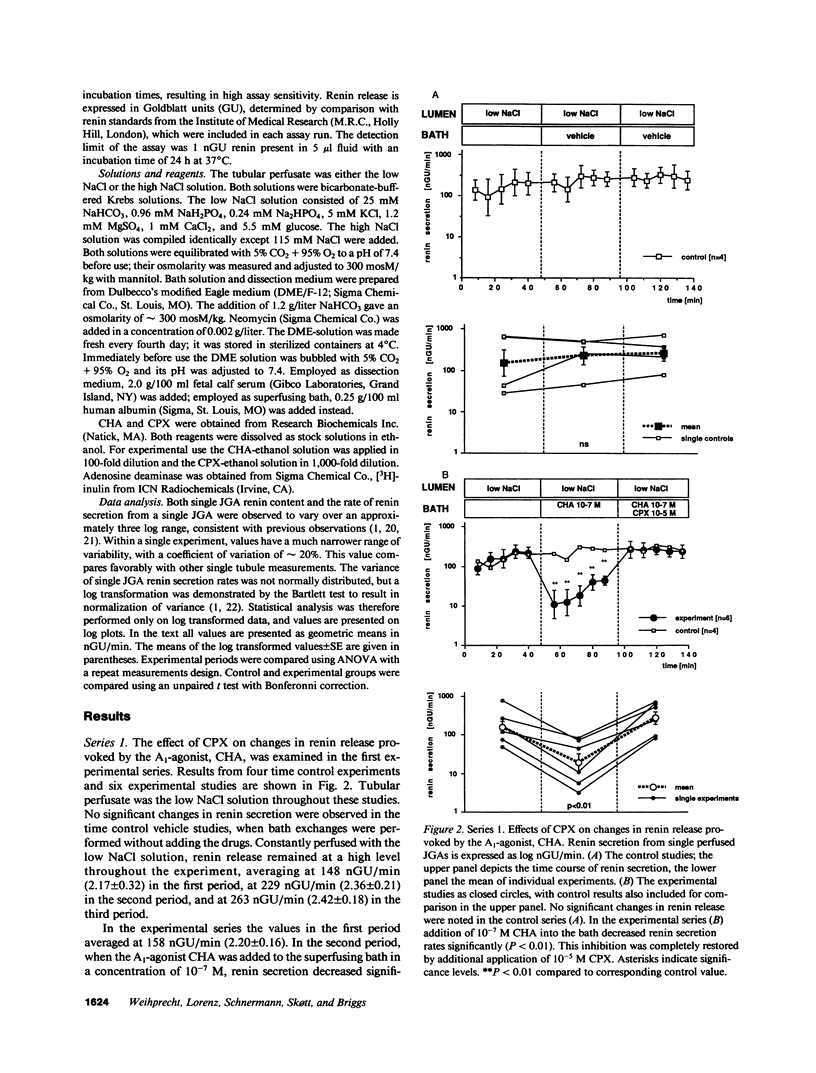
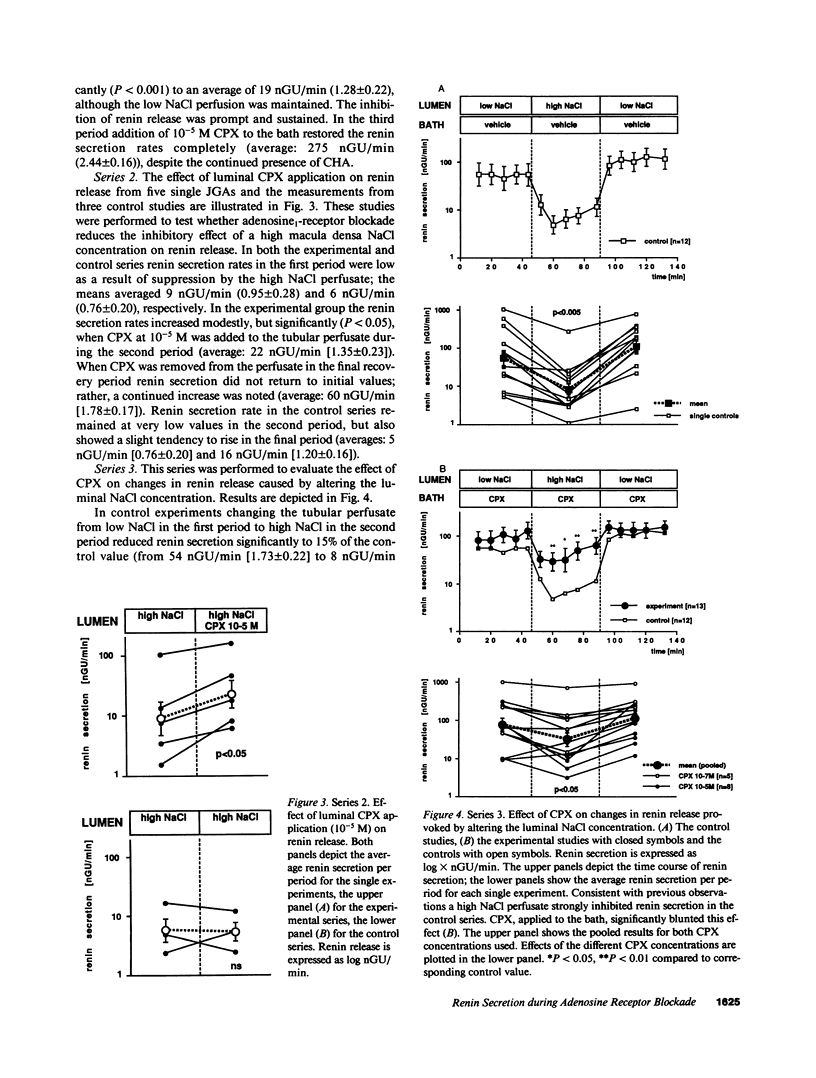
Abstract
Adenosine has been proposed to act within the juxtaglomerular apparatus (JGA) as a mediator of the inhibition of renin secretion produced by a high NaCl concentration at the macula densa. To test this hypothesis, we studied the effects of the adenosine1 (A1)-receptor blocker 8-cyclopentyl-1,3-dipropylxanthine (CPX) on renin release from single isolated rabbit JGAs with macula densa perfused. The A1-receptor agonist, N6-cyclohexyladenosine (CHA), applied in the bathing solution at 10(-7) M, was found to inhibit renin secretion, an effect that was completely blocked by adding CPX (10(-5) M) to the bath. Applied to the lumen, 10(-5) M CPX produced a modest stimulation of renin secretion rates suppressed by a high NaCl concentration at the macula densa (P less than 0.05). The effect of changing luminal NaCl concentration on renin secretion rate was examined in the presence of CPX (10(-7) and 10(-5) M) in the bathing solution and in vehicle control experiments. The control response to increasing luminal NaCl concentration was a marked suppression of renin secretion, that was maintained as long as luminal NaCl concentration was high and was promptly reversible when concentration was lowered. CPX did not alter renin release when luminal NaCl was low, but diminished the reduction caused by high NaCl (P less than 0.01). It is concluded that A1-receptors are located within the JGA, and that A1-receptor activation inhibits renin release. A high NaCl concentration at the macula densa appears to influence A1-receptor activation, but a low NaCl concentration does not. The findings support participation of adenosine in macula densa control of renin secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend L. J., Haramati A., Thompson C. I., Spielman W. S. Adenosine-induced decrease in renin release: dissociation from hemodynamic effects. Am J Physiol. 1984 Sep;247(3 Pt 2):F447–F452. doi: 10.1152/ajprenal.1984.247.3.F447. [DOI] [PubMed] [Google Scholar]
- Briggs J. P., Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol. 1987;49:251–273. doi: 10.1146/annurev.ph.49.030187.001343. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Churchill P. C., Churchill M. C. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther. 1985 Mar;232(3):589–594. [PubMed] [Google Scholar]
- Coulson R., Scheinman S. J. Xanthine effects on renal proximal tubular function and cyclic AMP metabolism. J Pharmacol Exp Ther. 1989 Feb;248(2):589–595. [PubMed] [Google Scholar]
- Freissmuth M., Hausleithner V., Tuisl E., Nanoff C., Schütz W. Glomeruli and microvessels of the rabbit kidney contain both A1- and A2-adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Apr;335(4):438–444. doi: 10.1007/BF00165560. [DOI] [PubMed] [Google Scholar]
- Gillies A., Morgan T. Renin content of individual juxtaglomerular apparatuses and the effect of diet, changes in nephron flow rate and in vitro acidification on the renin content. Pflugers Arch. 1978 Jun 21;375(1):105–110. doi: 10.1007/BF00584154. [DOI] [PubMed] [Google Scholar]
- Holz F. G., Steinhausen M. Renovascular effects of adenosine receptor agonists. Ren Physiol. 1987;10(5):272–282. doi: 10.1159/000173135. [DOI] [PubMed] [Google Scholar]
- Itoh S., Carretero O. A., Murray R. D. Possible role of adenosine in the macula densa mechanism of renin release in rabbits. J Clin Invest. 1985 Oct;76(4):1412–1417. doi: 10.1172/JCI112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton T. K., Campbell W. B. The pharmacologic alteration of renin release. Pharmacol Rev. 1980 Jun;32(2):81–227. [PubMed] [Google Scholar]
- Knabb R. M., Ely S. W., Bacchus A. N., Rubio R., Berne R. M. Consistent parallel relationships among myocardial oxygen consumption, coronary blood flow, and pericardial infusate adenosine concentration with various interventions and beta-blockade in the dog. Circ Res. 1983 Jul;53(1):33–41. doi: 10.1161/01.res.53.1.33. [DOI] [PubMed] [Google Scholar]
- Knabb R. M., Gidday J. M., Ely S. W., Rubio R., Berne R. M. Effects of dipyridamole on myocardial adenosine and active hyperemia. Am J Physiol. 1984 Nov;247(5 Pt 2):H804–H810. doi: 10.1152/ajpheart.1984.247.5.H804. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Bauer C. Role of cGMP as second messenger of adenosine in the inhibition of renin release. Kidney Int. 1988 Apr;33(4):798–803. doi: 10.1038/ki.1988.70. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Reddington M. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) inhibition of [3H]N-ethylcarboxamidoadenosine (NECA) binding allows the visualization of putative non-A1 adenosine receptors. Brain Res. 1986 Mar 19;368(2):394–398. doi: 10.1016/0006-8993(86)90589-5. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkegård S., Poulsen K. Ultramicroassay for plasma renin concentration in the rat using the antibody-trapping technique. Anal Biochem. 1976 Sep;75(1):250–259. doi: 10.1016/0003-2697(76)90076-2. [DOI] [PubMed] [Google Scholar]
- Martinson E. A., Johnson R. A., Wells J. N. Potent adenosine receptor antagonists that are selective for the A1 receptor subtype. Mol Pharmacol. 1987 Mar;31(3):247–252. [PubMed] [Google Scholar]
- Merrill G. F., Downey H. F., Jones C. E. Adenosine deaminase attenuates canine coronary vasodilation during systemic hypoxia. Am J Physiol. 1986 Apr;250(4 Pt 2):H579–H583. doi: 10.1152/ajpheart.1986.250.4.H579. [DOI] [PubMed] [Google Scholar]
- Murray R. D., Churchill P. C. Concentration dependency of the renal vascular and renin secretory responses to adenosine receptor agonists. J Pharmacol Exp Ther. 1985 Jan;232(1):189–193. [PubMed] [Google Scholar]
- Osswald H., Nabakowski G., Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12(1-2):263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Osswald H. Renal effects of adenosine and their inhibition by theophylline in dogs. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(1):79–86. doi: 10.1007/BF00501815. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Fastbom J., Wiederhold K. H., Probst A. Visualization of adenosine A1 receptors in the human and the guinea-pig kidney. Eur J Pharmacol. 1987 Jun 19;138(2):273–276. doi: 10.1016/0014-2999(87)90443-2. [DOI] [PubMed] [Google Scholar]
- Schnermann J. Effect of adenosine analogues on tubuloglomerular feedback responses. Am J Physiol. 1988 Jul;255(1 Pt 2):F33–F42. doi: 10.1152/ajprenal.1988.255.1.F33. [DOI] [PubMed] [Google Scholar]
- Skøtt O., Baumbach L. Effects of adenosine on renin release from isolated rat glomeruli and kidney slices. Pflugers Arch. 1985 Jul;404(3):232–237. doi: 10.1007/BF00581244. [DOI] [PubMed] [Google Scholar]
- Skøtt O., Briggs J. P. A method for superfusion of the isolated perfused tubule. Kidney Int. 1988 May;33(5):1009–1012. doi: 10.1038/ki.1988.101. [DOI] [PubMed] [Google Scholar]
- Skøtt O., Briggs J. P. Direct demonstration of macula densa-mediated renin secretion. Science. 1987 Sep 25;237(4822):1618–1620. doi: 10.1126/science.3306925. [DOI] [PubMed] [Google Scholar]
- Spielman W. S., Thompson C. I. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol. 1982 May;242(5):F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Tagawa H., Vander A. J. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ Res. 1970 Mar;26(3):327–338. doi: 10.1161/01.res.26.3.327. [DOI] [PubMed] [Google Scholar]
- Thompson L. P., Gorman M. W., Sparks H. V. Aminophylline and interstitial adenosine during sustained exercise hyperemia. Am J Physiol. 1986 Dec;251(6 Pt 2):H1232–H1243. doi: 10.1152/ajpheart.1986.251.6.H1232. [DOI] [PubMed] [Google Scholar]
- Thurau K. W., Dahlheim H., Grüner A., Mason J., Granger P. Activation of renin in the single juxtaglomerular apparatus by sodium chloride in the tubular fluid at the macula densa. Circ Res. 1972 Sep;31(9 Suppl):182–186. [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986 Oct;6(5):522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]