In an effort to answer many unresolved questions concerning orthotopic homotransplantation of the canine liver, a complete reappraisal of this preparation was under-taken in early 1964 in both the untreated and modified host. A particular effort was made to (A) reduce the operative mortality; (B) interpret the significance of pathologic changes in the homograft and in the recipient tissues; (C) define the presence or absence of a graft-host reaction; (D) study the effect of variations in therapy upon results; (E) assess the hepatotoxic properties of azathioprine; (F) determine if and with what regularity hepatic rejection could be reversed and chronic survival attained; and (G) find if and when a state of relative host-graft non-reactivity developed some time after homotransplantation.
METHODS
Mongrel dogs weighing 8.3 to 27.3 kilograms were used. All animals had hematologic and liver function determinations before and at regular intervals after the experiments were begun. Red cell survival studies were performed with a Cr51 technique.6 Tetracycline and chloramphenicol were routinely administered. Azathioprine* was employed in most experiments with a dose of 2 to 8 mg. per kilogram per day. Insofar as possible, the induction of leukopenia was avoided. Azathioprine and antibiotic therapy was discontinued at 120 days in all surviving animals. Other variations in therapy are described below. Tissues were examined with light and electron microscopy.
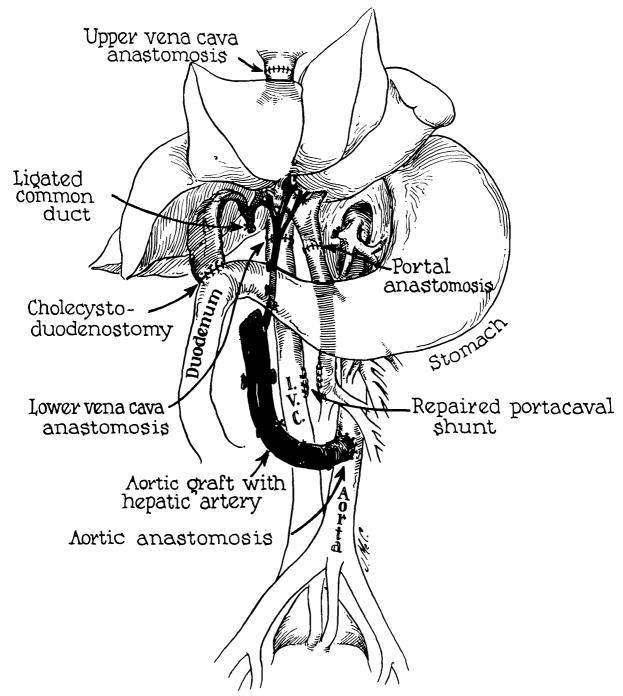
For homotransplantation, livers were obtained from donors of dissimilar appearance, but of approximately the same weight as the recipients. Prior to removal, the donor liver was cooled by perfusion of chilled Ringer’s lactate solution through the portal vein. The technique of transplantation24 resulted in an essentially normal blood supply (Fig. 1). The intervals of ischemia were almost all less than one hour. Cholecystoduodenostomy was established for internal biliary drainage.
Fig. 1.
Reconstruction after orthotopic liver homotransplantation. Internal biliary drainage is with a cholecystoduodenostomy. Note that the aorta is transplanted in continuity with the hepatic artery of the homograft.
Azathioprine toxicity study
In 18 non-transplanted dogs the effects of 40 days of azathioprine were studied. The animals were divided into 3 groups of 6 each which received:
Group I. Daily azathioprine (2 to 4 mg. per kilogram).
Group II. Daily azathioprine plus 1 Gm. intravenous L-methionine.* The methionine powder was dissolved in 100 ml. saline and given in 30 minutes.
Group III. Daily azathioprine plus intravenous S35-methionine every 5 days. Since the specific activity of the radioisotope varied, the amount of L-methionine ranged from 0.08 to 0.6 mg. for the S35 dose of 100 μc. Based upon the measured biologic half-life (18 to 28 days), and assuming equal distribution of S35 to all metabolizing cells, daily total-body irradiation was estimated to begin at 0.2 or 0.3 r per day, with equilibration at 1 r per day after 18 to 28 days.
Homotransplantation without azathioprine
Three groups of animals were studied after orthotopic homotransplantation:
Group A. Supportive operative care only: 7 dogs.
Group B. Daily intravenous L-methionine (1Gm.): 7 dogs.
Group C. S35-methionine every 5 days: 9 dogs.
Homotransplantation with azathioprine
Azathioprine was begun on the day of transplantation in all dogs except those of Group 5, which received pretreatment for 40 days. Since those animals lost during or shortly after operation invariably died from causes other than failure to control rejection, as many dogs were included in each series (except Group 5) as were necessary to obtain 11 which survived one week or longer. For statistical comparison of the effectiveness of differing regimens, only these selected 11 animals were included. Eight separate series were studied in the following chronology:
Group 1. Azathioprine, S35-methionine every 5 days, splenectomy: 17 dogs.
Group 2. Azathioprine, S35-methionine every 5 days: 16 dogs.
Group 3. Azathioprine, 1 Gm. per day L-methionine: 13 dogs.
Group 4. Azathioprine only: 16 dogs.
Group 5. Azathioprine pretreatment: 12 dogs which survived the 40 day azathioprine toxicity study and were subsequently provided with a homograft. (Postoperatively, 6 were treated as Group 2; 5 as Group 3; and 1 as Group 4. Only 7 survived a week or more, an early postoperative mortality so great that the study was discontinued.)
Group 6. Azathioprine, 2 mg. methionine every 5 days: 12 dogs. The dose schedule of methionine was comparable to those of Groups 1, 2, and 7, lacking only the radio-isotope.
Group 7. Azathioprine, 1 Gm. per day L-methionine, S35- methionine every 5 days, 10 mg. per kilogram choline per day: 17 dogs.
Group 8. Azathioprine only: 13 dogs. This series was treated exactly like Group 4, to determine if unrecognized variations in care had been introduced during the prolonged investigation.
RESULTS
Azathioprine toxicity study
Mortality and clinical course
Twelve of the 18 nontransplanted dogs lived through the toxicity study, 1 from Group I, 5 from Group 11, and 6 from Group 111. The other animals died after 18 to 33 days; 3 with bone marrow depression and pneumonitis, 2 from pneumonitis without leukopenia, and 1 of unknown cause. All dogs lost weight during the first three weeks (Fig. 2). Declines in hematocrit were noted in all 18 dogs (Fig. 2), affecting the three experimental groups equally.
Fig. 2.
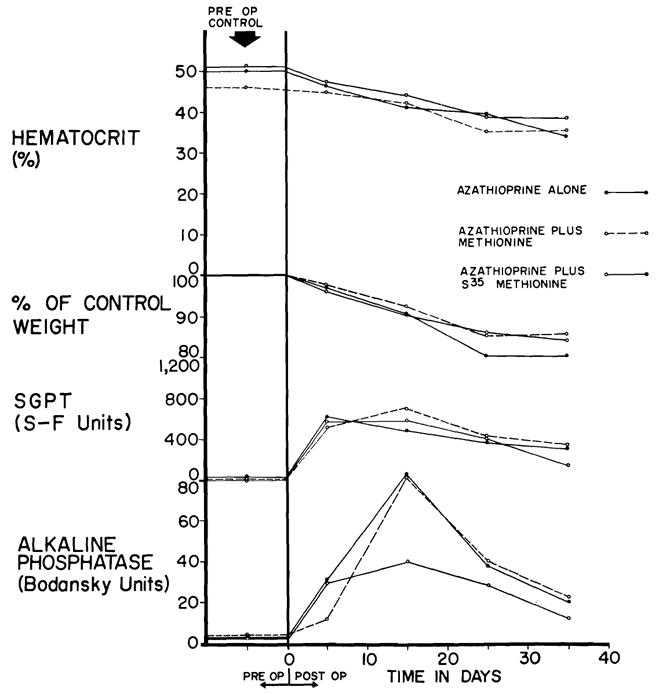
Toxicity of azathioprine when used alone, with S35-methionine, and with L-methionine. Six dogs were in each of the 3 test groups depicted. Despite the abnormalities of liver function, jaundice did not develop.
Biochemical changes
Although none of the animals became jaundiced, there was evidence of liver injury in all. Rises in SGPT, SCOT, and alkaline phosphatase (Fig. 2) were invariably observed within 2 to 7 days after beginning azathioprine. These changes were severe, usually peaking within 2 weeks and then declining toward normal, despite continuation of therapy. However, return to completely normal values did not occur in any of the dogs. The addition of L-methionine or radioactive methionine did not alter the pattern of injury (Fig. 2).
Pathologic changes in the liver (Studies done by K. A. P.)
Twelve of the 18 livers were abnormal. In all 12, the centrizonal area was most affected (Fig. 3), with either frank necrosis (7 dogs) or pallor of the hepatocytes (5 dogs). Five of the 7 most severely damaged livers had other findings; 5 with bile thrombi in the central and midzonal canaliculi, 2 with intracytoplasmic fat in the midzone, and 2 with scattered focal necrosis. The centrilobular changes were noted in all 6 livers of Group I, 5 of the 6 in Group II, and only 1 of Group III. Mononuclear cell infiltration was not observed.
Fig. 3.
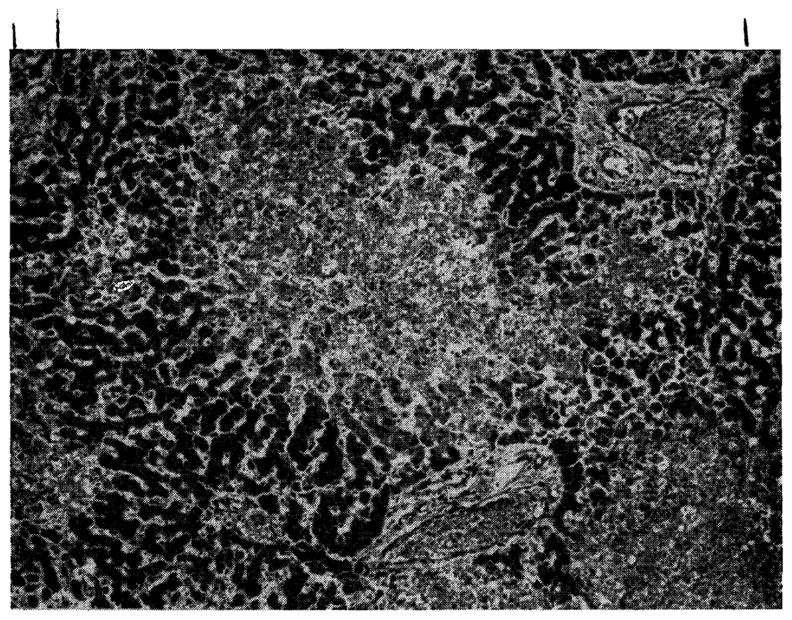
Liver of a dog treated with azathioprine for 26 days. There is centrilobular necrosis of hepatocytes but no cellular infiltration. (Hematoxylin and eosin. Original magnification ×40.)
Four livers were examined electron microscopically, 3 of which had the centrizonal changes note above. In one, which had appeared normal under light microscopy, the hepatocytes around the central veins had a decreased glycogen, a reduction in rough endoplasmic reticulum, and an increase in lipofuscin granules. In the other 3, these changes were more pronounced, extending in one liver to the midzonal area. In 2 of the livers there was dilatation of the bile canaliculi, with thinning and shortening of their microvilli.
Changes in other organs (Studies done by K. A. P.)
Six animals had complete autopsy after 18 to 33 days of azathioprine therapy. Three that died of marrow aplasia had severe depletion of large and small lymphocytes in the lymphoid tissue. Three others had moderate atrophy and hyperemia of the lymph nodes and spleen, with normal thymus and bone marrow. Pneumonitis and pulmonary congestion were present in 5 animals. Megakaryocytes were often seen lodged in the alveolar capillaries. The other organs were unaffected.
Homotransplantation without azathioprine
Operative mortality and survival
The only operative death resulted from hemorrhage. The remaining 22 animals survived for at least 2 days and 19 lived for 6 days or longer. All dogs were dead at 10 days. The mean survival was 7.1 ± 2.2 (S.D.) days. Many animals had pathologic processes other than rejection which contributed to death: pneumonitis (12 cases), intussusception (6 cases), and multiple gastrointestinal ulcerations (2 cases). The three subgroups were all comparable, animals receiving no therapy having no difference in survival than those treated with L-methionine or radioactive methionine (Table I).
Table I.
Survival after hepatic homotransplantation to recipient dogs treated with azathioprine
| Group | No. dogs | Survival |
Mean survival of dogs living 2 days or more | Range (days) | S.D. | |
|---|---|---|---|---|---|---|
| 2 days or more | 6 days or more | |||||
| I (no therapy) | 7 | 7 | 6 | 7.0 | 2–10 | 2.5 |
| II (methionine) | 7 | 7 | 6 | 7.7 | 3–10 | 2.4 |
| III (S35-methionine) | 9* | 8 | 7 | 6.6 | 3–10 | 2.0 |
| 23 | 22 | 19 | 7.1 | 2–10 | 2.2 | |
One dog did not survive operation and is therefore not included in the statistical analysis
Clinical course
The clinical course and biochemical alterations were no different than those previously described in the pioneer studies of Moore and co-workers16 and in our experience.25 By the fourth day, all animals had marked increases in alkaline phosphatase, SGOT, and SGPT, and by the fifth day, 17 of 19 dogs had hyperbilirubinemia.
Pathologic changes in the liver (Studies done by K. A. P.)
Interstitial edema, prominence of Kupffer cells, and necrosis of a few hepatocytes in areas of centrilobular congestion were present in the first 48 hours, changes which are nonspecific, since they have also been seen in liver autografts.13 From the third day onward, the histologic features of rejection1, 13, 16, 25 were present in all but 2 homografts; these consisted of mononuclear cell infiltrations around and within the walls of small portal and central veins, centrilobular and midzonal hepatocyte necrosis, distortion of the centrally located sinusoids (often with mononuclear cells adhering to the endothelium), hypertrophy of bile- or hemosiderin-laden Kupffer cells, and intrahepatic bile stasis in association with destruction of bile duct epithelium. The severity of damage was related to duration of survival, and was not influenced by methionine therapy. In 14 animals only a thin rim of hepatic cells remained around the portal tracts and some of the longest survivors had reticulin collapse and early fibrosis around the central veins. With time, the character of the mononuclear cell infiltration changed. At first, approximately 40 percent of these cells possessed pyroninophilic cytoplasm, but few were typical mature plasma cells. The majority were difficult to classify and were the cells variously called “large lymphoid,”22 “hemocytoblasts,”7 and “immunoblast.”4 After the sixth day the infiltrate was very dense in all the small portal tracts and contained a higher proportion of plasma cells. Fibrinoid necrosis in the walls of small hepatic arteries was seen in only 5 of the 23 dogs.
Changes in other organs. (Studies done by K. A. P.)
In the 19 animals dying after the third day the mesenteric lymph nodes were swollen, partly as a result of an intense proliferation of large cells with pyroninophilic cytoplasm. Macrophages containing hemosiderin and debris were present in larger numbers than normal. In those animals dying after the seventh day, the medullary cords were crammed with plasma cells. Similar changes were found in the spleens. Pulmonary congestion, hemorrhage, and bronchopneumonia were common in these animals, while focal thickening of the alveolar septa and interstitial infiltration by mononuclear cells were seen in only a few dogs. Megakaryocytes were found trapped in the alveolar capillaries in all lungs. Myocardial and renal lesions were not seen, and fibrinoid necrotic lesions of arterial walls were not encountered in organs other than the liver.
Homotransplantation with azathioprine
Operative mortality and survival
Thirty-two of the 116 recipients (27.5 percent) died at operation or during the first postoperative week (Table II), most commonly of pneumonitis (or atelectasis) and intussusception (Table III). These 32 acute failures were considered operative deaths and were eliminated from analysis of the efficacy of immunosuppression.
Table II.
Survival after hepatic homotransplantation to recipient dogs treated with azathioprine
| Group | No. dogs | Survival |
Mean survival of dogs living 7 days or more (50 day limit)* | S.D. (50 day limit) | |||
|---|---|---|---|---|---|---|---|
| 7 days or more | 25 days or more | 50 days or more | |||||
| 1† | 17 | 11 | 8 | 4 | 33.4 | 72.3 | 16.4 |
| 2† | 16 | 11 | 7 | 5 | 31.7 | 68.8 | 19.3 |
| 3† | 13 | 11 | 7 | 3 | 29.5 | 63.1 | 15.0 |
| 4† | 16 | 11 | 4 | 2 | 24.2 | 39.1 | 14.5 |
| 5 | 12 | 7 | 2 | 1 | 19.7 | 28.6 | 15.6 |
| 6 | 12 | 11 | 5 | 3 | 26.5 | 32.2 | 18.5 |
| 7 | 17 | 11 | 7 | 3 | 28.9 | 37.1 | 15.6 |
| 8 | 13 | 11 | 4 | 3 | 26.1 | 30.8 | 18.1 |
| 116 | 84 | 44 | 24 | 27.9 | 47.3 | 16.5 | |
The dogs surviving longer than 50 days are described individually in Table V, the greatest longevity now being 324 days. Statistical evaluation of different regimens take into account only the first 50 days. This avoids introduction of unfair bias in the event of unusually protracted survival, and allows comparison of different series of animals undergoing operation in the chronology shown. In all of the groups, the actual mean survival without the artificial 50 day limit has been brought up to date to Feb. 10, 1965, and is just to the right of the artifactually low values.
In at least one dog from each group, all therapy, including azathioprine, was discontinued after 120 days.
Table III.
Homotransplantation with azathioprine; nonimmunologic conditions which contributed to or were the direct cause of death of 32 animals during operation or in the first postoperative week. Several animals had more than one complication
| Pneumonitis or atelectasis | 13 |
| Intussusception | 9 |
| Hemorrhagic gastroenteritis | 5 |
| Hepatic arterial occlusion | 5 |
| Pulmonary emboli | 2 |
| Operative hemorrhage | 2 |
| Portal vein occlusion | 1 |
| Total | 37 |
In the 84 remaining dogs (72.5 percent) which lived for 7 days or longer, there was a high incidence of similar complications (Table IV), but there was usually also biochemical or histologic evidence that some degree of homograft rejection was present. Of the 84 dogs culled for definitive analysis, 44 lived for 25 days and 24 lived for 50 days or longer. Eight more died between days 50 and 120 of causes listed in Table V. Abnormal hepatic function was present in all 8, although other lethal complications were present in each. Another animal which died after discontinuance of azathioprine therapy is discussed below. Fifteen dogs are still alive, from 62 to 324 days after operation (Table V).
Table IV.
Homotransplantation with azathioprine; complications other than rejection observed in dogs dying 7 or more days after homotransplantation of the liver. (Sixty-nine of the 84 animals died at some time after the first week and the figures below are based on the autopsy findings; 15 animals are still alive)
| Pneumonitis or atelectasis | 46 |
| Bone marrow depression | 11 |
| Generalized gastrointestinal hemorrhage | 6 |
| Liver abscess | 3 |
| Generalized peritonitis | 3 |
| Subdiaphragmatic abscess | 3 |
| Intra-abdominal hemorrhage | 3 |
| Perforated viscus | 3 |
| Intussusception | 3 |
| Bleeding duodenal ulcer | 3 |
| Pulmonary emboli | 2 |
| Hepatic artery occlusion | 2 |
| Wound dehiscence | 1 |
| Pyelonephritis | 1 |
| Leg gangrene | 1 |
| Thermal burn of back | 1 |
| Total | 92 |
Table V.
Homotransplantation with immunosuppression (fate of 24 animals living 50 days or longer; the survival figures for dogs still living is brought up to Feb. 10, 1965)
| Group | No. dogs | Survival (days) | Gross cause of death | Last bilirubin (mg. %) | Last alkaline phosphatase (B.U.*) | Last SGOT (S-F.U.†) |
|---|---|---|---|---|---|---|
| 1 | 1 | 324 | Alive‡ | 0.1 | 1.9 | 23 |
| 2 | 98 | Pneumonitis; liver failure | 5.7 | 503 | 760 | |
| 3 | 116 | Alive | 0.3 | 58 | 62 | |
| 4 | 90 | Alive | 0.3 | 172 | 94 | |
| 2 | 1 | 120 | Gastrointestinal hemorrhage; liver failure | 3.7 | 296 | 870 |
| 2 | 58 | Pneumonitis | 1.3 | 300 | 220 | |
| 3 | 228 | Alive‡ | 0.1 | 73 | 470 | |
| 4 | 70 | Gastrointestinal hemorrhage; liver failure | 5.9 | 516 | 1660 | |
| 5 | 181 | Alive‡ | 4.1 | 361 | 1080 | |
| 3 | 1 | 218 | Alive‡ | 0.1 | 30 | 400 |
| 2 | 101 | Pneumonitis; liver failure | 5.6 | 436 | 580 | |
| 3 | 201 | Alive‡ | 0.1 | 16 | 650 | |
| 4 | 1 | 161 | Bleeding duodenal ulcer; liver failure‡ | 4.7 | 166 | 110 |
| 2 | 103 | Post biopsy hemorrhage; liver failure | 5.7 | 746 | 380 | |
| 5 | 1 | 112 | Alive | 6.2 | 692 | 1000 |
| 6 | 1 | 69 | Pneumonitis; liver failure; gastrointestinal hemorrhage | 3.9 | 657 | 1200 |
| 2 | 50 | Pneumonitis; liver failure | 4.8 | 354 | 250 | |
| 3 | 93 | Alive | 3.9 | 268 | 1068 | |
| 7 | 1 | 82 | Alive | 2.8 | 527 | 1900 |
| 2 | 81 | Alive | 0.2 | 188 | 360 | |
| 3 | 77 | Alive | 6.1 | 184 | 500 | |
| 8 | 1 | 72 | Alive | 4.3 | 530 | 1100 |
| 2 | 68 | Alive | 4.4 | 140 | 37 | |
| 3 | 62 | Alive | 7.0 | 473 | 1500 |
Bodansky units.
Sigma-Frankel units.
Azathioprine stopped after 116 to 123 days; subsequent course without immunosuppression.
Hematologic changes
In virtually every animal a decline in hematocrit was observed, similar to that observed in nontransplant animals receiving azathioprine. The 5 dogs surviving longest started with a mean preoperative hematocrit of 47.5 percent, which had fallen to 36.8 percent by the time azathioprine was discontinued on postoperative day 120. Forty days later the mean hematocrit had returned to 43.5 percent. The increase in hematocrit after discontinuance of azathioprine is strong evidence that this agent was primarily responsible for the chronic anemia, presumably from inhibition of erythropoiesis.
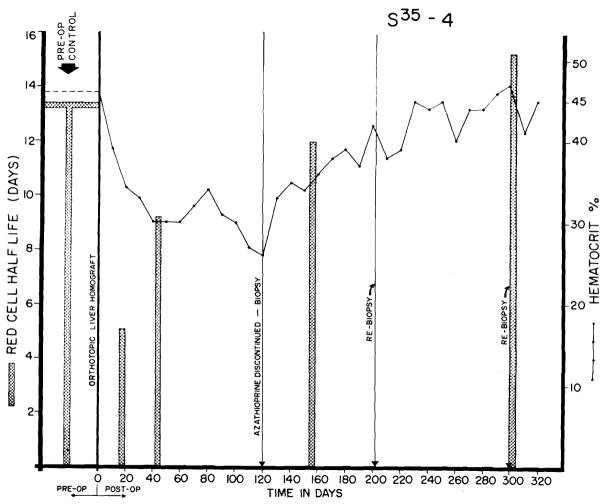
In addition, a contributory early factor seemed to be increased red cell destruction, which may have been due to erythroclastic activity of the liver,9 in view of the prominent hemosiderin deposits (to be described subsequently) in many of the homografts. Seven animals studied from 7 to 27 days after homotransplantation had a red cell half-life of 6.7 ± 3 (S.D.) days (normal for this laboratory, 14 days). After the first month, the half-life gradually returned toward normal (Fig. 4).
Fig. 4.
Red cell survival and hematocrit values in a dog that is still alive 324 days after orthotopic transplantation. Note the sharp reduction in red cell half-life in the first postoperative month, with gradual return toward normal. Red cell survival was not altered by withdrawal of azathioprine a t the end of 4 months, but the depressed hematocrit rose sharply during the succeeding months.
Clinical course, biochemical changes, and reversal of rejection
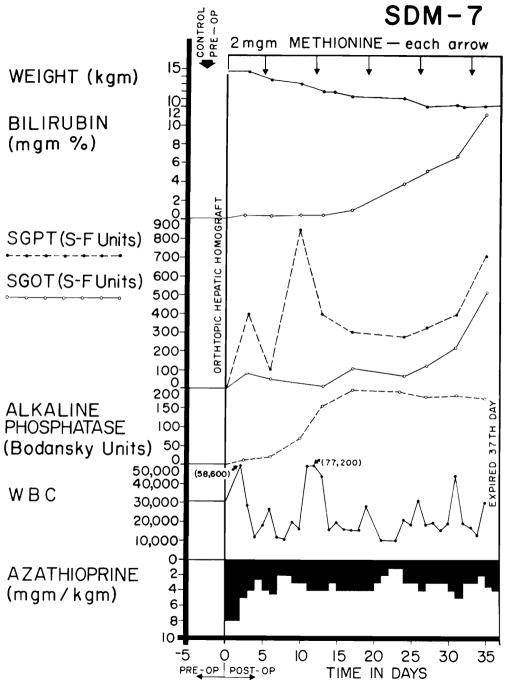
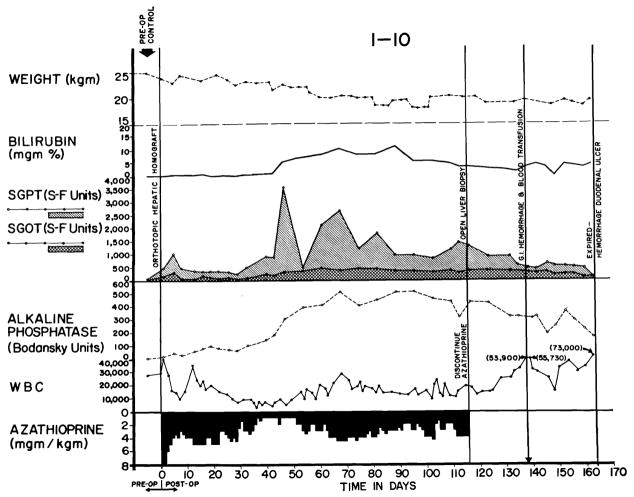
The course after homotransplantation was extremely variable. In 24 (28.6 percent) of the 84 definitive test animals rejection was uncontrollable. In exorable jaundice developed (Fig. 5), with anorexia and rapid weight loss. Rises in SGOT, SGPT, and alkaline phosphatase (Fig. 5) were similar to, but of generally greater magnitude, than those produced by azathioprine administration to the nontransplanted dog. Terminal convulsions or coma, intractable vomiting, and hypoglycemia were common. All 24 animals died in less than 41 days, with a mean survival of only 15 days.
Fig. 5.
An example of inexorable rejection despite immunosuppressive therapy. Determination of the serum bilirubin was the most useful measurement for following the course after homotransplanation since the other abnormalities of liver function depicted can also be caused by azathioprine. Group 6 series.
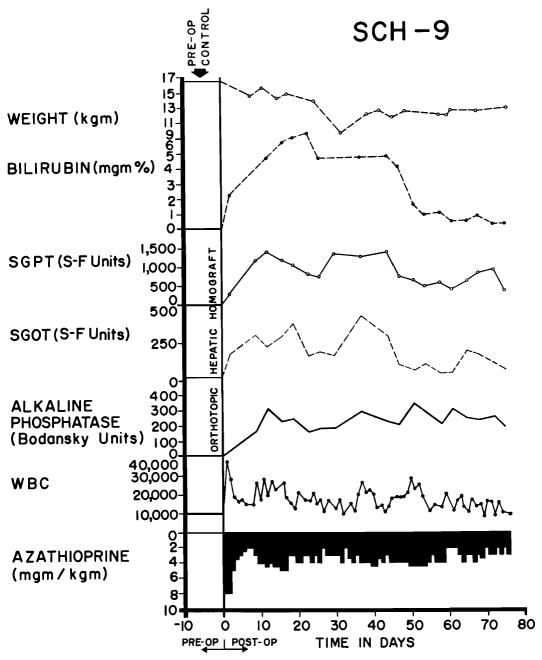
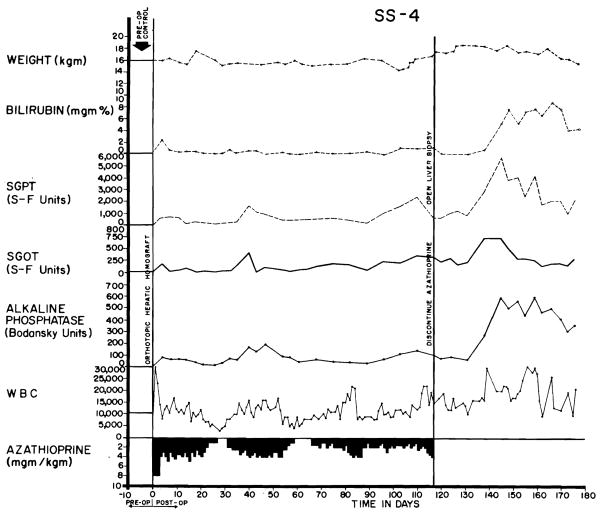
A more benign rejection was observed in 41 of 84 experiments (48.3 percent) despite the fact that hyperbilirubinemia and rises in SGOT, SGPT, and alkaline phosphatase were also present (Figs. 6 and 7), sometimes to the same degree as in the 24 animals noted above. However, the biochemical indices of hepatic injury were partially (Fig. 7), or even relatively completely, reversible (Fig. 6). At the peak of rejection, which lasted as long as 6 weeks in some cases, these dogs often appeared to be in terminal condition, with clay-colored stools, dark urine, profound anorexia, and rapid weight loss. As liver chemistries subsequently improved, most of the animals had a return of appetite. A number of the dogs ultimately died from complications of chronic liver insufficiency, such as gastrointestinal hemorrhage, or from pneumonitis, but others continued to live for many weeks or months with gradually improving liver function. The longest survival of any animal with this type of reversible rejection was 161 days (Fig. 7). Sixteen others lived for 50 days or more, indicating that protracted survival under these unfavorable circumstances was not rare.
Fig. 6.
Virtually complete reversal of severe hepatic rejection. Note the severe jaundice which ultimately completely disappeared. The animal is still alive. Reversal was accomplished without any alteration in immunosuppressive therapy. The animal received adjuvant S35-methionine, L-methionine, and choline (Group 7).
Fig. 7.
Reversal o f rejection in an animal treated with azathioprine. Serum bilirubin rose to more than 10 mg. percent and then declined. Note that the improvement in liver chemistries continued after discontinuance of azathioprine therapy at 116 days. Cause of death 45 days later was massive hemorrhage from a large duodenal ulcer. Group 4 series.
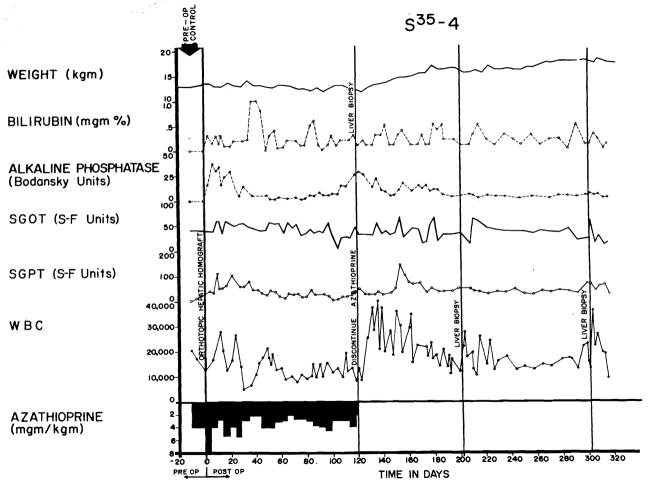
In the other 19 dogs (22.6 percent), overt rejection did not occur. Although there were rises in alkaline phosphatase, SGOT, and SGPT, the serum bilirubin did not exceed 2.5 mg. percent at any time during postoperative azathioprine treatment (Fig. 8). Weight loss and anorexia were not pronounced. Seven dogs died from other causes than rejection before 25 days, so that failure to develop jaundice may have been more a function of the short survival than anything else. The other 12 all lived for more than 25 days. Five died after 28 to 35 days without developing hyperbilirubinemia, and the other 7 are still alive after 90, 116, 181, 201, 218, 228, and 324 days (Table V). Five dogs from this favored group had azathioprine discontinued at 116 to 123 days as described below.
Fig. 8.
Course of an animal which never exhibited any clinical evidence of homograft rejection. Note the rapid weight gain following cessation of therapy at 4 months. The pronounced leukocytosis after withdrawal of immunosuppression was commonly seen. This animal received adjuvant S35-methionine.
Chronic liver function
The last routine liver chemistry studies done on all dogs living beyond 50 days are shown in Table V. In 6 of these animals other determinations were performed from 98 to 300 days postoperatively. Bromsulfonphthalein retention at 45 minutes was 5 percent or less in 4, 8.2 percent in 1, and 34 percent in 1. Prothrombin times were 52 to 90 percent. Total proteins were 4.8 to 5.8 Gm. percent.
Host-graft nonreactivity
Five nonjaundiced animals had all therapy discontinued after 116–123 days. All are still alive 63, 81, 100, 109, and 204 days later. One had a delayed rejection, beginning 2 weeks after stopping azathioprine, but the hyperbilirubinemia and enzyme increases ultimately stabilized and reversed without treatment (Fig. 9). The other 4 have had no deterioration of homograft function in the ensuing 81 to 204 days (Fig. 8). In all cases, irregular increases in the peripheral leukocyte count were observed during the first 2 months after cessation of therapy.
Fig. 9.
Course of an animal which did not have clinically evident rejection during the first 4 postoperative months. After azathioprine was discontinued, there was deterioration of all liver chemistries, despite which the animal appeared healthy and had little weight loss. The late rejection partially reversed without reinstitution of therapy.
A sixth animal had serious liver malfunction while under azathioprine therapy (Fig. 7), a maximum bilirubin of 10 mg. percent being present on postoperative day 90. By day 120 this was decreasing and continued to decline after azathioprine therapy was stopped. The animal ultimately died of a bleeding duodenal ulcer 161 days posttransplant.
Effect of adjuvant therapy
Mean survival of the various test groups is shown in Table II. For analytic purposes, no animal was credited with more than 50 days. Within this arbitrary time limit, none of the regimens had a statistically significant superiority. In Groups 1, 2, 3, and 7 in which either S35-methionine and/or 1 Gm. per day L-methionine were administered, mean survival exceeded that of Groups 4 and 8, in which these agents were not used, and was greater than Group 6, in which 2 mg. non-radioactive methionine was given every 5 days. However, even comparison of the series combinations (Groups 1, 2, 3, 7, versus 4, 6, 8; or 1, 2, 3, 7 versus 4, 8) did not show a statistically significant advantage of the adjuvant therapy (p = 0.18 and 0.17).
Group 5, which differed from the other 7 series in that 40 days of azathioprine pretreatment was employed, had the shortest mean survival. This seemed attributable to a heightened incidence of infection, a septic complication being the cause of death in 9 of the 11 failures. Thus, azathioprine pretreatment had an adverse effect after hepatic homotransplantation in contrast to an apparent benefit previously described with renal homograft.27
Pathologic changes after homotransplantation with azathioprine. (Studies done by K. A. P.)
Homografts in animals dying within one week
Twenty-seven livers were examined and showed the central venular and sinusoidal congestion, variable centrizonal necrosis, interstitial edema, and Kupffer cell swelling already described in the untreated dogs dying in the first 2 postoperative days. In addition, centrilobular bile stasis was usual. Portal and central cellular infiltration was appreciable in only 7 and 2 of the homografts, respectively, consisting of mixed neutrophilic and mononuclear cells, with pyroninophilic cytoplasm being rare in the latter. In several specimens, the perivenular lymphatics were dilated.
Electron microscopic examination of a homograft at 6 days showed a few lymphoid cells around the small portal veins and adhering to the sinusoidal endothelium. Some of these had abundant free cytoplasmic ribosomes arranged in rosettes. The hepatocytes adjacent to the central vein were necrotic; those cells just peripheral to this zone contained many fat droplets and lipofuscin granules, lacked glycogen and rough ergastoplasm, and contained swollen mitochondria. Hepatocytes in the middle and peripheral zones were normal.
Homografts in animals dying in 7 to 15 days
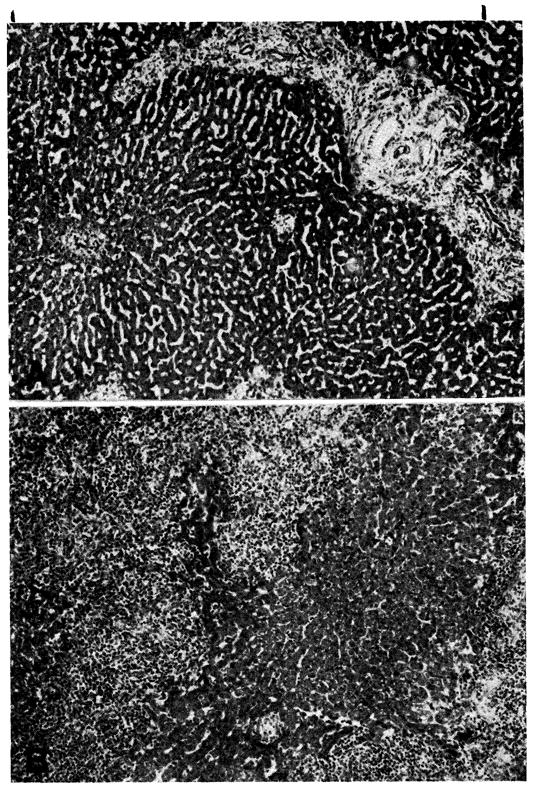
Twenty-seven homografts were examined; central necrosis was present in 22, had extended to the midzone in 17 (Fig. 10), and was often associated with mild to moderate central congestion or sinusoidal distortion. With severe hepatocyte loss, central reticulin collapse had occurred, and in 5 the condensed reticulin bands connected adjacent central veins. Interstitial edema, cholestasis, destruction of bile canalicular epithelium, and bile- or hemosiderin-containing Kupffer cells were also present. In every liver the small, and often the large, portal tracts were moderately (8 cases) or extensively (19 cases) infiltrated by mononuclear cells (Fig. 10), 30 to 40 percent of which possessed pyroninophilic cytoplasm. As in the untreated animals, many were the primitive “large lymphoid” cells; others resembled plasma cells. A similar, but lesser infiltrate was noted in and around the walls of the central veins in 17 instances.
Fig. 10.
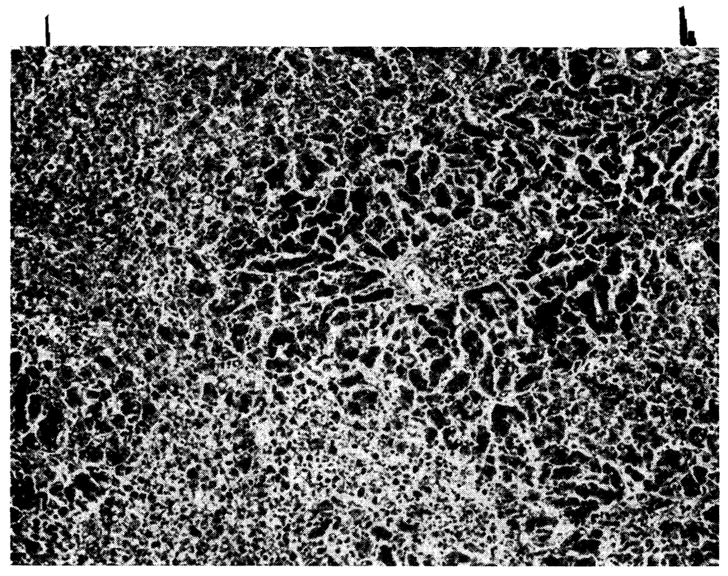
Hepatic homograft which has been rejected by 15 days despite azathioprine treatment. There is widespread destruction of hepatocytes in the central and middle zones of the lobules. Only a rim of liver cells remain around the small portal tract, which is heavily infiltrated with mononuclear cells. (Hematoxylin and eosin. Original magnification ×40.
Preliminary ultrastructural analysis of 3 homografts at 8 to 10 days revealed an extension of the findings noted above in the 6-day specimen. The lymphoid cells in the portal tracts and sinusoids were now joined by plasma cells and their precursors with abundant rough endoplasmic reticulum, and the sinusoidal endothelium had disintegrated in many places. Dilatation and destruction of bile canaliculi with microvillar loss and obstruction by masses of bile pigment were common. Many intact hepatocytes exhibited changes in the cytoplasmic organelles as well as in the nuclei.
Homografts in animals dying in 16 to 25 days
In 14 of the 17 livers, mononuclear infiltration around the portal and central veins was observed, but in all but 3 the cells were fewer than had been present after 7 to 15 days and had a decreased percentage with pyroninophilic cytoplasm. The majority were small lymphocytes and plasma cells with some hemosiderin-laden macrophages. Centrilobular hepatocyte loss was seen in 15 homografts but it appeared to be recent only in the 3 specimens with heavy cellular infiltration. The surviving liver cells around necrotic areas often contained fat droplets, bile pigment particles, and excess lipofuscin. Centrizonal reticulin collapse and deposition in these areas of collagen fibers was common, and in 3 livers, bands of connective tissue had linked adjacent central veins. Periportal connective tissue was not increased. Centrilobular bile stasis and hemosiderin deposition were prominent. In one homograft there was focal fibrinoid necrosis of the walls of small hepatic artery branches. (This was the only example of that change in the 101 azathioprine-treated hepatic homografts examined microscopically.)
Three homografts were examined electron-microscopically at 16, 17, and 25 days. Two of these, which happened to come from the atypical specimens with heavy cellular infiltration, were similar to those in the 7 to 15 day group. The third had only a few numbers of infiltrating cells which were mostly lymphocytes and mature plasma cells. The other findings also confirmed those observed with light microscopy, and revealed that many centrilobular canaliculi had microvillar loss and were plugged with bile pigment. Fat-laden hepatocytes adjacent to areas of necrosis lacked rough ergastoplasm.
Homografts in animals dying in 26 to 50 days
Four of the 18 livers were completely free of invasion, and in the others the quantity and character of the cellular infiltrate was comparable to that of the previous group. The centrilobular hepatocyte loss was also similar, being present in 15 homografts, but with recent necrosis in only 4. The central reticulin condensation was present in 17, and in 7, connective tissue bands had linked the central veins (Fig. 11). The portal reticulin and connective tissue were also increased in 4 livers, and in one of these there was proliferation of small bile ductules. One homograft had small regeneration nodules lacking normal lobular architecture. The intrahepatic cholestasis and hemosiderin deposition described in the earlier specimens were usually present.
Fig. 11.
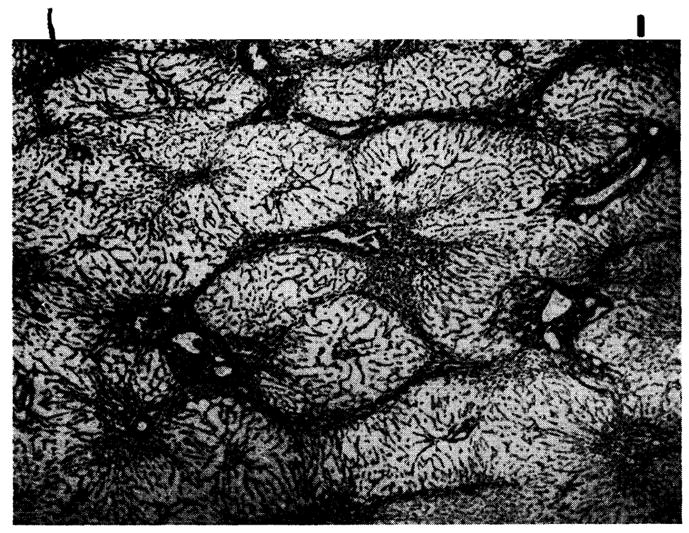
Hepatic homograft at 32 days from a dog treated with azathioprine. The reticulin framework of the lobules has collapsed around the central veins and bands of reticulin now connect central veins to each other and to portal tracts. (Reticulin stain. Original magnification ×20.)
Homografts in animals dying in 51 to 120 days
Infiltration around the portal and central veins was mild in 4 of the homografts and marked in the 3 others. In the former, the cells were almost all lymphocytes or plasma cells, but in the latter, many primitive cells with abundant free cytoplasmic ribosomes were also present. Nonacute central hepatocyte loss with associated reticulin collapse and collagen deposition were similar to that described in the two preceding sections. In 6 of these, connective tissue linked the central vein, and in 3, several central veins were occluded with fibrous tissue. Portal fibrosis had occurred in all 7 homografts, and some fibrous and reticulin bands connected these areas to the central veins. Regeneration nodules were present in 2 homografts, and in all livers peripheral hepatocytes showed increased basophilia, occasional mitoses, and polyploidy. Centrilobular bile stasis and Kupffer cell hemosiderosis were conspicuous in all the livers.
Effect of withdrawing therapy upon homograft histology
In 3 dogs the homograft was biopsied at 120 to 123 days when all immunosuppressive drugs were withdrawn. A further biopsy was then obtained 77 to 84 days later. One animal was biopsied for a third time 182 days after withdrawal of therapy. The animal whose liver was sampled on 3 occasions was unique in that all specimens appeared to be within normal limits, both with light (Fig. 12) and electron-microscopy.
Fig. 12.
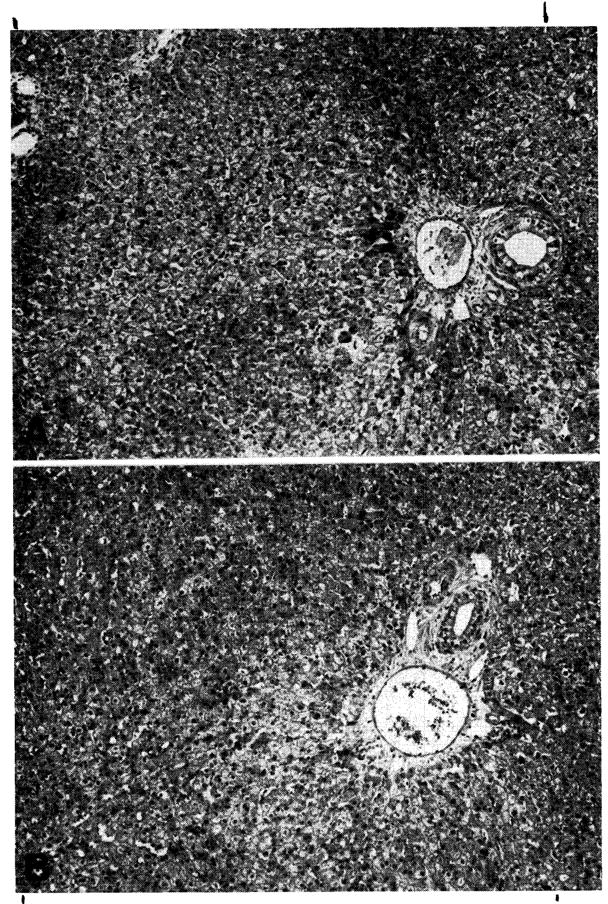
Two biopsies from a hepatic homograft. The first (A) was taken after the host had been receiving azathioprine for 120 days. There is evidence of regeneration of hepatocytes at the periphery of the lobules, but no other abnormality. All immunosuppressive drugs were then stopped and 182 days later, 302 days after transplantation, sample B was obtained. The homograft appears normal. (Hematoxylin and eosin. Original magnification ×40.)
A second dog had an abnormal hepatic homograft at the time treatment was discontinued (Fig. 13, A) with moderate infiltration of mixed mononuclear cells (10 percent pyroninophilic) and neutrophiles in the portal areas, and a few mononuclear cells around the central veins. The lobular pattern was distorted with fibrous and reticulin bands which linked portal tracts to each other and occasionally to central veins; by reticulin and collagen condensation around thickened central veins; and by pseudolobules of actively regenerating liver cells. The centrilobular hepatocytes had disappeared and in some areas or were atrophic in others. Many remaining liver cells had decreased basophilia, increased lipofuscin, and pale, foamy cytoplasm; ultrastructurally they contained lipid droplets and lacked glycogen and rough ergastoplasm. In the portal tracts, there was proliferation of small ductules, accumulations of hemosiderin- and lipofuscin-containing macrophages, and occasional nonfibrotic intimal thickening of small hepatic arteries. Bile plugs were not seen. Seventy-seven days later (Fig. 13, B), the cellular infiltration was lighter and lacked neutrophiles, although the percentage of pyronine-positive cells was unchanged. Active proliferation of peripheral liver cells was continuing, but there were fewer regeneration nodules and the hepatocytes appeared to be more normal with both light and electron-microscopy. The fibrotic alterations persisted, but the over-all picture was one of improvement.
Fig. 13.
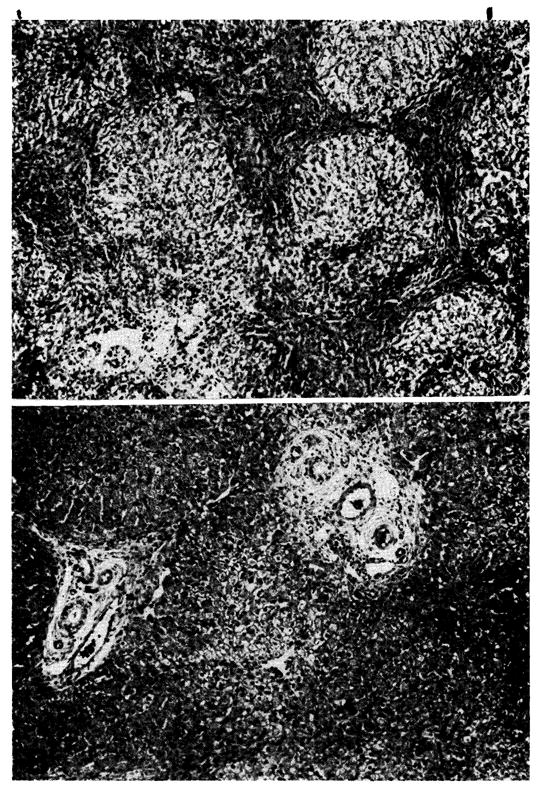
Two biopsies from a hepatic homograft. The first (A) was taken after the host had been receiving azathioprine for 121 days. The lobular architecture is distorted by thick bands of connective tissue which link portal tracts to each other and to central veins. Hepatocytes in the pseudolobules of regenerating liver contain much lipid. Azathioprine therapy was then discontinued, and 77 days later, 198 days after transplantation, the second biopsy (B) was taken. There has been a striking improvement in the general liver architecture. Connective tissue bands are no longer so obvious and the liver cells look more healthy. (Hematoxylin and eosin. Original magnification ×40.)
A homograft in a third dog had comparable abnormalities at the time therapy was withdrawn (Fig. 14, A) differing only in that fibrosis was somewhat less and regeneration nodules were absent. Eighty-four days later (Fig. 14, B) there was further homograft damage in which many centrally located hepatocytes contained fat droplets. Intrahepatic cholestasis was not present. Cellular infiltration was increased, chiefly due to an increased number of neutrophiles. The deterioration appeared to be due, at least in part, to the effects of cholangitis.
Fig. 14.
Two biopsies from a hepatic homograft. The first (A) was taken after the dog had been treated with azathioprine for 123 days. There is some increase in portal connective tissue, proliferation of small biliary ductules, and a slight cellular infiltration. Treatment with immunosuppressive drugs was stopped, and 84 days later, 207 days after transplantation, the second biopsy (B) was taken. There has been a marked deterioration. The portal tracts are very heavily infiltrated with a mixture of mononuclear and neutrophilic leukocytes. (Hematoxylin and eosin. Original magnification ×40)
Effect of methionine upon homograft histology
Centrilobular necrosis occurred in 40 percent of animals treated with adjuvant methionine plus azathioprine, compared to 64 percent when azathioprine was used alone. The incidence of cellular infiltration in both groups was 85 percent.
Changes in host tissues in 92 experiments
Changes in the recipient’s lymph nodes and spleen roughly paralleled those in the homograft. In 16 experiments in which a heavy infiltrate of pyroninophilic cells was found in the liver, “large lymphoid” and other smaller pyronine-positive cells were found actively proliferating around postcapillary venules in the lymph nodes and around small arteries of the spleen, as well as large numbers of plasma cells in the medullary cords and red pulp. The hyperemic lymph nodes had lost their normal follicular architecture.
In 24 experiments in which moderate numbers of pyroninophilic cells were invading the homograft, the degree of proliferation of similar cells in host lymphoid tissue was variable. Fifty-two homografts had mild or no infiltration of the liver, and here the recipient spleen and lymph nodes resembled those described earlier in animals receiving azathioprine alone, in that “large lymphoid” cells and small lymphocytes were either normal or Sewer in number.
Bone marrow changes usually seemed to be related to azathioprine administration. In several animals there was extreme myeloid hypoplasia or even aplasia. There was some evidence of thymic hyperplasia in a few of the dogs that rejected the hepatic homograft after 24 days. The lungs often showed congestion, edema, and intra-alveolar hemorrhage. Bronchopneumonia was a frequent complication. Alveolar wall thickening and sequestration of megakaryocytes were seen. A few of the kidneys showed either small areas of chronic pyelonephritis or scars suggestive of past infection with canine leptospirosis. Necrotic changes in the walls of arteries and arterioles in organs other than the liver were very rare.
DISCUSSION
The present canine studies provide increased hope that orthotopic hepatic homotransplantation may ultimately have use in the treatment of human liver disease. The overwhelming early mortality previously encountered in dogs 14–16, 25, 26, 28 has been substantially reduced. Many long-term survivors were obtained, proving that control of hepatic rejection is not uniquely difficult, and that results can be achieved which are similar to those previously reported for the kidney.2, 3
Instead, the past discouraging experiences seem ascribable to other factors which play an extremely prominent cumulative role. Technical failures are common until considerable experience is acquired. Hemorrhagic gastroenteritis, acute peptic ulceration, and intussusception are frequent. The susceptibility to pneumonitis appeared to be accentuated by the subdiaphragmatic presence of the large homograft. Finally, the hepatotoxicity of azathioprine has a special significance, since the target tissue of rejection is also injured by the drug used to prevent this process.
Because elevations in SGOT, SGPT, and alkaline phosphatase are caused both by azathioprine and by rejection, the serum bilirubin was the most useful test with which to serially study homograft function in the treated canine host. The regularity with which reversal of hepatic rejection could be documented by this means is noteworthy, examples being seen in half of the technically successful experiments. Of great interest is the fact that reversal was not dependent upon intensification of immunosuppressive therapy, since neither increased doses of azathioprine nor additional drugs were used, and in one instance the phenomenon was noted in the absence of all therapy (Fig. 9). It seems probable that rejection of any tissue is characterized by a tendency to remission. Although prednisone,8, 12, 29 and less certainly actinomycin C2 or local irradiation,10 may be of life-saving value in promoting the process after renal homotransplantation, the use of such additional measures is probably not requisite in some instances regardless of the type of homograft used. The spontaneity with which reversal may occur makes caution necessary in attributing this effect to any preceding variation in therapy.
A related problem in evaluating treatment in mongrel animals or man is the extreme variability of host response to the foreign tissue. In the present study, this ranged from complete absence of rejection to uncontrollable repudiation of the liver homograft, a spectrum which undoubtedly conformed to the degree of chance antigen matching between donors and recipients. The presence in outbred populations of this powerful and unpredictable influence makes conclusions about minor therapeutic alterations meaningless unless hundreds or even thousands of experiments are performed.
This criticism applies to the study of adjuvant lipotropic agents described in this communication. I t would not be surprising if these substances were of value after hepatic homotransplantation, since they are said to prevent (or facilitate recovery from) liver injury due to other causes,5, 19 an effect which Schwarz, Stesney, and Foltz21 attribute in part to tracer quantities of selenium in the sulfur-containing amino acids. The additional factor of irradiation was introduced in those animals receiving S35-methionine, although the calculated dose to the liver or whole body was far too small to be of predictable biologic importance. With testing, the lipotropic materials had no measureable influence on azathioprine hepatotoxicity, did not potentiate survival after homo transplantation to the otherwise untreated host, and did not cause a statistically significant prolongation of posttransplant survival when combined with azathioprine. Nevertheless, it is interesting that the longest average survival after homotransplanation to azathioprine-treated recipients was in those 4 groups which also received L-methionine or S35-methionine, a finding which was uninterpretable because the range of results was so great in every group. This may have been due to the system of testing, in which the variables of uncontrolled donor-recipient selection were of far greater importance in the individual experiment than the therapeutic variable being examined.
Although the early recovery after liver homotransplantation has many hazards, chronic convalescence is no more difficult than after the transplantation of less complicated organs. Indeed, the frequency and rapidity with which dogs could be withdrawn from immunosuppression without an ensuing fatal rejection is remarkable. Liver homografts in 5 of the 6 dogs which had therapy stopped after 116 to 123 days have provided life-sustaining function for 63 to 204 subsequent days and the cause of death of the sixth animal was from other causes than progressive hepatic failure. The consistency of this state of host-graft nonreactivity and the rapidity with which it seemed to develop exceeds that reported after canine renal homotransplantation.17, 18, 23, 30 The explanation for this is not apparent, but conceivably the large antigenic mass could play a role or, alternatively, perhaps the liver with its enormous regenerative capacity is simply capable of sustained function in the face of continuing but minimal chronic rejection. Findings in the serial biopsies obtained after discontinuance of therapy do not support the latter hypothesis, but the amount of histologic material available is still too scanty to warrant conclusions. It is also important to note that cessation of therapy was not followed by a graft-host reaction. None of the animals developed “secondary homologous disease” and the shortened red cell half-life noted in the early postoperative period did not recur.
The correlation between liver chemistry determinations and the pathologic findings in the homografts was excellent. In the untreated animal, the abnormalities of function and the anatomic injury were both progressive until death. Administration of azathioprine delayed the onset of rejection in virtually all animals but in most dogs dying within 7 to 15 days the histologic and biochemical alterations were indistinguishable from those of the control experiments. From this time onward, most of the livers appeared to have survived the first assault and were settling down to repair and regeneration; delayed death was commonly due to pneumonitis or other nonhepatic causes. Phagocytic activity of Kupffer cells and macrophages was prominent for several months. The centrilobular localization of the original necrosis seemed responsible for the most conspicuous features of healing, including the centrizonal bile canalicular dilatation and cholestasis, the connective tissue bridges between the central veins, and the regenerating nodules which sometimes contributed to a pseudolobular pattern.
Histologic evidence indicting a vascular component of hepatic rejection was particularly sought in view of previous hypotheses that a reduction in blood flow was immunologically induced at either the sinusoidal level,13, 25 or more proximally in the larger arteries of the portal tracts.14 The findings in the present study are compatible only with the former possibility. There was evidence in some of the electron micrographs of damage to the sinusoidal endothelium following contact with host lymphoid cells, a finding analogous to that reported in renal homografts,11, 20 and one which could plausibly explain the centrilobular localization of the hepatocyte necrosis. The possibility of occlusion of larger vessels is unsupported by the results reported here, since the degree of tissue injury was relatively homogeneous, without areas of selective destruction. Furthermore, injury to the hepatic artery or portal vein was not a prominent feature in the untreated series and was virtually never seen in the treated animals.
An attempt was also made to define those histologic abnormalities in the host which were related to the presence of the homograft, since varying interpretations have been published. From our laboratories1, 25 findings described after homotransplantation to unmodified recipients included lymph node enlargement with cortical thinning, decreased follicles, and increased numbers of lymphocytes and plasma cells. These alterations, as well as histologic abnormalities in the bone marrow, kidneys, and lungs, were thought to be part of a “general host reticuloendothelial response to the antigenic stimulus of the homograft.” McBride and associates13 contended that the host changes (with the possible esception of those in the lymph nodes and spleen) were nonspecific, since in their experience most were also present after autotransplantation.
The findings in the present study support McBride’s conclusion that changes in organs other than the host’s lymphoid tissue are unlikely to be directly related to the response of the host to the homograft. They also, however, provide evidence that a general lymphoid reaction to the hepatic homograft always occurs in the untreated recipient and can be seen in the treated animal when immunosuppression is inadequate. In these circumstances, the spleen and lymph nodes are sites of intense proliferation of large primitive lymphoid cells and later of plasma cells which are contemporaneously found in the homograft. These changes resemble those occurring in the nodes draining a skin homograft22 and in the spleen and lymph nodes after a renal homograft.20
SUMMARY
Without azathioprine therapy, the operative risk with orthotopic liver transplantation is small. Twenty-two of 23 animals survived 2 days or more, and 19 for 6 days or longer. All eventually died of rejection within 10 days. Changes in homograft histology and function were similar to those previously reported, with cellular infiltration and hepatocyte necrosis which was heavily concentrated in the centrilobular areas. In individual experiments, there was little evidence of immunologically induced segmental hepatic arterial or portal venous occlusion; hepatocyte loss was homogeneous, and fibrinoid vascular lesions were uncommon. There was, however, some evidence of damage to the sinusoidal endothelium by adherent mononuclear cells. The changing character of the mononuclear infiltration of the homograft was reflected by widespread proliferation of similar cells in the host lymphoid tissue. Specific changes in other host organs were not noted.
Some of the biochemical and histologic alterations caused by unmodified rejection can also be produced by azathioprine. In 18 nontransplanted dogs, acute rises in SGOT, SGPT, and alkaline phosphatase, unaccompanied by hyperbilirubinemia, were noted within a few days after beginning administration of this agent. Although these abnormalities tended to regress within the 40 day period of observation, more than two thirds of the livers showed histologic evidence of centrilobular hepatocyte damage or necrosis—often with intrahepatic cholestasis, but always without mononuclear cell infiltration. The hepatotoxicity was not prevented by methionine. Weight loss and progressive anemia also occurred. Lymphoid tissue was depleted. The mortality from the toxicity study was 33 percent.
The use of azathioprine to mitigate rejection increased the early mortality after homotransplantation, 32 of 116 dogs dying within the first week (28 percent), most commonly of pulmonary complications. The 84 animals living longer than 7 days had a greatly potentiated homograft survival, exceeding 25 days in 44 dogs, and 50 days in 24. Fifteen animals are still alive from 62 to 324 days postoperatively. Six dogs had all drugs stopped after 116 to 123 days. Only 1 has had a clinically evident late rejection and 5 are still alive from 63 to 204 days later. Three of these animals had repeat biopsies 77 to 182 days after cessation of therapy; one homograft which was normal at 4 months remained so 6 months later, another had an improved histologic appearance, and the third had deteriorated. The longest mean survival was in those animals receiving adjuvant therapy with L-methionine or S35-methionine, but the variability of the results was so great that a statistically significant advantage of these agents could not be demonstrated. Soon after operation red cell survival was decreased, but in chronic survivors there was no evidence of a graft-host reaction.
There was great variability in the vigor of rejection, ranging from the uncontrollable (29 percent) to the clinically undetectable (23 percent). Most of the animals (49 percent) had some biochemical evidence of rejection which proved to be spontaneously reversible, to a greater or lesser degree, since intensification of immunosuppressive therapy was not required. These findings correlate well with the histologic studies. In virtually all animals, azathioprine delayed the onset of rejection but in those dying in the second and third postoperative weeks, the pathologic stigmas of rejection were very similar to the untreated controls. As in the untreated animals, the number of proliferating large pyroninophilic cells in the host’s lymphoid tissues was roughly proportional to the number of mononuclear cells invading the homograft liver. After this time, the predominant histologic features in most animals were those of repair and regeneration, with either absent or relatively minor degrees or continuing destruction. Since the major rejection damage was centrizonal, the healing was most prominent in these areas with interconnecting fibrosis around the central veins, centrilobular bile canalicular dilatation and cholestatis, and pseudolobule formation. In some of the homografts, increased connective tissue was also present in the portal tracts, but in others including the longest survivor there were no residual abnormalities whatever.
In azathioprine-treated animals, damage to the vessels in the homograft portal tracts was found in only one liver. With elect on microscopy there was some evidence of damage to the sinusoidal endothelium by adherent mononuclear cells, a finding which could be analogous to that described by Kountz and co-workers11 in the peritubular capillaries of renal homografts. If immunologically mediated hemodynamic alterations play an important role in liver homograft rejection by interrupting the blood supply to the hepatocytes, it seems most likely that they occur at this intrasinusoidal capillary level rather than in the larger vessels.
Acknowledgments
Aided by Grants AM 06283, AM 06344, HE 07735, AM 07772, AI 04152 and FR 00051 from the United States Public Health Service; and by a grant from the Medical Research Council of Great Britain.
Footnotes
Presented at the Twenty-sixth Annual Meeting of the Society of University Surgeons, Philadelphia, Pa., Feb. 11–13, 1965.
Imuran supplied by Burroughs Wellcome and Company, Inc., Tuckahoe, N. Y.
X-ray spectrographic analysis by Dr. Fred Leaver (Denver Veterans Administration Hospital) revealed a selenium concentration of approximately 150 μg per gram methionine.
References
- 1.Brock DR, Starzl TE. Histopathologic alterations associated with the transplanted homologous dog liver. Exper & Molecular Path. 1962;1:187. doi: 10.1016/0014-4800(62)90020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calne RY, Alexandre GPJ, Murray JE. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann New York Acad Sc. 1962;99:743. doi: 10.1111/j.1749-6632.1962.tb45358.x. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Murray JE. Inhibition of the rejection of renal homografts in dogs by Burroughs Wellcome 57–322, S. Forum. 1961;12:118. [PubMed] [Google Scholar]
- 4.Dameshek S. “Immunoblasts” and “immunocytes”—an attempt at a functional nomenclature. Blood. 1953;21:243. [PubMed] [Google Scholar]
- 5.Drill VA. Hepatotoxic agents: Mechanism of action and dietary interrelationship. Pharmacol Rev. 1952;4:1. [PubMed] [Google Scholar]
- 6.Ebaugh FG, Emerson CP, Ross JF. The use of radioactive chromium51 as an erythrocyte-tagging agent for the determination of red cell survival in vivo. J Clin Invest. 1953;32:1260. doi: 10.1172/JCI102855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagraeus A. Nomenclature of immunologically competent cells. Ciba Foundation Symposium on Cellular Aspects of Immunity; Boston. Little, Brown & Company; 1960. p. 3. [Google Scholar]
- 8.Goodwin WE, Kaufman JJ, Mims MM, Turner RD, Glassock R, Goldman R, Maxwell MM. Human renal transplantation: Clinical experiences with 6 cases of renal homotransplantation. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 9.Jacob HS, MacDonald RA, Jandl JH. The regulation of spleen growth and sequestering function. J Clin Invest. 1962;41:1367. doi: 10.1172/JCI104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman HM, Jr, Cleveland RJ, Dwyer JJ, Lee HM, Hume DM. Prolongation of renal homograft function by local graft radiation. Surg Gynec & Obst. 1965;120:49. [PubMed] [Google Scholar]
- 11.Kountz SL, Williams MA, Williams PL, Kapros C, Dempster WJ. Mechanism of rejection of homotransplanted kidneys. Nature (London) 1963;199:257. doi: 10.1038/199257a0. [DOI] [PubMed] [Google Scholar]
- 12.Marchioro TL, Axtell HK, La Via MF, Waddell WR, Starzl TE. The role of adrenocortical steroids in reversing established homograft rejection. Surgery. 1964;55:412. [PMC free article] [PubMed] [Google Scholar]
- 13.McBride RA, Wheeler HB, Smith LL, Moore FD, Dammin GJ. Homotransplantation of the canine liver as an orthotopic vascularized graft. Am J Path. 1962;41:501. [PMC free article] [PubMed] [Google Scholar]
- 14.Moore FD, Birtch AG, Dagher F, Veith F, Krisher JA, Order SE, Shucart WA, Dammin GJ, Couch NP. Immunosuppression and vascular insufficiency in liver transplantation. Ann New York Acad Sc. 1964;120:729. doi: 10.1111/j.1749-6632.1964.tb34765.x. [DOI] [PubMed] [Google Scholar]
- 15.Moore FD, Smith LL, Burnap TK, Dallenbach FD, Dammin GJ, Gruber VF, Shoemaker WC, Steenburg RW, Ball MR, Belko JS. One-stage homotransplantation of the liver following total hepatectomy in dogs. Transplant Bull. 1959;6:103. doi: 10.1097/00006534-195901000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 17.Murray JE, Ross Sheil AG, Moseley R, Knight P, McGavic JD, Dammin GJ. Analysis of mechanism of immunosuppressive drugs in renal homotransplantation. Ann Surg. 1964;160:449. doi: 10.1097/00000658-196409000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierce JC, Varco RL. Effects of long-term 6-mercaptopurine treatment upon kidney homotransplants in dogs. Surgery. 1963;54:124. [PubMed] [Google Scholar]
- 19.Popper H, Schaffner F. Liver: Structure and function. New York: McGrawHill Book Company, Inc; 1957. p. 500. [Google Scholar]
- 20.Porter KA, Joseph NH, Rendall JM, Stolinski C, Hoehn RJ, Calne RY. The role of lymphocytes in the rejection of canine renal homotransplants. J Lab Invest. 1964;13:1080. [PubMed] [Google Scholar]
- 21.Schwarz K, Stesney JA, Foltz CM. Relation between selenium traces in L-cystine and protection against dietary liver necrosis. Metabolism. 1959;8:88. [PubMed] [Google Scholar]
- 22.Scothorne RJ, MacGregor IA. Cellular changes in lymph nodes following skin homografting in the rabbit. J Anat. 1955;89:283. [PMC free article] [PubMed] [Google Scholar]
- 23.Starzl TE. Philadelphia. W. B. Saunders Company; 1964. Experience in renal transplantation; p. 164. [Google Scholar]
- 24.Starzl TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RU. Reconstructive problems in canine liver homotransplantation with special reference to the post operative role of hepatic venous flow. Surg Gynec & Obst. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Kaupp HA, Brock DR, Linman J, Moss WT. Studies on the rejection of the transplanted homologous dog liver. Surg Gynec & Obst. 1961;112:135. [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Marchioro TL, Huntley RT, Rifkind D, Rowlands DT, Jr, Dickinson TC, Waddell WR. Experimental and clinical homotransplantation of the liver. Ann New York Acad Sc. 1964;120:739. doi: 10.1111/j.1749-6632.1964.tb34766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Marchioro TL, Rifkind D, Holmes JH, Rowlands DT, Jr, Waddell WR. Factors in successful renal transplantation. Surgery. 1964;56:296. [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynec & Obst. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 30.Zukoski CF, Callaway JM. Adult tolerance induced by 6-methyl mercaptopurine to canine renal homograft. Nature. 1963;198:706. doi: 10.1038/198706a0. [DOI] [PubMed] [Google Scholar]