Abstract
The effect of FK 506 on regeneration of the liver was studied in rats after a two-thirds partial hepatectomy after 60 min of ischemia of the unresected liver. The animals were divided into three distinct groups of 10 rats each. Group 1 (controls) received 0.5 ml saline solution intravenously 30 min after the induction of ischemia. Groups 2 and 3 were injected with FK 506 (0.3 mg/kg) intravenously 30 min after and 24 min before the induction of hepatic ischemia, respectively. The hepatic content of ATP and serum levels of ALT and lactate dehydrogenase were determined on each animal. In addition, the histological appearance and mitotic activity of the remnant liver was determined at regular 24-hr intervals after hepatic ischemia. All 10 control animals died within 72 hr. Treatment with FK 506 resulted in improved survival in groups 2 and 3 (30% and 80%, respectively). The improved survival seen in the FK 506–treated animals was reflected by a restoration of hepatic ATP content, a reduction in the serum levels of ALT and lactate dehydrogenase, an amelioration of hepatic necrosis and neutrophilic infiltration and an increase in the mitotic activity of the liver. These results suggest that FK 506 ameliorates the hepatic injury associated with ischemia/reperfusion and has a potent stimulatory effect on liver cell regeneration that may make it valuable as a hepatoprotective agent when administered to organ donors before graft harvesting.
In recent years, orthotopic liver transplantation (OLT) has been accepted as a reasonable therapy for patients with end-stage liver disease (1). However, the allograft liver is subject to a variety of insults as a result of ischemia experienced during the organ harvest, reperfusion at the time of organ engraftment, and eventually as part of the recipient’s immunological response to the allograft after transplantation. The ability of the liver to withstand these injuries and then to regenerate and regulate its ultimate size is crucial after clinical liver transplantation.
Recently, it has been reported that FK 506 (FK), in addition to being a powerful immunosuppressive agent, is also hepatotrophic agent (2, 3). To further clarify this issue, this study was performed. Specifically the efficacy of FK in protecting the liver against the injury associated with ischemia/reperfusion in rats subjected to a two-thirds hepatectomy after 60-min of ischemia was examined.
MATERIALS AND METHODS
Surgical Procedure
Adult male Lewis rats weighing between 200 and 300 gm were used in the study. Anesthesia was induced using ether and was maintained with inhalation of metofane. Through a midline abdominal incision a 60-min period of ischemia of the right lateral lobe of the liver was induced by placing noncrushing microvascular clamps around the appropriate branches of the portal vein and hepatic artery using an operative microscope. On releasing the clamps and restoring blood flow to the right lateral lobe, the median and left lateral lobes of the liver were excised according to the method of Higgins and Anderson (4). This technique avoids total occlusion of the portal vein and hepatic artery during experimental hepatic ischemia, leading to splanchnic pooling of blood and death of the animal, which is unrelated to the ischemic injury produced (5, 6). The animals were allowed to recover spontaneously, and subsequent survival was determined at 12-hr intervals for 7 days.
All animals used in these studies received humane care as defined by The Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publications no. 80-23, revised 1978).
Experimental Protocol
All animals were acclimatized to the animal research laboratory for 5 days before being used and were allowed free access to food and water both before and after the operation. The rats so prepared were assigned to one of three different experimental groups (10 rats each). Group 1 animals were injected intravenously with 0.5 ml saline solution and served as the controls. Group 2 animals received FK (0.3 mg/kg) through the inferior vena cava 30 min before hepatic reperfusion. Group 3 animals were injected intravenously with FK (0.3 mg/kg) 24 hr before induction of the experimental hepatic ischemia.
In the second experiment, five to six rats in each of the three groups already described were killed at 24-hr intervals, beginning from time 0 (end of surgical procedure) and ending with 72 hr postoperatively. Immediately before the animals were killed, blood samples were taken from the inferior vena cava for biochemical analysis. At the same time a portion of the remnant liver was removed under freeze-clamping using liquid nitrogen for determination of the ATP content. All specimens were kept at − 70° C until the time of analysis. Another portion of the remnant liver was removed for histological examination and counting the number of mitoses.
Biochemical Analyses
For each rat, serum ALT levels and lactate dehydrogenase (LDH) levels were determined using standard laboratory methods and Sigma diagnostic kits (Sigma Chemical Co., St. Louis, MO). Measurement of the ATP content in the liver was accomplished using the methods of Bergmeyer (7). A weighed portion of the frozen liver was ground in liquid nitrogen in 5 vol precooled 4% perchloric acid (wt/vol), and the mixture was thoroughly homogenized using a precooled polytron homogenizer (Beckman Instruments, Fullerton, CA). The resultant sample was centrifuged at 3,000 rpm for 10 min at 4° C. The supernatant was removed and adjusted to pH 6.5 by the addition of 6 N K2 CO3 and respun at 3,000 rpm for 5 min at 4° C. The hepatic ATP content was quantitated enzymatically using ultraviolet spectrophotometry and a Sigma diagnostic kit.
Histological Examination
Liver specimens were fixed in 10% formalin, dehydrated, embedded in paraffin, cut at 5 μm and stained for histological examination with hematoxylin and eosin. The number of mitoses, as an index of hepatocyte regeneration, was determined/10 high-power field, and the extent of any liver necrosis and hepatocyte damage was estimated semiquantitatively on a 0 to 3 + scale.
Statistical Analysis
Data are reported as mean values ± S.E.M. Mean values were compared using ANOVA and the Tukey multiple comparison procedure. The χ2 test with Yates’ correction was used to test for differences in proportions. A p value of < 0.05 was considered to be statistically significant.
RESULTS
The 7-day survival rate for each of the three groups of animals studied is shown in Figure 1. As may be seen, all group 1 controls died within 72 hr. The survival in the animals receiving 0.3 mg/kg FK 30 min before reperfusion in group 2 was 30%. In contrast, the survival for group 3 animals receiving the same dose of FK (0.3 mg/kg) but administered 24 hr before the induction of hepatic ischemia was 80% (p < 0.01).
Fig. 1.
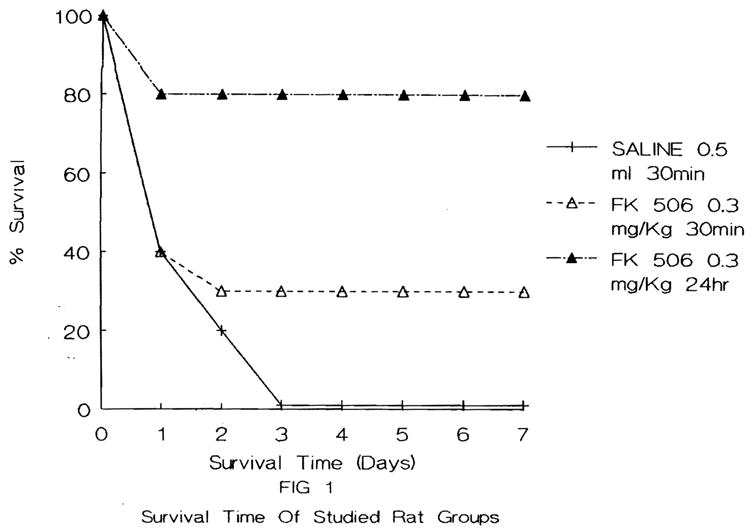
Survival time course of the animals studied.
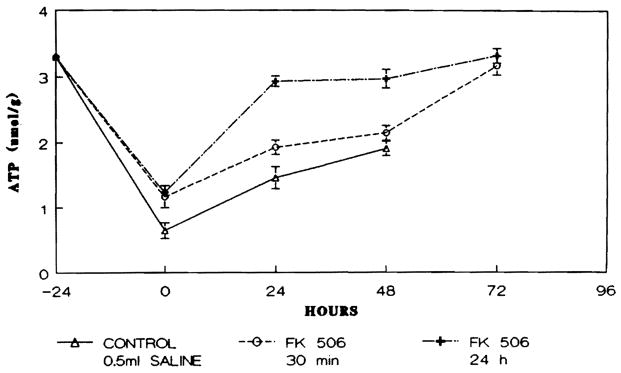
At each time interval studied, the concentration of ATP in the reperfused ischemic remnant of the liver was significantly higher in both groups of rats receiving FK than in the group 1 control animals (p < 0.05). Furthermore, as may be seen in Figure 2, in group 3 the ATP content reached the preischemic value within 24 hr postoperatively and was maintained at that level through 72 hr; in group 2 rats, ATP content reached preischemic levels by 72 hr.
Fig. 2.
Hepatic ATP content (μmol/gm dry liver weight) in the animals studied. Points represent mean level and brackets indicate S.E.M.
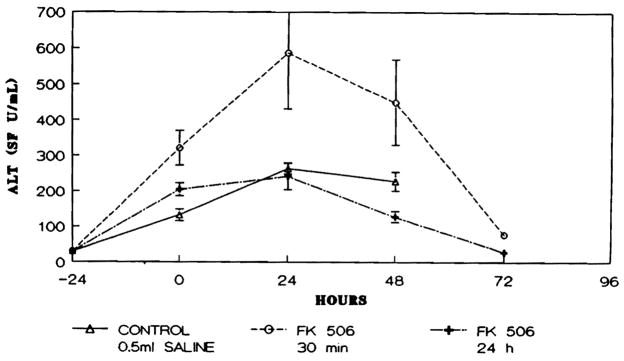
Serum ALT values in all groups of animals studied increased after the ischemia, peaked at 24 hr and declined thereafter. Statistically significant differences were found between groups 2 and 3 at 0 and at 48 hr postoperatively, with lower ALT values seen in the FK-pretreated group 3 animals (p < 0.05) (Fig. 3).
Fig. 3.
Serum ALT (U/ml) levels in animals studied. Points represent mean level and brackets indicate S.E.M.
As illustrated in Figure 4, the values for LDH increased markedly in all groups studied immediately after ischemia (0 hr) but declined thereafter only in the rats that received FK (groups 2 and 3). The LDH level was significantly lower in the group 3 animals that were pretreated with FK 24 hr before induction of ischemia as compared with the controls both at 24 and 48 hr after ischemia (p < 0.05).
Fig. 4.
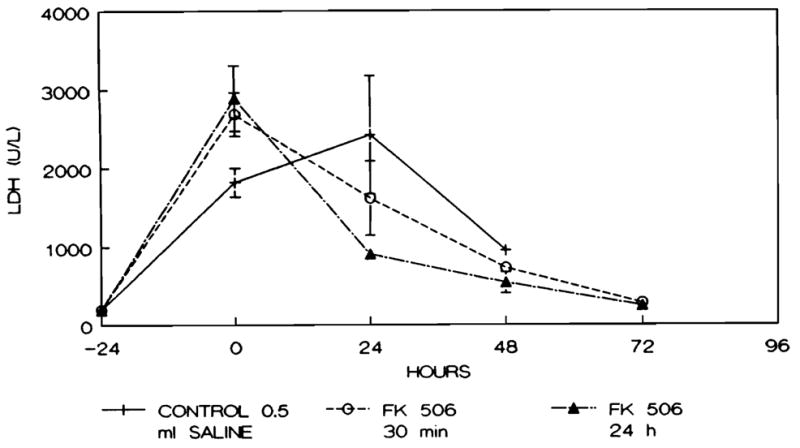
Serum LDH (U/L) levels in animals studied. Points represent mean level and brackets indicate S.E.M.
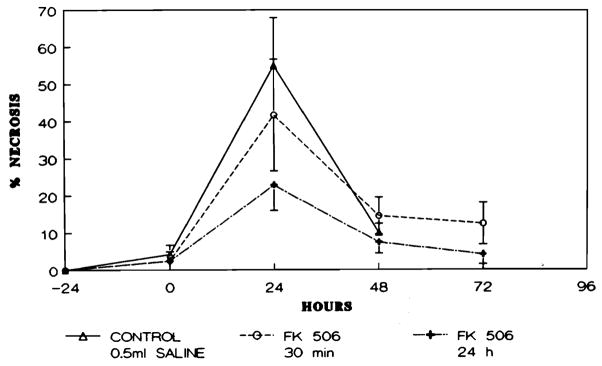
The degree of hepatic necrosis in each group of animals studied is presented in Figure 5. In all groups the greatest degree of necrosis was seen at 24 hr after ischemia when the transaminase levels also reached their maximal value. However, the animals treated with FK (groups 2 and 3) had lower levels than did those in the control group. At 48 hr, the extent of hepatic necrosis was less in the group 3 animals as compared with both the animals in groups 1 and 2.
Fig. 5.
Percentage of the hepatic parenchyma demonstrating necrosis in animals studied across time.
The mitotic activity present within the liver remnant reached a maximal level 48 hr after the ischemic period in the control group and the FK-treated animals. The mitotic activity in the group 3 animals increased further on the third day (72 hr) and achieved a peak value that was double the level seen in the group 2 animals at 72 hr. This difference was statistically significant (p < 0.02) (Fig. 6).
Fig. 6.
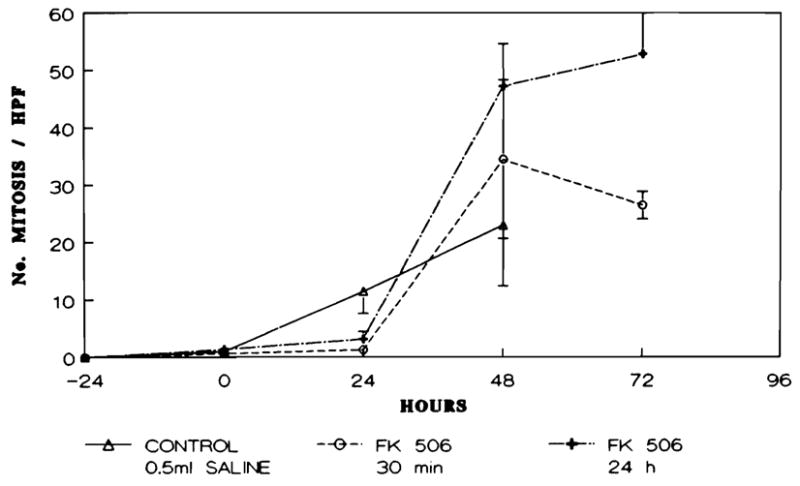
Number of mitoses/10 high-power field in the animals studied across time.
The histological alterations present in representative livers are illustrated in Figure 7. In the control group (group 1), extensive necrosis with neutrophilic infiltration was seen 24 hr after ischemia (Fig. 7A). The residual parenchymal cells were swollen, had cytoplasmic vacuoles and demonstrated nuclear pyknosis. At 24 and 48 hr, increased mitotic activity was evident in the control rat hepatocytes that escaped necrosis (Fig. 7B). In contrast, in rats treated with FK (groups 2 and 3) a lesser degree of hepatic necrosis was seen at 24 hr postoperatively as shown in Figure 7C. Moreover, the specimens obtained showed a higher mitotic activity in the hepatocytes of group 3 at 48 hr compared with groups 1 and 2 (Fig. 7D). The histological changes in the number of viable hepatocytes in the FK-treated groups were quite similar to those in the control group, but these changes occurred to a lesser degree and with minimal or absent neutrophilic infiltration, particularly at day 3 (72 hr).
Fig. 7.
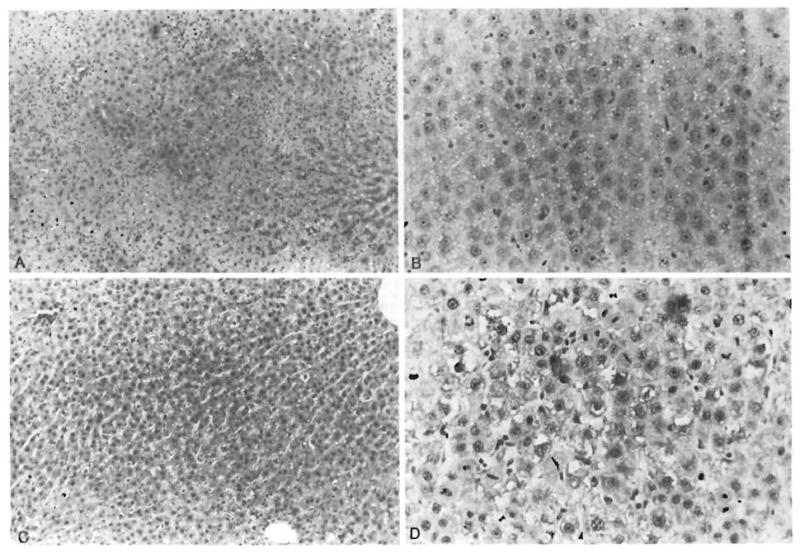
Histological appearance of representative livers obtained from animals studied. (A) Medium-power photomicrograph of hepatic lobule in group 1 animal. Wide swaths of coagulative necrosis are infiltrated by neutrophils. Residual preserved hepatocytes extend into the center of the field (H & E, original magnification × 100). (B) Residual hepatocytes in group 1 animal. Single mitosis is identified in the center of the photograph. Other animals in this group had higher mitotic activity (H & E, original magnification × 250). (C) Lobular field from group 3 animal. Even at this power, sublethal hepatocyte damage in the form of cytoplasmic vacuolization is observed. However, no overt necrosis is seen (H & E, original magnification × 100). (D) Brisk mitotic activity is seen in hepatocytes from this group 3 animal (H & E, original magnification × 250).
DISCUSSION
Recent improvement in patient survival has resulted in the widespread application of OLT as a reasonable therapy for end-stage liver disease (8). Attempts to prevent or to diminish the allograft injury associated with organ harvesting, reperfusion and rejection have already involved the use of several different pharmacological agents. Recently, experimental studies obtained in animals have focused considerable attention on the hepatotrophic activities of cyclosporin A (9-14) and FK (2, 3, 15), two potent immunosuppressive agents currently being used after clinical OLT.
In this study, rats were subjected to a standard two-thirds hepatectomy after 60-min of ischemia of the unresected right hepatic lobe. The regenerative response of the residual ischemic right hepatic lobe was therefore critical for subsequent animal survival.
Pretreatment of the rats with FK at a dose of 0.3 mg/kg, 24 hr before the induction of the experimental ischemia (group 3) had a survival rate of 80% at 24 hr that was maintained through the subsequent 7 days (Fig. 1). The ATP content in the liver of these same animals was restored at 24 hr and was maintained for the subsequent 72 hr (Fig. 2). A similar strong association between postischemic hepatic ATP content and subsequent recovery has been demonstrated in other models of hepatic (16) and renal (17) ischemia. The improved survival of the group 3 animals pretreated with FK 24 hr before the initiation of ischemia was reflected also by lower serum levels of ALT (Fig. 3) and LDH (Fig. 4) and by an amelioration of the liver necrosis (Fig. 5) and neutrophilic infiltration produced as a result of the ischemia as compared with the saline-treated controls. Moreover, the mitotic activity of the residual hepatocytes in the FK-treated animals was greater than that seen in the saline-treated control group (Fig. 7).
A link between the immune system and hepatic regeneration has been reported recently (11, 12, 18–21). It is therefore of some considerable interest to determine the precise relationship between the status of the immune system and subsequent hepatic regeneration. In the present model the immune system is activated by both the two-thirds partial hepatectomy (22–25) and the experimental hepatic ischemia (12). Structural alterations of the surface of hepatocytes caused by the ischemic injury presumably initiate a cytotoxic immune reaction against autologous hepatocytes. It is also possible that the activated immune system has an independent inhibitory effect on hepatocyte cell mitosis and that this action is unmasked by the powerful T cell–specific immunosuppression exhibited by both cyclosporin A and FK. In agreement with this latter hypothesis is the observation of Takahashi, Takeshita and Yokomuro (26) that activated lymphocytes exert an important effect in suppressing hepatocyte division. Additionally, liver regeneration recently has been conceptualized as a phenomenon regulated by T lymphocytes (27, 28). In fact, regeneration is inhibited rather than enhanced by nonspecific immunosuppressive agents such as azathioprine (29) and glucocorticoids (30).
Another possible mechanism for the beneficial effect of FK in terms of the effects herein observed is that by inhibiting interleukin-2 production and binding (31, 32), FK impairs the secretion of liver regulatory factors, either by an as yet unknown immune mechanism (15) or possibly by pathways that are not connected with the immune system at all (2).
It is of some interest to mention that in this work, FK-treated rats showed minimal or absent neutrophilic infiltration compared with control animals. This effect may contribute to the efficacy of FK in enhancing the regenerative response and recovery of the experimental animals because these cells release oxygen free radicals and other materials that can injure liver cells and thereby impair their regeneration.
From a clinical point of view, perhaps one of the most promising strategies for the amelioration of the reperfusion injury after organ engraftment is reduction of oxygen free radical production (33, 34). It is currently unknown whether FK inhibits the generation of oxygen free radicals by granulocytes or by activated enzymes within the ischemic tissue. Either action would be likely to enhance graft function after transplantation (35).
Based on these data, it can be concluded that FK maintains hepatic ATP levels and reduces cytosolic enzyme loss after hepatic ischemia. Furthermore, it appears to reduce the subsequent hepatic necrosis and neutrophilic infiltration and to stimulate hepatocyte proliferation. As a result, the liver is able to recover and subsequent survival is enhanced.
Acknowledgments
This work was supported in part by grants from the NIDDK 32556 and 39789 and NIAAA 06601.
Footnotes
Mahmoud F. Sakr and Tarek I. Hassanein were each recipients of a peace fellowship from the Agency of International Development.
References
- 1.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: liver transplantation (first of two parts) N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francavilla A, Barone M, Todo S, Zeng Q, Porter KA, Starzl TE. Augmentation of rat liver regeneration by FK 506 compared with cyclosporin. Lancet. 1989;25:1248–1249. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Scotti-Foglieni CL, Porter KA, Trejo Bellido J, Carrieri G, Todo S, Fung JJ, et al. Studies of the hepatotrophic qualities of FK 506 and CyA. Transplant Proc. 1990;22(suppl 1):93–95. [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins GM, Anderson RM. Experimental pathology of the liver. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 5.Meer C, Kley GA, Valkenburgh PW. Studies on the cause of death after permanent and temporary occlusion of the portal vein in rats. Cire Shock. 1976;3:191–193. [Google Scholar]
- 6.Farber JL, Chien KR, Mittnacht S., Jr The pathogenesis of irreversible cell injury in ischemia. Am Assoc Pathol. 1981 Feb;:271–281. [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmeyer HU. Nucleotides, coenzymes and related compounds. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. pp. 2097–2100. [Google Scholar]
- 8.Demetris AJ, Qian S, Sun H, Fung JJ. Liver allograft rejection: an overview of morphologic findings. Am J Surg Pathol. 1990;14(suppl 1):49–63. [PubMed] [Google Scholar]
- 9.Kim YI, Salvini P, Auxilia F, Calne RY. Effect of cyclosporin A on hepatocyte proliferation after hepatectomy in rats: comparison with standard immunosuppressive agents. Am J Surg. 1988;155:245–249. doi: 10.1016/s0002-9610(88)80705-0. [DOI] [PubMed] [Google Scholar]
- 10.Kim YI, Caine RY, Nagasue N. Cyclosporin A stimulates proliferation of the liver cells after partial hepatectomy in rats. Surg Gynecol Obstet. 1988;166:317–322. [PubMed] [Google Scholar]
- 11.Yushimira S, Kamada N. Effect of cyclosporin A on liver regeneration following partial hepatectomy in the mouse. Transplant Proc. 1989;21(1):911–912. [PubMed] [Google Scholar]
- 12.Kawano K, Kim YI, Kaketani K, Kobayashi M. The beneficial effect of cyclosporin on liver ischemia in rats. Transplantation. 1989;48(5):759–764. doi: 10.1097/00007890-198911000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kahn D, Lain HS, Makowka L, Van Thiel D, Starzl TE. Cyclosporin A augments the regenerative response after partial hepatectomy in the rat. Transplant Proc. 1988;20(3):850–852. [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzaferro V, Porter KA, Scotti-Foglieni CL, Venkataramanan R, Makowka L, Rossaro L, Francavilla A, et al. The hepatotrophic influence of cyclosporine. Surgery. 1990;107(5):533–539. [PMC free article] [PubMed] [Google Scholar]
- 15.Francavilla A, Barone M, Starzl TE, Zeevi A, Scotti C, Carrieri G, Mazzaferro V, et al. FK 506 as a growth control factor. Transplant Proc. 1990;23(1 Suppl 1):90–92. [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger J, Lauterburg BH. Postischemic ATP levels predict hepatic function 24 hours following ischemia in the rat. Experimentia. 1988;44:455–457. doi: 10.1007/BF01940546. [DOI] [PubMed] [Google Scholar]
- 17.Stromski ME, Cooper K, Thulin G, Gaudio KM, Siegel NJ, Shulman RG. Chemical and functional correlates of postischemic renal ATP levels. Proc Natl Acad Sci USA. 1986;83(4):6142–6145. doi: 10.1073/pnas.83.16.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craddock CG, Nakai GS, Fukuta H, Vanslager LM. Proliferative activity of the lymphatic tissue of rats as studied with tritium-labeled thymidine. J Exp Med. 1964;120:389–412. doi: 10.1084/jem.120.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai A, Muller-Berat CN, Debray-Sachs M, Crevon MC. Effects of partial hepatectomy on the immune response to sheep red cells. Acta Pathol Microbiol Scand. 1970;78:652–654. doi: 10.1111/j.1699-0463.1970.tb04352.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakai A, Pferrermann R, Kountz SL. Liver regeneration and lymphocyte activation. Surg Gynecol Obstet. 1976;143:914–918. [PubMed] [Google Scholar]
- 21.Miyahara S, Yokomuro K, Takahashi H, Kimura Y. Regeneration and the immune system. I. In vitro and in vivo activation of lymphocytes by liver regeneration and the role of Kupffer cells in stimulation. Eur J Immunol. 1983;13:878–883. doi: 10.1002/eji.1830131104. [DOI] [PubMed] [Google Scholar]
- 22.Craddock CG, Rytomaa T, Nakai GS. The transfer of labeled DNA from blood cells to regenerating liver. In: Good RA, Gabrielsen AE, editors. The thymus in immunology, structure, function and role in disease. New York: Harper & Row; 1964. pp. 317–331. [Google Scholar]
- 23.Flye MW, Yu S. Augmentation of cell-mediated cytotoxicity following 50% partial hepatectomy. Transplantation. 1990;49:581–587. doi: 10.1097/00007890-199003000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Sakai A, Tanaka S. Liver and immune response. V. Regenerating liver cells activate syngeneic lymphocytes. Cell Differentiation. 1979;8:285–290. doi: 10.1016/0045-6039(79)90004-6. [DOI] [PubMed] [Google Scholar]
- 25.Weeds AG, Taylor RS. Stimulation of hepatocytes and lymphocytes in vitro by liver regeneration. Nature. 1974;250:667–669. doi: 10.1038/257053a0. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H, Takeshita T, Yokomuro K. Suppression of liver regeneration resulting from intravenous injection of splenic glass adherent cells activated by poly I: C. Immunology. 1988;176:217–223. doi: 10.1016/S0171-2985(88)80054-8. [DOI] [PubMed] [Google Scholar]
- 27.Babaeva AG, Kraskina NA, Iudina NV. Stimulation of the mitotic activity of hepatocytes and Kupffer cells in the liver of nonoperated mice by thymus derived and bone marrow derived lymphocytes from partially hepatectomized syngeneic donors. Bull Exp Biol Med. 1980;89:69–70. [PubMed] [Google Scholar]
- 28.Sobotka L, Simek J, Dvorackova I, Nouza K. Influence of antithymocytic serum on the regeneration activity of the liver after partial hepatectomy in mice. Czech Med. 1980;3:271–279. [PubMed] [Google Scholar]
- 29.Gonzalez EM, Krejczy K, Malt RA. Modification of nucleic acid synthesis in regenerating liver by azathioprine. Surgery. 1970;68:254–259. [PubMed] [Google Scholar]
- 30.Guzek JW. Effect of adrenocorticotrophic hormone and cortisone on the uptake of tritiated thimidine by regenerating liver tissue in the white rat. Nature. 1964;201:930–931. doi: 10.1038/201930a0. [DOI] [PubMed] [Google Scholar]
- 31.Goto T, Kino T, Hatanaka H, Nishiyama M, Okuhara M, Kohsaka M, Aoki H, et al. Discovery of FK 506: a novel immunosuppressant isolated from streptomyces tsukubaensis. Transplant Proc. 1987;19(suppl 16):4–8. [PubMed] [Google Scholar]
- 32.Borel JF. The history of cyclosporin A and its significance. In: White DJG, editor. Cyclosporine A: proceedings of an international conference on cyclosporine A; Amsterdam: Elsevier Biomedical Press; 1982. pp. 5–17. [Google Scholar]
- 33.Marubayashi S, Dohi K, Ezaki H, Hayashi K, Kawasaki T. Preservation of ischemic rat liver mitochondrial functions and liver viability with CoQ 10. Surgery. 1982;91:631–637. [PubMed] [Google Scholar]
- 34.Marubayashi S, Dohi K, Ochi K, Kawasaki T. Role of free radicals in ischemic rat liver injury: prevention of damage by alphatocopherol administration. Surgery. 1986;99:184–191. [PubMed] [Google Scholar]
- 35.Ohhori I, Izumi R, Yabushita K, Watanabe T, Hashimoto T, Takamori M, Hirowawa H, et al. Prevention of liver damage by using free radical scavengers and changes in plasma PG levels during liver ischemia. Transplant Proc. 1989;21:1309–1311. [PubMed] [Google Scholar]