Abstract
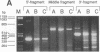
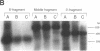
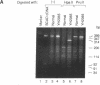
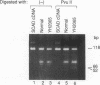
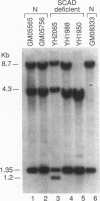
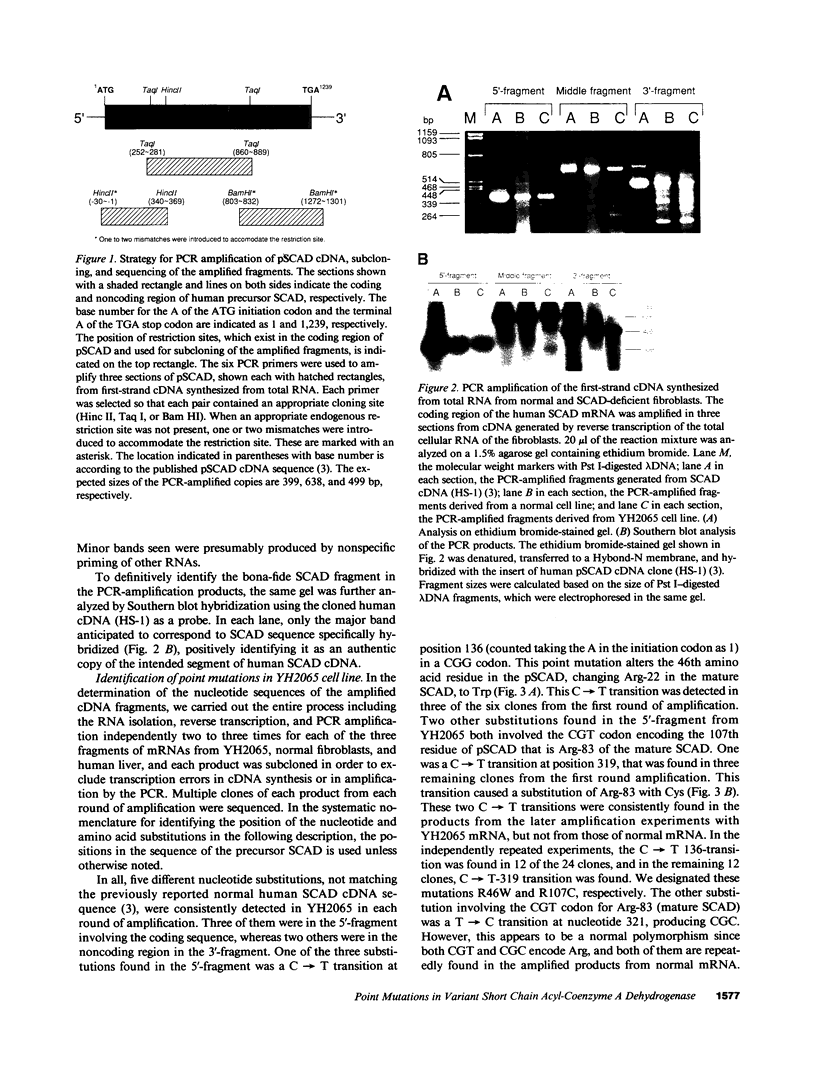
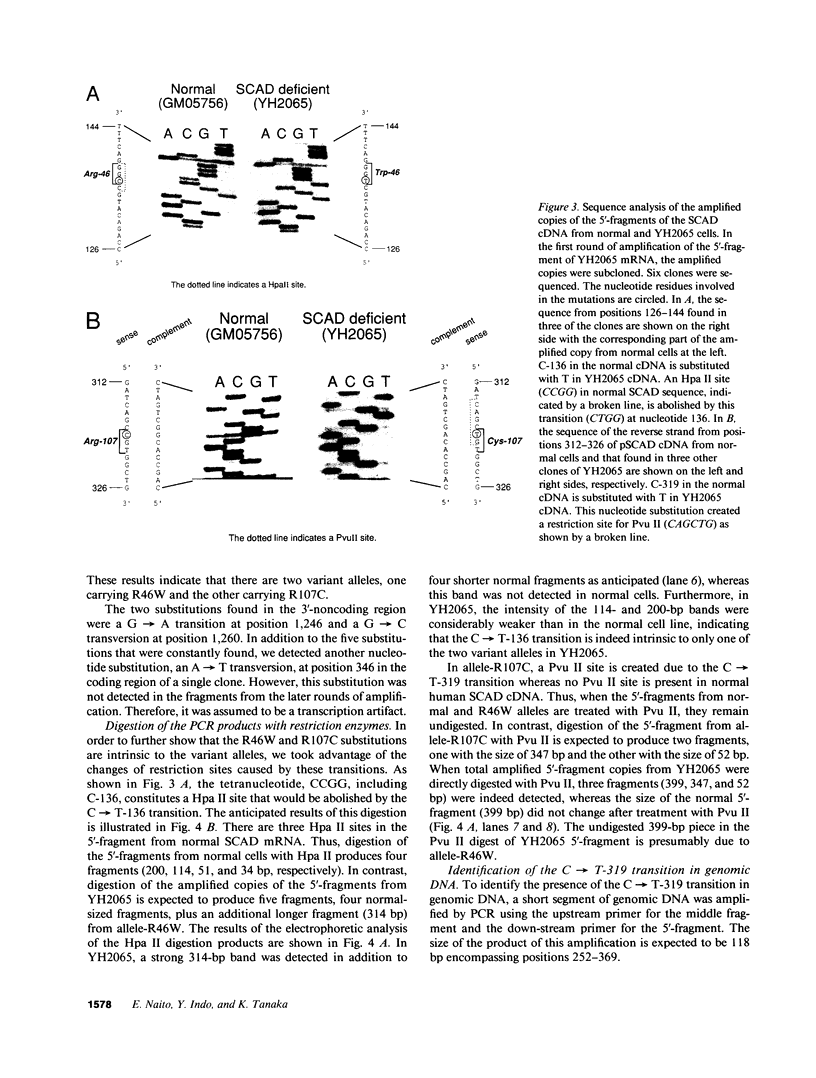
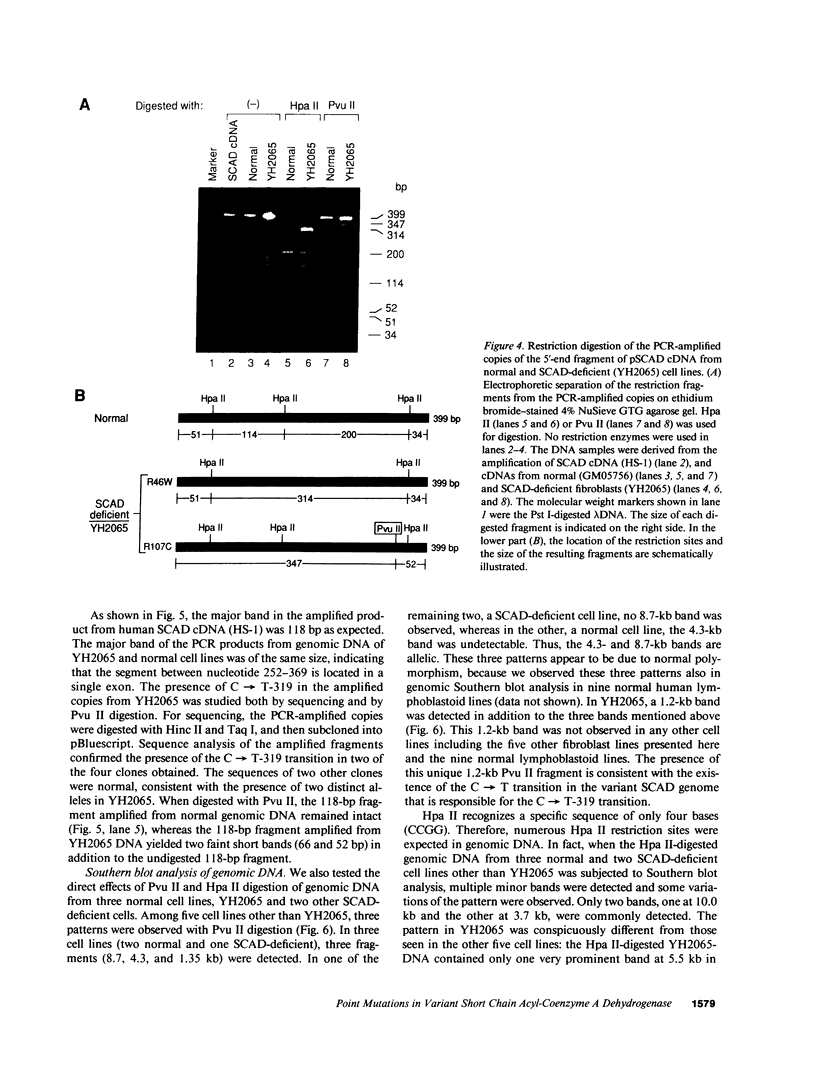
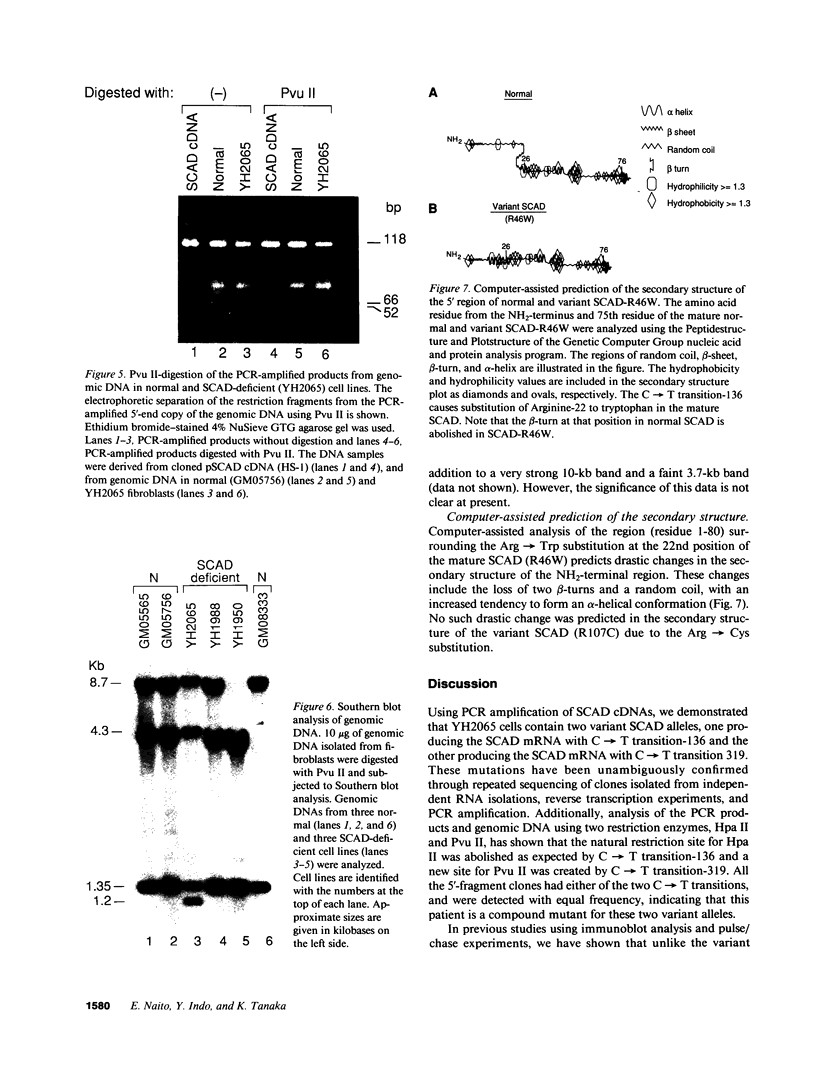
Two distinct mutant alleles of the precursor (p) short chain acyl-CoA dehydrogenase (SCAD) gene were identified in a SCAD-deficient patient (YH2065) using the polymerase chain reaction to amplify cDNA synthesized from total RNA from her fibroblasts. Cells from this patient had previously been shown to synthesize a labile variant SCAD in contrast to the normal stability of variant SCADs in two other SCAD-deficient cell lines (Naito, E., Y. Indo, and K. Tanaka. 1989. J. Clin. Invest. 84:1671-1674). In the present study, both mutant alleles of YH2065 were found to contain a C----T transition, one at position 136 and the other at position 319 of the coding region of pSCAD cDNA. Clones of cDNA amplified from this region showed only one of the C----T transitions, indicating that each mutation was derived from different pSCAD alleles. Each of these mutations altered a known restriction endonuclease site, and restriction analysis of additional cDNA clones from amplified mutant cDNA and Southern blotting of mutant genomic DNA confirmed the presence of two unique mutant alleles in YH2065, indicating YH2065 is a compound heterozygote. These C----T transitions result in the substitution of Arg-22 and Arg-83 of the mature SCAD with Trp and Cys, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amendt B. A., Greene C., Sweetman L., Cloherty J., Shih V., Moon A., Teel L., Rhead W. J. Short-chain acyl-coenzyme A dehydrogenase deficiency. Clinical and biochemical studies in two patients. J Clin Invest. 1987 May;79(5):1303–1309. doi: 10.1172/JCI112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Coates P. M., Hale D. E., Finocchiaro G., Tanaka K., Winter S. C. Genetic deficiency of short-chain acyl-coenzyme A dehydrogenase in cultured fibroblasts from a patient with muscle carnitine deficiency and severe skeletal muscle weakness. J Clin Invest. 1988 Jan;81(1):171–175. doi: 10.1172/JCI113290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finocchiaro G., Ito M., Tanaka K. Purification and properties of short chain acyl-CoA, medium chain acyl-CoA, and isovaleryl-CoA dehydrogenases from human liver. J Biol Chem. 1987 Jun 15;262(17):7982–7989. [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Shuman M. A., Lawn R. M. Identification of a missense mutation in the factor VIII gene of a mild hemophiliac. Science. 1986 Jun 13;232(4756):1415–1416. doi: 10.1126/science.3012775. [DOI] [PubMed] [Google Scholar]
- Hata A., Setoyama C., Shimada K., Takeda E., Kuroda Y., Akaboshi I., Matsuda I. Ornithine transcarbamylase deficiency resulting from a C-to-T substitution in exon 5 of the ornithine transcarbamylase gene. Am J Hum Genet. 1989 Jul;45(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Keese S. M., Fenton W. A., Tanaka K. Biosynthesis of four rat liver mitochondrial acyl-CoA dehydrogenases: in vitro synthesis, import into mitochondria, and processing of their precursors in a cell-free system and in cultured cells. Arch Biochem Biophys. 1987 Feb 1;252(2):662–674. doi: 10.1016/0003-9861(87)90072-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Okamura-Ikeda K., Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. J Biol Chem. 1985 Jan 25;260(2):1311–1325. [PubMed] [Google Scholar]
- Kelly D. P., Kim J. J., Billadello J. J., Hainline B. E., Chu T. W., Strauss A. W. Nucleotide sequence of medium-chain acyl-CoA dehydrogenase mRNA and its expression in enzyme-deficient human tissue. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4068–4072. doi: 10.1073/pnas.84.12.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A., Spence J. E., O'Brien W. E., Nussbaum R. L. Characterization of point mutations in the same arginine codon in three unrelated patients with ornithine transcarbamylase deficiency. J Clin Invest. 1988 Oct;82(4):1353–1358. doi: 10.1172/JCI113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara Y., Indo Y., Naito E., Ozasa H., Glassberg R., Vockley J., Ikeda Y., Kraus J., Tanaka K. Molecular cloning and nucleotide sequence of cDNAs encoding the precursors of rat long chain acyl-coenzyme A, short chain acyl-coenzyme A, and isovaleryl-coenzyme A dehydrogenases. Sequence homology of four enzymes of the acyl-CoA dehydrogenase family. J Biol Chem. 1989 Sep 25;264(27):16321–16331. [PubMed] [Google Scholar]
- Matsubara Y., Kraus J. P., Ozasa H., Glassberg R., Finocchiaro G., Ikeda Y., Mole J., Rosenberg L. E., Tanaka K. Molecular cloning and nucleotide sequence of cDNA encoding the entire precursor of rat liver medium chain acyl coenzyme A dehydrogenase. J Biol Chem. 1987 Jul 25;262(21):10104–10108. [PubMed] [Google Scholar]
- McBride L. J., McCollum C., Davidson S., Efcavitch J. W., Andrus A., Lombardi S. J. A new, reliable cartridge for the rapid purification of synthetic DNA. Biotechniques. 1988 Apr;6(4):362–367. [PubMed] [Google Scholar]
- Naito E., Indo Y., Tanaka K. Short chain acyl-coenzyme A dehydrogenase (SCAD) deficiency. Immunochemical demonstration of molecular heterogeneity due to variant SCAD with differing stability. J Clin Invest. 1989 Nov;84(5):1671–1674. doi: 10.1172/JCI114346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E., Ozasa H., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of complementary DNAs encoding human short chain acyl-coenzyme A dehydrogenase and the study of the molecular basis of human short chain acyl-coenzyme A dehydrogenase deficiency. J Clin Invest. 1989 May;83(5):1605–1613. doi: 10.1172/JCI114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Modrow S., Motz M., Jameson B. A., Hermann G., Förtsch B. An integrated family of amino acid sequence analysis programs. Comput Appl Biosci. 1988 Mar;4(1):187–191. doi: 10.1093/bioinformatics/4.1.187. [DOI] [PubMed] [Google Scholar]