles bound per unit area.
-
2.
The fusion efficiency, expressed as the fraction of vesicles that fuse after docking, is easily obtained from direct observation.
-
3.
The mean fusion delay time after docking, i.e., the time from docking to membrane merger, is obtained from histograms of distributions of measured individual Δtfus.
-
4.
The number of particles (interpreted as the number of SNARE complexes) forming a fusion site is determined.
-
5.
The individual activation rates of the particles are relatively easy to extract from kinetic modeling of the Δtfus data.
Effect of DOPS on docking and fusion
Although most investigators include some fraction of PS in their fusion assays, we find inclusion of 5–15 mol % PS progressively decreasing fusion and 15 mol % causing nonspecific docking in our system. Contrary to common belief, this result is not too surprising to us. Negatively charged surfaces should electrostatically repel each other, even at approximately physiological ionic strength as used in the present experiments. It is likely that additional proteins such as synaptotagmin in conjunction with Ca2+ are required to overcome this electrostatic repulsion.
If this is true, why then is fusion observed between reconstituted proteoliposomes with just neuronal SNAREs and in the presence of PS, but without synaptotagmin?
We think that the answer lies in the more than four orders of-magnitude different speeds of fusion between the liposome and single vesicle planar membrane fusion assays and possibly the much higher protein concentrations that are typically used in the bulk liposome fusion assay. Under these conditions, occasional dockings may happen with low frequency and then lead to slow productive fusion even at 15–25% PS. These slow events are not detected in our assay. In fact, when we included 15% PS, we observed many nonspecific docking events of Syb2 vesicle binding to protein-free target membranes. In the case of 15% PS in the target membrane, these events may have been mediated by an excess of basic residues in the juxtamembrane region and the zero-layer Arg of Syb2 interacting with the target membrane. Because we believe that these interactions are nonphysiological, we did not further pursue studies at PS concentrations >5 mol %.
DOPE increases the docking efficiency, decreases the fusion efficiency, decreases the number of SNARE complexes per fusion site, and leaves the fusion rate unchanged
DOPE (× mol %) in a background of POPC/DOPS/Chol (75-×:5:20) exerts a complex behavior on docking and fusion when analyzed at the single vesicle level. We have performed a detailed analysis of a total of 4388 single fusion events recorded in symmetric and asymmetric membranes containing increasing amounts of PE up to 30 mol %, which is approximately physiological for the cytoplasmic leaflets of the plasma and vesicle membranes.
The result that PE increases the efficiency of docking of Syb2 vesicles to acceptor SNARE complexes in planar target membranes is new and was not expected. It is not immediately clear why PE should increase the docking efficiency by approximately two- to threefold. A possible explanation for this effect may be a change in the clustering of syntaxin 1a and acceptor SNARE complexes in the target membrane and Syb2 in the vesicle membrane that may be modulated by PE. We have shown previously that Syx1a forms clusters in cholesterol-containing model membranes and that these clusters can be disrupted by acidic lipids (12). The effect of PE on the clustering of Syx1a and Syb2 is not known, but it is conceivable that PE also unclusters Syx1a and/or Syb2 to some extent, and thereby makes more acceptor sites available for Syb2 vesicle binding. If, for example, one acceptor complex was sufficient for docking of Syb2 vesicles, even a partial unclustering of SNAREs may offer more binding sites and thereby explain the observed results (Fig. 4).
Figure 4.
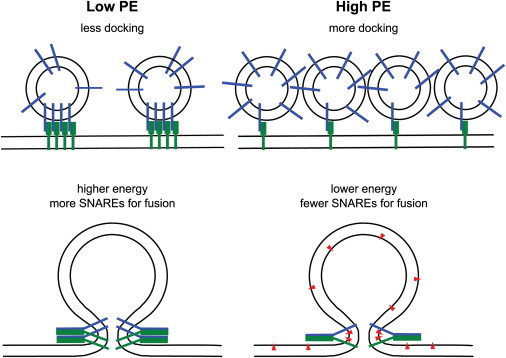
Models explaining the effects of PE on docking and fusion. In the absence of PE, we hypothesize that SNAREs may be more clustered in cholesterol-containing target and vesicle membranes leading to fewer docking sites. The membrane-bending energy in the absence of PE is higher requiring more SNAREs for fusion. In the presence of PE, SNARE clusters may be (partially) dispersed leading to more docking sites. The inverted cone shape of PE (red triangles) facilitates curved intermediate membrane structures (including hemifusion stalk, not shown) and thus lowers the energy of fusion intermediates requiring fewer SNAREs for fusion.
A similar scenario might explain the approximately threefold decreased fusion efficiency at the higher PE concentrations. If a certain minimal number of SNARE complexes is required for the membrane merger to proceed at a fast rate (see below), we would expect that even a mild dispersing effect of PE on the clusters could leave fewer sites with enough acceptor SNARE complexes or Syb2 present for fast fusion to occur. However, once fusion occurs at a site with a sufficient number of SNARE complexes, it would be expected to be still as fast as in the absence of PE, i.e., to still exhibit an ∼20-ms fusion delay, which is exactly what we have observed.
The most significant change was that the number of SNARE complexes that generate a productive fusion site decreased with increasing PE concentration in the membranes. In asymmetric membranes, we observed that the number of SNARE complexes per fusion site decreased from 7 ± 2 to 3 ± 2 complexes upon addition of 30% PE (Fig. 4). A similar effect was observed when PE was included symmetrically in both membranes, but in this case 10% PE was sufficient to decrease the number to 3 ± 1 SNARE complexes per fusion site. The finding that lower numbers of SNARE complexes are sufficient to fuse PE-containing membranes likely reflects the need for a lower activation energy to fuse membranes with PE compared to membranes without PE, as will be discussed in the next section.
Role of PE on fusion intermediate states
It is well known that, due to its cone-shaped average structure, DOPE induces negative spontaneous curvature in the membranes (37). It is also generally accepted that fusion proceeds through some sort of stalk intermediate before the first narrow fusion pore opens (38,39). Both the lipid stalk and the initial fusion pore require bending of one or both leaflets of the bilayer and therefore are energetically unfavorable. Lipid stalks have negative overall curvature and therefore are energetically less costly to form in the presence of a negative curvature-promoting lipid like DOPE. The opposite is true for transforming the lipid stalk to a narrow initial fusion pore, which, if purely lipidic, has positive overall curvature. Even though opposite curvatures dominate different fusion intermediates, the energy penalty of the lipid stalk is thought to be greater than that of the initial fusion pore. Therefore, the energy penalty of the stalk dominates and DOPE should promote formation of a stalk intermediate and, to a lesser degree, should also promote overall fusion (Fig. 4). Lipid stalks are topologically equivalent to hemifusion intermediates and, therefore, should be experimentally detectable by lipid mixing before contents mixing or lipid mixing of the proximal membrane leaflets before lipid mixing of the distal leaflets.
SNARE-mediated hemifusion has been observed in bulk liposome, in single vesicle, and in single-vesicle-to-planar-membrane-fusion experiments in vitro (23,26,40), as well as in vacuole fusion and flipped SNARE assays performed with natural membranes (41,42). For example, Liu et al. (23) took a similar approach to ours and studied the role of DOPE on SNARE-mediated membrane fusion in a single vesicle planar membrane format. In those studies Syb2 vesicles fused either directly without a visible fusion intermediate in ∼20–30 ms, or in ∼1 s through a hemifused intermediate state, which itself took ∼60–80 ms to develop. Almost no hemifusion intermediates were observed in the absence of DOPE, but hemifusion intermediates developed in ∼40% of all events in the presence of 40–60% DOPE. Why vesicles that went through the observable hemifusion pathway fused 30–50 times slower than those that underwent complete spontaneous fusion was unexplained in that study.
The hemifused intermediate states were detected by Liu et al. (23) by the occurrence of two successive dequenching peaks in the time-resolved single vesicle fusion traces. In none of our traces did we detect stepwise fusion. Therefore, the hemifusion intermediate must occur in our assay at a rate that is too fast to detect in our experimental system, i.e., within the first 4 ms of fusion. We do not know why the results of Liu et al. are different from ours, but as mentioned before, there are several significant differences in experimental protocol that may explain the differences:
-
1.
The planar supported bilayers were formed by different methods in the two studies. We used a combined Langmuir-Blodgett/vesicle fusion technique to reconstitute our acceptor SNARE complexes into supported bilayers, whereas Liu et al. (23) used the direct vesicle fusion method. The latter produces bilayers with topologically randomly distributed SNAREs across the membrane that are laterally immobile. Therefore, the membrane preparations of Liu et al. are likely more heterogeneous and more static than ours.
-
2.
Liu et al. used reconstituted coexpressed syntaxin 1a and SNAP25 of unknown stoichiometry at a protein/lipid ratio of 1:20,000, whereas we reconstituted the stabilized 1:1 acceptor SNARE complex at a ratio of 1:3000.
-
3.
The fusing membranes of Liu et al. contained no cholesterol, but 15% DOPS and in some cases high concentrations of DOPE (up to 60%). In contrast, our vesicles and planar bilayers contained 20% Chol and in many cases 5% DOPS and up to 30% DOPE. Furthermore, many of our target membranes were asymmetric in terms of lipid composition whereas those of Liu et al. were symmetric.
A currently hotly debated issue regards the number of SNARE complexes that contribute to the formation of a single fusion pore. One recent bulk liposome fusion study reported that one SNARE complex may be sufficient to drive membrane fusion (29). On the other hand, our previous studies indicated the requirement for 6–9 complexes to drive this reaction with millisecond kinetics on planar target membranes (28). (A number of 5–10 contributing SNARE complexes was later confirmed in a similar assay, but with lower time resolution (43).) In this article, we have shown that the number of SNARE complexes supporting fast membrane fusion can be reduced to as few as three complexes when the lipid composition is more favorable for fusion. If the formation of each SNARE complex releases 18–35 kT in folding energy (44–46), the energy from three complexes would be sufficient to overcome the energy barrier of forming a fusion stalk, which has been estimated to be of ∼45–100 kT (47–50).
Why then is it possible to fuse membranes with a single SNARE complex (29)? We think that this may only be possible for slow fusion events that are part of the wide distribution of fusion times observed in the bulk fusion assay. In these liposome assays, local membrane fluctuations may occasionally happen on a slow timescale that are sufficient to fuse two liposomes that are tethered together with a partially zippered single SNARE complex. If one only waits long enough, even untethered highly curved pure lipid vesicles will eventually fuse and form larger vesicles with less curvature strain. This explanation of fast fusion times requiring more SNARE complexes than slow fusion times is actually consistent with our observations. We always observe long tails of fusion times that extend even beyond our typical recording times. These tails are fit with the second slower components in our model and the slow components (although usually not numerous) always require fewer SNARE complexes per fusion site than the fast components in our fits (see also (28)).
Conclusions
In this work, we have set up a highly reliable fast fusion assay that is under tight control of the involved lipids and proteins. Very interestingly, a small number of SNAREs is sufficient to promote fusion under close to physiological lipid conditions. However, fusion is a relatively rare event under these conditions and even rarer when the PS concentration is further increased toward physiological. This opens up the interesting prospect of finally gaining control of fast neuronal SNARE-mediated fusion by the action of synaptotagmin and calcium.
If fast fusion is indeed restricted by electrostatic or other repulsion of the two fusing membranes, synaptotagmin may overcome this critical energy barrier, and coreconstitution with synaptotagmin may allow us to eventually disentangle the many conflicting reports about the mechanism of action of this important regulator of neuronal exocytosis.
Acknowledgments
We thank the members of the Tamm laboratory and Dr. Reinhard Jahn for many helpful discussions.
This work was supported by grant No. P01 GM72694 from the National Institutes of Health, Bethesda, MD.
Supporting Material
References
- 1.Sutton R.B., Fasshauer D., Brunger A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 2.Fasshauer D. Structural insights into the SNARE mechanism. Biochim. Biophys. Acta. 2003;1641:87–97. doi: 10.1016/s0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R., Scheller R.H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 4.Rizo J., Rosenmund C. Synaptic vesicle fusion. Nat. Struct. Mol. Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pobbati A.V., Stein A., Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 6.Weber T., Zemelman B.V., Rothman J.E. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 7.Schuette C.G., Hatsuzawa K., Jahn R. Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc. Natl. Acad. Sci. USA. 2004;101:2858–2863. doi: 10.1073/pnas.0400044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H., Coleman J., Tareste D. Protein determinants of SNARE-mediated lipid mixing. Biophys. J. 2010;99:553–560. doi: 10.1016/j.bpj.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salaün C., James D.J., Chamberlain L.H. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang T., Bruns D., Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieber J.J., Willig K.I., Lang T. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 12.Murray D.H., Tamm L.K. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z., Hui E., Jackson M.B. Phosphatidylserine regulation of Ca2+-triggered exocytosis and fusion pores in PC12 cells. Mol. Biol. Cell. 2009;20:5086–5095. doi: 10.1091/mbc.E09-08-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Paolo G., Moskowitz H.S., De Camilli P. Impaired PtdIns4,5 P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 15.Hay J.C., Fisette P.L., Martin T.F. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 16.Aikawa Y., Martin T.F. ARF6 regulates a plasma membrane pool of phosphatidylinositol4,5 bisphosphate required for regulated exocytosis. J. Cell Biol. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen H.L., Hoy M., Gromada J. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic β-cells. Proc. Natl. Acad. Sci. USA. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong L.W., Di Paolo G., Toomre D. Phosphatidylinositol phosphate kinase type I γ regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. USA. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milosevic I., Sørensen J.B., Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam A.D., Tryoen-Toth P., Stuenkel E.L. SNARE-catalyzed fusion events are regulated by Syntaxin1A-lipid interactions. Mol. Biol. Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fix M., Melia T.J., Simon S.M. Imaging single membrane fusion events mediated by SNARE proteins. Proc. Natl. Acad. Sci. USA. 2004;101:7311–7316. doi: 10.1073/pnas.0401779101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T., Tucker W.C., Weisshaar J.C. SNARE-driven, 25-millisecond vesicle fusion in vitro. Biophys. J. 2005;89:2458–2472. doi: 10.1529/biophysj.105.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T., Wang T., Weisshaar J.C. Productive hemifusion intermediates in fast vesicle fusion driven by neuronal SNAREs. Biophys. J. 2008;94:1303–1314. doi: 10.1529/biophysj.107.107896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowen M.E., Weninger K., Chu S. Single molecule observation of liposome-bilayer fusion thermally induced by soluble N-ethyl maleimide sensitive-factor attachment protein receptors (SNAREs) Biophys. J. 2004;87:3569–3584. doi: 10.1529/biophysj.104.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H.K., Yang Y., Yoon T.Y. Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science. 2010;328:760–763. doi: 10.1126/science.1187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon T.Y., Okumus B., Ha T. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA. 2006;103:19731–19736. doi: 10.1073/pnas.0606032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner M.L., Tamm L.K. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domanska M.K., Kiessling V., Tamm L.K. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J. Biol. Chem. 2009;284:32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bogaart G., Holt M.G., Jahn R. One SNARE complex is sufficient for membrane fusion. Nat. Struct. Mol. Biol. 2010;17:358–364. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasshauer D., Margittai M. A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J. Biol. Chem. 2004;279:7613–7621. doi: 10.1074/jbc.M312064200. [DOI] [PubMed] [Google Scholar]
- 31.Fasshauer D., Antonin W., Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]
- 32.Stein A., Radhakrishnan A., Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat. Struct. Mol. Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 33.Kalb E., Frey S., Tamm L.K. Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim. Biophys. Acta. 1992;1103:307–316. doi: 10.1016/0005-2736(92)90101-q. [DOI] [PubMed] [Google Scholar]
- 34.Wagner M.L., Tamm L.K. Tethered polymer-supported planar lipid bilayers for reconstitution of integral membrane proteins: silane-polyethyleneglycol-lipid as a cushion and covalent linker. Biophys. J. 2000;79:1400–1414. doi: 10.1016/S0006-3495(00)76392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon T.Y., Lu X., Shin Y.K. Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 2008;15:707–713. doi: 10.1038/nsmb.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand R.P., Fuller N.L., Parsegian V.A. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 1990;29:76–87. doi: 10.1021/bi00453a010. [DOI] [PubMed] [Google Scholar]
- 38.Chernomordik L.V., Kozlov M.M. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Chernomordik L.V., Kozlov M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y., Zhang F., Shin Y.K. Hemifusion in SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- 41.Giraudo C.G., Hu C., Rothman J.E. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J. Cell Biol. 2005;170:249–260. doi: 10.1083/jcb.200501093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese C., Mayer A. Transition from hemifusion to pore opening is rate limiting for vacuole membrane fusion. J. Cell Biol. 2005;171:981–990. doi: 10.1083/jcb.200510018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karatekin E., Di Giovanni J., Rothman J.E. A fast, single-vesicle fusion assay mimics physiological SNARE requirements. Proc. Natl. Acad. Sci. USA. 2010;107:3517–3521. doi: 10.1073/pnas.0914723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li F., Pincet F., Tareste D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 2007;14:890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 45.Liu W., Montana V., Mohideen U. Single molecule measurements of interaction free energies between the proteins within binary and ternary SNARE complexes. J. Nanoneurosci. 2009;1:120–129. doi: 10.1166/jns.2009.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiederhold K., Fasshauer D. Is assembly of the SNARE complex enough to fuel membrane fusion? J. Biol. Chem. 2009;284:13143–13152. doi: 10.1074/jbc.M900703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Markin V.S., Albanesi J.P. Membrane fusion: stalk model revisited. Biophys. J. 2002;82:693–712. doi: 10.1016/S0006-3495(02)75432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozlovsky Y., Kozlov M.M. Stalk model of membrane fusion: solution of energy crisis. Biophys. J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen F.S., Melikyan G.B. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J. Membr. Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- 50.Malinin V.S., Lentz B.R. Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys. J. 2004;86:2951–2964. doi: 10.1016/S0006-3495(04)74346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


