Abstract
Despite the capacity of chaperones and other homeostatic components to restore folding equilibrium, cells appear poorly adapted for chronic oxidative stress that increases in cancer and in metabolic and neurodegenerative diseases. Modulation of endogenous cellular defense mechanisms represents an innovative approach to therapeutic intervention in diseases causing chronic tissue damage, such as in neurodegeneration. This article introduces the concept of hormesis and its applications to the field of neuroprotection. It is argued that the hormetic dose response provides the central underpinning of neuroprotective responses, providing a framework for explaining the common quantitative features of their dose–response relationships, their mechanistic foundations, and their relationship to the concept of biological plasticity, as well as providing a key insight for improving the accuracy of the therapeutic dose of pharmaceutical agents within the highly heterogeneous human population. This article describes in mechanistic detail how hormetic dose responses are mediated for endogenous cellular defense pathways, including sirtuin and Nrf2 and related pathways that integrate adaptive stress responses in the prevention of neurodegenerative diseases. Particular attention is given to the emerging role of nitric oxide, carbon monoxide, and hydrogen sulfide gases in hormetic-based neuroprotection and their relationship to membrane radical dynamics and mitochondrial redox signaling. Antioxid. Redox Signal. 13, 1763–1811.
Proteotoxicity, Cellular Stress Response, and the Vitagene Network
Sirtuins and the Integration of Adaptive Stress Responses in Neurons
The Kelch-Like ECH-Associated Protein 1/Nrf2/Antioxidant Response Element Pathway
Adaptive ER Stress Responses: Calcium and Protein Chaperones
-
Neuro Gas Biology and the Roles of CO, NO, and Hydrogen Sulfide in Brain Physiopathology
I. Introduction
The adaptation and survival of cells and organisms requires the ability to sense proteotoxic insults and to coordinate protective cellular stress response pathways and chaperone networks related to protein quality control (310). The toxic effects that stem from the misassembly or aggregation of proteins or peptides, in any cell type, are collectively termed proteotoxicity, whereas neurotoxicity is a term that refers to general toxic effects observed in neurons. To understand the relevance of proteotoxicity to neurotoxicity and to neurodegeneration is important, a comprehensive understanding of the mechanisms that lead to the development of neurodegenerative diseases. To date, these mechanisms are poorly understood; however, it is clear that protein aggregation is tightly linked to the emergence and development of neurodegenerative diseases. Molecular chaperones are known to disrupt aggregates and this represents the basis of the therapeutic potential of heat shock proteins (Hsps), which prevent protein misfolding and aggregation. However, despite the abundance and apparent capacity of chaperones and other components of homeostasis to restore folding equilibrium, the cell appears poorly adapted for chronic proteotoxic stress that increases in cancer and in metabolic and neurodegenerative diseases (311). In these conditions, a decline in biosynthetic and repair activities that compromises the integrity of the proteome is strongly influenced by protective genes called vitagenes that control aging, thus linking stress and protein homeostasis with the health and life span of the organism (99, 100). The disruption of protein folding quality control results in the accumulation of a nonnative protein species that can form oligomers, aggregates, and inclusions indicative of neurodegenerative disease (312). Pharmacological modulation of cellular stress response pathways has emerging implications for the treatment of human diseases, including neurodegenerative disorders, cardiovascular disease, and cancer (102).
A critical key to successful medical intervention is getting the dose right. Achieving this goal can be extremely challenging due to human interindividual variation as affected by age, gender, diet, exercise, genetic factors, and health status. Getting the dose right can also be affected by exposure to other drugs as well as factors such as circadian rhythms. For instance, it has been shown that exercise promotes longevity and ameliorates type 2 diabetes mellitus and insulin resistance. However, exercise also increases mitochondrial formation of presumably harmful reactive oxygen species (ROS). Consistent with the concept of mitohormesis, exercise-induced oxidative stress ameliorates insulin resistance and causes an adaptive response promoting endogenous antioxidant defense capacity. Importantly, recent finding indicates that supplementation with antioxidants may preclude these health-promoting effects of exercise in humans (372). Another issue that can affect clinical success is the nature of the dose response in and adjacent to the therapeutic zone(58,60). Over the past decade considerable advances have been made in the understanding of the nature of the dose response, especially in the low-dose zone (88, 116, 204, 280, 291, 292, 398). These findings challenge previous concepts of the dose response, suggesting that the commonly accepted threshold and linear dose–response models upon which society has based its toxicological and pharmacological predications for drug and chemical effects often fail to provide reliable estimates of responses in the low-dose zone, that is, below the threshold (55, 61, 69–71, 90, 91). Failure to accurately predict the effects of drugs and chemicals can lead to failed clinical trials, inadequate patient care, and potentially harmful governmental regulations (40, 73, 135, 143). While traditional dose–response models have failed to accurately predict responses in the low-dose zone in large-scale studies, the hormetic dose response performed extremely well, lending considerable support to other studies (56, 57, 65, 66, 73–86, 92) that have suggested that the process of drug development and chemical hazard/risk assessment could be improved in significant ways by a consideration of the hormetic dose response in the design, execution, and analysis of toxicological and pharmacological investigations. Consequently, this article will introduce the hormetic dose–response concept, including its scientific foundations, toxicological and pharmacological implications, and its applications to the field of neuroprotection and their mechanistic foundations.
II. Hormesis
Hormesis is a dose–response phenomenon characterized by a low-dose stimulation and a high-dose inhibition (Fig. 1). It may be graphically represented by either an inverted U-shaped dose response or by a J- or U-shaped dose response. The term hormesis was first presented in the published literature in 1943 by Southam and Ehrlich, who reported that low doses of extracts from the red cider tree enhanced the proliferation of fungi with the overall shape of the dose response being biphasic. However, credit for experimentally demonstrating the occurrence of hormesis goes to Hugo Schulz (396), who reported biphasic dose responses in yeast after exposure to a large number of toxic agents. The work of Schulz inspired a large number of investigators in diverse fields to assess whether such low-dose effects may be a general feature of biological systems. In fact, similar types of dose–response observations were subsequently reported by numerous researchers assessing chemicals (49) and radiation (41, 50–53, 246, 307, 313, 367, 381, 397, 431,432) with investigators adopting different names such as the Arndt-Schulz Law, Huppe's Rule, and other terms to describe these similar dose–response phenomena (368). Despite the rather substantial historical literature concerning hormetic dose responses, this concept had a difficult time being incorporated into routine safety assessment and pharmacological investigations, principally because it (a) required more rigorous evaluation in the low-dose zone, (b) failure of investigators to understand its clinical significance, (c) failure to appreciate the quantitative features of the hormetic dose response, (d) failure to understand the limitations of its implications for commercial applications in agriculture as well as medicine, (e) because of the predominant interest in responses at relatively high doses during most of the 20th century, and (f ) the continuing, yet inappropriate, tendency to associate the concept of hormesis with the medical practice of homeopathy (64, 89, 91). However, from the late 1970s (423, 433) there has been a growing interest in hormetic-like biphasic dose responses across the broad spectrum of biomedical sciences. This resurgence of interest resulted from a variety of factors, including the capacity to measure progressively lower doses of drugs and chemicals, the adoption of cell culture methods, which has permitted more efficient testing of numerous doses and the need to reexamine the validity of linearity at low-dose modeling of cancer risks due to their enormous cost implications for regulations (379), as well the astute observations of independent investigators and their capacity to generalize their findings across biological systems (267, 423).
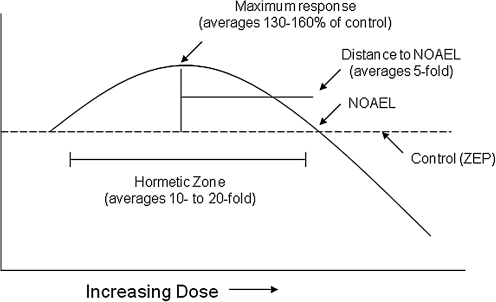
FIG. 1.
Dose–response curve depicting the quantitative features of hormesis. NOAEL, No Observed Adverse Effect Level; ZEP, zero equivalent point.
What has emerged from these research initiatives from highly diverse biomedical areas is the recognition that hormetic dose responses were common and highly generalizable, being independent of biological model, endpoints measured, and chemical class and/or physical agent studied (50–54, 68, 306, 448). This was an unexpected finding as hormetic responses were often considered by many in the so-called mainstream branches of toxicology and pharmacology to be paradoxical, not commonly expected and being of questionable reliability with a lack of capacity for replication. The casual dismissal of the hormesis concept during the mid decades of the last century is reflected in the general absence of the hormesis concept from the leading toxicological and biomedical textbooks. This situation has radically changed such that hormesis is now incorporated into all leading textbooks of toxicology (e.g., ref. 167) encyclopedias (89, 94) and other leading monographs. In fact, while the terms hormetic and hormesis were cited only about 160 times during the entire decade of the 1980s within the Web of Science database, in 2008 alone these terms were cited nearly 2300 times.
Of further significance were observations that these broad-ranging dose–response relationships also shared the same general quantitative features. More specifically, the low-dose stimulation that becomes manifested immediately below the pharmacological and toxicological thresholds is modest in magnitude, being at most only about 30%–60% greater than the control group response. The width of the hormetic stimulation is usually about 10–20-fold starting immediately from the zero equivalent dose (i.e., estimated threshold) (Fig. 1). The hormetic dose response may result from either a direct stimulation or via an overcompensation stimulatory response after disruption in homeostasis (49, 50). Regardless of the mode of action by which the stimulation occurs, the quantitative features of hormetic dose responses are similar. These observations are based on copious data derived from the published literature ranging from plants to humans (68, 93), involving numerous receptor systems (61–64). These findings have recently led to nearly 60 biomedical scientists recommending that biological stress responses, including those of pre- and postconditioning, be integrated within an hormetic context, along with the adoption of a terminology that would be based within an interdisciplinary framework (72).
The hormetic dose response confers a new set of interpretations for the dose response. At high doses within a toxicological setting, the typical endpoints measured indicate cellular damage. However, as the dose decreases below the threshold, the low-dose stimulation more likely represents a manifestation of an adaptive response that conforms to a measure of biological performance as may be seen in the cases of modest increases in cognition, growth, longevity, bone density, and other biomedical endpoints of interest (424).
The consistency of the vast array of hormetic findings suggests strongly that this dose response may be a manifestation of the plasticity of biological systems. That essentially all biological models respond to imposed stress with the same quantitative features of the dose response is a central finding within the biological sciences that has not been previously recognized. These findings suggest that the hormetic dose response would have been broadly selected for and highly conserved. This adaptive response not only enhances survival by conferring resistance to environmental stress, but also represents a way to regulate the allocation of biological resources in a manner that ensures cellular and organismal stability. The consistency of the hormetic dose response across the vast array of biological models, at different levels of biological organization, regardless of the type of stress and health status of the individual, indicates that hormesis provides a quantitative index of biological plasticity across multiple levels of biological organization, making it a central biological element for enhancing survival.
These quantitative features of the hormetic dose response have important medical implications. Most significantly, the hormetic dose response imposes constraints upon the magnitude of a drug to induce a desired effect. For example, if a drug increased cognitive performance in an elderly patient by approximately 25%–30%, the hormetic model suggests that this level of performance could not be further increased using a new drug combination. This concept has been supported in a variety of studies on hormesis and drug interaction. Flood (173–176) has demonstrated that the hormetic response for memory was bounded by the 30%–60% increase even when several drugs were used in combination that were designed to maximize memory outcome. This response magnitude constraint has been reported for immune stimulation, bacterial growth, increases in hair growth, plant growth, decrease in anxiety, decreases in tumor incidence, and numerous other endpoints (73).
This limitation in the magnitude of the stimulatory response is a critical implication of the hormesis dose–response concept. It is an observation that is based on extensive findings, and it is a controlling feature that defines what pharmaceutical companies can expect to achieve with drugs that are designed to enhance performance. However, the limitation in the magnitude of response is also potentially important with respect to the capacity to detect a desirable response. This may not be a particularly important issue when using highly inbred animal models or cell cultures where experimental conditions can be highly controlled. However, attempting to measure a low-dose hormetic stimulation within the context of a clinical trial can be problematic. Given the likelihood of considerable human variation in response to a drug, it is possible that the test population may have their responsiveness distributed over a range of responses that includes toxicity, optimal response, and a group in which the dose is ineffective. The data from all subjects in such studies would normally be averaged together leading to a marked dilution of an overall positive treatment effect in the optimal response zone subgroup. This suggests a possible reason why drugs that were very successfully tested in preclinical studies with highly inbred strains of animals could and often have failed during the clinical trial. Of particular note is that investigators may have to modify doses based upon the sensitivity or susceptibility of the subjects. Calabrese and Baldwin (59) have shown that the hormetic dose response is often expressed in the broad range of subjects independent of their susceptibility. As expected, those individuals who are very resistant to the drug or chemical treatment would have their hormetic response shifted to the right on the dose–response graph, whereas those individuals with greater-than-normal susceptibility would have their hormetic response shifted to the left. The hormetic dose response therefore imposes considerable challenges to the biomedical community that is interested in the development of drugs that are concerned with improvements in human performance.
The hormetic dose response can also have undesirable effects. This may be most readily seen in the case of drugs that are designed to suppress growth or kill cells or organisms at higher doses. For example, there is now substantial evidence that low doses of many antitumor drugs can stimulate the proliferation of such cells at lower concentrations (67). This also been shown to be the case with antibiotics, including penicillin (366) and streptomycin (366, 465). This phenomenon has also been reported with selected cardiac glycosides that have effects on nontarget tissues such as the prostate, where it is able to enhance the proliferation of smooth muscle cells by about 30% with clinically relevant doses (1, 127). Such a 30% increase in prostate smooth muscle was considered likely to impede urination in males. The failure to consider the possibility of the hormetic response not only can lead to a lack of recognition of a desirable drug-induced response but also can result in failure to prevent an adverse effect of drug treatment.
Since hormetic dose responses have now been widely reported in biological systems, there is the desire to develop a new subfield of hormetic mimetics. These mimetics are agents that can activate hormetic pathways with the intention of producing a desirable clinical effect. For example, it would be desirable to develop agents that can induce the desirable adaptive hormetic response without having risks associated with the exposures as in the case of certain chemical agents and radiation.
In the ensuing sections of this article we consider several of the major cellular and molecular systems that mediate adaptive stress responses/hormesis, with a focus on the nervous system in health and disease.
III. Membrane Radical Dynamics
To elucidate the roles of hormesis in protecting cells and organs against disease, it is important to understand the nature of the potentially damaging stresses to which cells are subjected. While our focus in this article is on neurodegenerative conditions, many of the mechanisms we describe are also operative in disorders of other organ systems. The plasma membrane serves as the proximate sensor of extrinsic stressors. Membranes serve vital functions in all cells, not only by maintaining the ionic and molecular (proteins, nucleic acids, etc.) compositions of subcellular compartments, but also by serving as signal transduction platforms for a range of extracellular (hormones, growth factors, neurotransmitters, lipid messengers, and others) and intracellular (inositol phospholipids, kinases and their substrates, etc.) signaling molecules. Lipids that comprise membranes (phospholipids, cholesterol, sphingomyelins, and others) and the various proteins associated with membranes (receptors, ion channels, cell adhesion proteins, and others) encounter a range of ROS including hydrogen peroxide, hydroxyl radical, superoxide, nitric oxide (NO), and others. Excessive attack by free radicals can impair fundamental functions of membranes, resulting in cell damage and death. Such oxidative damage to membranes is believed to play major roles in a range of disorders, including myocardial infarction (172), stroke and traumatic brain injury (254), and Alzheimer's disease (AD) (286). However, as described below, lower amounts of membrane-associated oxidative stress may activate adaptive stress response pathways.
IV. Lipid Peroxidation and Sphingomyelin Metabolism
The unsaturated (double) bonds in the fatty acid chains of the membrane lipid bilayer are prone to attack by free radicals, particularly hydroxyl radical and peroxynitrite (282, 459). Hydroxyl radical is formed from hydrogen peroxide in the Fenton reaction that is catalyzed by Fe2+ or Cu+, whereas peroxynitrite is generated when superoxide interacts with NO (150). Both hydroxyl and peroxynitrite radicals can abstract a hydrogen atom from a fatty acid, quenching the original radical but generating a lipid radical that interacts with oxygen to form a lipid peroxyl radical. The peroxyl radical can then interact with neighboring fatty acids, thereby setting in motion a self-propagating lipid peroxidation reaction. Lipid peroxidation can occur in any cellular membranes (mitochondria, endoplasmic reticulum [ER], nucleus, etc.), but for the purposes of the present article we will focus on the plasma membrane. Lipid peroxidation generates lipid cleavage products, including aldehydes of various chain lengths and isoprostanes. Among the products of lipid peroxidation, 4-hydroxynonenal (HNE) has received considerable attention for its possible contributions to the pathogenesis of several major disorders, including cardiovascular, renal, and neurodegenerative diseases (226, 252, 411). For example, evidence from studies of experimental models of Alzheimer's disease has shown that HNE can impair the function of ion-motive ATPases (sodium and calcium pumps) (277), glucose transporters (279), and GTP-binding proteins coupled to muscarinic acetylcholine receptors (37) in neurons.
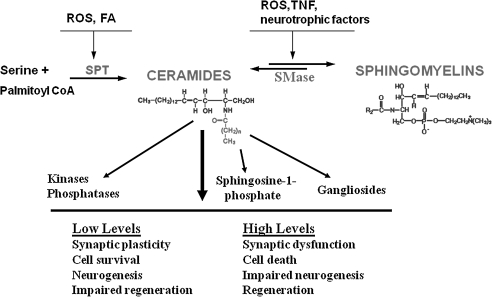
In addition to lipid peroxidation, membrane-associated oxidative stress can activate sphingomyelinases (basic and acidic sphingomyelinases), which cleave sphingomyelin to generate ceramides (140) (Fig. 2). Sphingomyelinases are also activated by tumor necrosis factor (TNF) and some growth factors. Ceramides can activate signaling pathways involved in the regulation of cell growth and survival. However, excessively high amounts of ceramide can trigger a form of programmed cell death called apoptosis (415). Ceramides generated in response to membrane-associated oxidative stress are implicated in the dysfunction and death of cells in a range of disorders, including diabetes (428), emphysema (352), ischemic stroke (476), Alzheimer's disease (141), and amyotrophic lateral sclerosis (142).
FIG. 2.
Membrane sphingomyelin and ceramide metabolism pathways involved in adaptive stress responses and neurodegenerative conditions. Modified from Cutler et al. (142). FA, fatty acids; ROS, reactive oxygen species; SMase, sphingomyelinase; SPT, serine palmitoyl CoA transferase; TNF, tumor necrosis factor.
V. Plasma Membrane Redox System
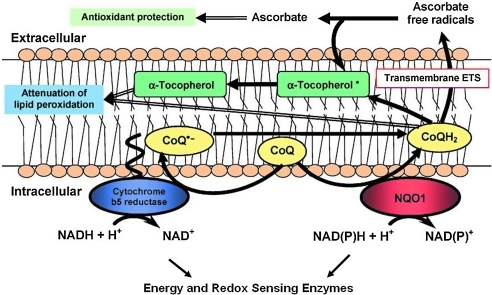
An understudied redox system of enzymes is associated with the plasma membrane where it is involved in modulating levels of oxidative stress (210). The plasma membrane redox system (PMRS) includes the following enzymes reduced nicotinamide adenine dinucleotide (Fig. 3): (a) reduced nicotinamide adenine dinucleotide (NADH)-ascorbate free radical reductase; (b) NAD(P)H-quinone oxidoreductase 1 (NQO1), (c) NADH-ferrocyanide reductase; (d) NADH-coenzyme Q10 reductase; and (e) NADH-cytochrome c reductase. In addition, the lipophilic antioxidants α-tocopherol and coenzyme Q10 are involved. The PMRS is therefore similar to the redox system of the inner mitochondrial membrane, which is involved in electron transport and energy metabolism. The PMRS plays an important role in the response of cells to membrane-associated oxidative stress by transferring electrons from reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H] and ascorbate to extracellular free radicals/oxidants. Within the membrane, coenzyme Q10 (CoQ) can be reduced by either NQO1 or NADH-cytochrome b5 reductase. Dietary CoQ is important for the maintenance of sufficient levels of reduced CoQ and α-tocopherol in the plasma membrane, thereby protecting against lipid peroxidation. Expression and activity of the PMRS enzymes can be altered in response to physiological challenges. For example, activity and protein levels of NADH-ascorbate free radical reductase; NQO1, NADH-ferrocyanide reductase; and NADH-coenzyme Q10 reductase are decreased in brain cells during normal aging and are increased in response to dietary energy restriction (211). The PMRS is therefore ideally poised to mediated hormetic responses of neurons and other cells to a range of stimuli that increase oxidative stress.
FIG. 3.
The plasma membrane redox system is a conserved sensor of cellular redox status and energy metabolism. Modified from Hyun et al. (210). CoQ, coenzyme Q10; ETS, electron transport system; NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; NAD(P)H, reduced nicotinamide adenine dinucleotide phosphate; NQO1, NAD(P)H quinone oxidoreductase 1.
VI. Membrane-Related Hormesis Mechanisms
Evidence is emerging to support hormetic roles for low and transient increases in membrane oxidative stress. Levels of membrane lipid peroxidation are relatively low under most normal conditions. However, in some types of cells, lipid peroxidation increases considerably during periods of increased energy demand. For example, during vigorous physical exercise there is a marked increase in production of superoxide and hydrogen peroxide, hydroxyl radical, peroxynitrite, and lipid peroxidation (377). Evidence suggests that free radicals and products of lipid peroxidation generated during moderate exercise play important roles in hormetic effects of exercise on muscles, including changes in energy metabolism pathways, mitochondrial biogenesis, and upregulation of protein chaperones and antioxidant systems (377). Benefits of exercise on the cardiovascular system may also involve membrane oxidative stress-related mechanisms. Thus, it was reported that HNE activates nuclear factor erythroid 2-related factor 2 (Nrf2) and antioxidant gene expression in vascular cells (412). HNE may also activate other adaptive stress response pathways that promote the survival and plasticity of cells (349).
Ceramide is also believed to mediate hormetic effects of moderate/transient increases in membrane-associated oxidative stress. For example, pretreatment of neurons with subtoxic concentrations of ceramide results in increased resistance of the neurons to subsequent high levels of oxidative stress (191). Other studies have provided evidence for a pivotal role for ceramide in the cardioprotective effect of preconditioning ischemia in animal models of myocardial infarction (16, 149). Preconditioning ischemia is a classic example of hormesis, wherein exposure of cells to a moderate transient stress protects them against more severe stresses. Changes in the PMRS in response to stress may also allow cells to adapt to potentially damaging conditions. A dramatic example comes from a study in which the mitochondria of cells were rendered dysfunctional, and the cells were able to survive because of a compensatory upregulation of PMRS enzyme activities (212).
VII. Mitochondria: A Hub of Cellular Redox Processes
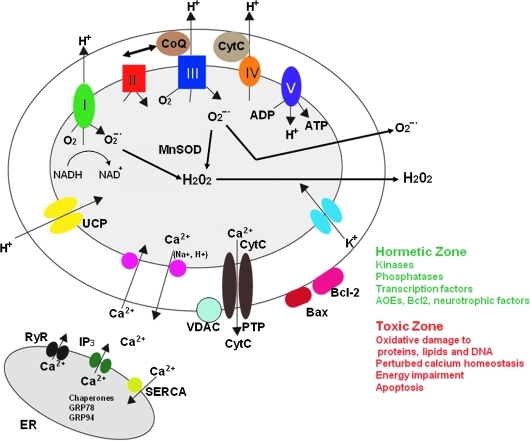
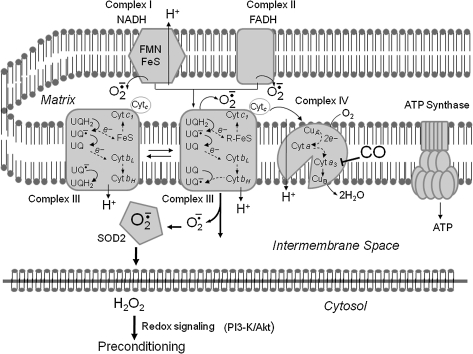
Mitochondria are the major source of energy (ATP and oxidized nicotinamide adenine dinucleotide [NAD+]) production in eukaryotic cells, and produce correspondingly large amounts of superoxide anion radical through abstraction of an electron from oxygen in the so-called electron transport chain at the inner mitochondrial membrane (291). Mitochondria may produce superoxide in relatively constant amounts, or may elicit spontaneous or environmentally induced superoxide flashes (462). Much of the superoxide produced is efficiently converted to hydrogen peroxide via the activity of superoxide dismutases, including cytoplasmic (SOD1) and mitochondrial (SOD2) forms of the enzyme. Being a highly reactive free radical, superoxide can damage molecules (DNA, proteins, and lipids), and so its conversion to hydrogen peroxide protects cells. However, in the presence of even very low concentrations of Fe2+ or Cu+, hydrogen peroxide can generate hydroxyl radical, which is a potent inducer of membrane lipid peroxidation (Fig. 3). In addition, NO (which is generated in response to activation of the enzyme NO synthase by Ca2+/calmodulin) can interact with mitochondrial superoxide to generate the highly reactive free radical peroxynitrite. Methods have been developed to detect and quantify oxidative damage to proteins, lipids, and DNA, with antibodies that selectively recognize proteins modified by the lipid peroxidation product 4-hydroxynonenal (37, 242, 279) and by nitration (124, 220) being particularly useful. In addition, fluorescent probes for imaging relative levels of overall mitochondrial redox status and superoxide have been used to elucidate roles for mitochondrial ROS in a range of physiological and pathological processes (178, 243, 261, 462). The damaging effects of excessive production of ROS are believed to contribute to a wide range of diseases, including cancers, cardiovascular disease, and inflammatory conditions such as arthritis (2, 250, 296). Neurons may be particularly vulnerable to mitochondrial ROS because of their high energy demands and their excitability, and because they are postmitotic and are therefore in most cases irreplaceable (284, 291). However, lower subtoxic levels of mitochondrial ROS can activate signaling pathways that protect cells against injury and disease.
VIII. Hormesis, Mitochondria, and Neuroprotection
Recent findings have overturned the long-held belief that mitochondrial ROS have only a negative impact on cell function and survival. It is now clear that mitochondrial superoxide and hydrogen peroxide play important roles in a range of cellular functions, and can also activate signaling pathways that promote cell survival and disease resistance. Exposure of hippocampal neurons to subtoxic levels of hydrogen peroxide triggers the release of Ca2+ from the ER by causing the opening of both IP3 and ryanodine receptor channels (184). Interestingly, superoxide enhances long-term potentiation of synaptic transmission in hippocampal CA1 neurons by a mechanism requiring activation of ryanodine receptors and extracellular regulated kinases (207). Mitochondrial ROS may also play roles in recovery from injury. For example, superoxide can stimulate neurite outgrowth by directly activating protein kinase C (192). Moreover, peroxynitrite can promote the phosphorylation and nitration of regulatory sites within receptor tyrosine kinases, thereby activating cell survival signaling pathways, including those involving phosphatidylinositol 3-kinase (PI3K)–protein kinase B (Akt) and mitogen-activated protein (MAP) kinases (38). Nitration of proteins involved in synaptic vesicle trafficking can enhance glutamate release, suggesting a potential role for superoxide and peroxynitrite in regulating neurotransmission (151).
Mitochondrial superoxide production is believed to contribute to damage of neurons in conditions ranging from chronic intermittent cerebral hypoxia (402) to Alzheimer's disease (225). However, it has been widely reported that transient exposure of neurons to low levels of superoxide that are converted into hydrogen peroxide can protect the neurons against a subsequent exposure to what would have otherwise been a lethal level of stress. This neuroprotective effect of a subtoxic increase in cellular oxidative stress has been termed “preconditioning” by neuroscientists who study stroke (162), but clearly falls under the broad umbrella of hormesis (72). Although the involvement of oxidants in many signaling pathways is well documented, the cellular strategies for conferring pathway specificity to such reactive molecules have remained more recondite. Recent studies now suggest that cells may spatially restrict oxidant production to allow microdomain-specific signaling (446). The specific molecular mechanisms by which mitochondrial ROS elicit hormetic responses in neurons are poorly understood, but emerging evidence suggests important roles for certain transcriptional regulators. For example, we showed that the transcription factor NFκB is activated in neurons in response to oxidative stress and plays a pivotal role in the adaptive response that protects the neurons against more severe oxidative stress [see ref. (288) for review]. NFκB induces expression of genes encoding several proteins that protect mitochondria against oxidative stress, including SOD2 and Bcl-2 (46, 115, 281). We also found that, contrary to the view that TNF exerts only detrimental effects on neurons, TNF upregulates expression of Mn-SOD via an NFκB-mediated mechanism and can thereby protect neurons against excitotoxic, ischemic, and oxidative injury (45, 47).
While mitochondrial H2O2 may activate adaptive stress response pathways in neurons, they may also play neuroprotective roles by acting on other cell types in the nervous system. For example, microglial activation, which occurs in response to increased oxidative stress, can have either beneficial or detrimental effects on neurons and neural progenitor cells depending upon the type and amounts of cytokines and growth factors secreted by the microglia (168). A final example of trans-cellular hormesis mediated by ROS comes from studies showing that oxidative stress can stimulate angiogenesis in the brain (217, 295), a process that could be very important in restoring blood flow to neurons during the days and weeks after a stroke.
IX. Proteotoxicity, Cellular Stress Response, and the Vitagene Network
Protein thiols play a key role in redox sensing, and regulation of cellular redox state is a crucial mediator of multiple metabolic signaling and transcriptional processes (97, 108). More in general, protein quality control, which is a critical feature of intracellular homeostasis (100), is maintained by a highly complex network of molecular interactions that balances protein biosynthesis, folding, translocation, assembly/disassembly, and clearance (98, 312). The ability to ensure proper protein folding is critical for cellular function and organismal viability. In cells undergoing division, damaged and oxidized proteins can be sequestered and retained in mother cells, enabling daughter cells to have a pristine, undamaged proteome. However, in postmitotic cells, such as most neurons, protein quality control must be maintained by other, possibly more complex, mechanisms. Further, particular neuronal populations are more vulnerable to proteotoxicity, whereas others are more able to tolerate the misfolding and aggregation of disease proteins. Thus, the cellular environment must play a significant role in determining whether disease proteins are converted into toxic or benign forms. The endomembrane neuronal network divides brain cells into different subcellular compartments that possess distinct sets of molecular chaperones and protein interaction networks (164). Cells buffer proteotoxic events related to intracellular protein misfolding via chaperone-mediated partitioning of nonnative conformers between pathways for proper folding, inclusion body formation, and degradation. Chaperones, in fact, act as agonists and antagonists of disease protein aggregation to prevent the accumulation of toxic intermediates in the aggregation pathway. Interacting partners can also modulate the conformation and localization of disease proteins and thereby influence proteotoxicity. Thus, interplay between these protein homeostasis network components can modulate the self-association of disease proteins and determine whether they elicit a toxic or benign outcome (164, 203). Polypeptides that fail to fold properly, along with damaged and oxidized mature proteins, are targeted for degradation by specialized cellular degradation machineries. A failure to prevent the misfolding and aggregation of one protein can destabilize the proteome, resulting in uncontrolled aggregation of other polypeptides. When conformationally challenged aggregation-prone proteins are expressed, the resulting unfolded or misfolded proteins are rapidly degraded via the ubiquitin–proteasome pathway. However, in some cases, protein aggregation leads to the development of the so-called conformational diseases. Among the conformational diseases are the human neurodegenerative diseases, such as Alzheimer's (AD), Parkinson's (PD), and Huntington's (HD). In AD, dual digestion of amyloid precursor protein (APP) releases the aggregation-prone peptides that are collectively termed amyloid-β (Aβ). The accumulation and aggregation of Aβ (particularly the highly aggregative Aβ1–42) is associated with AD. Similarly, aberrant aggregation of α-synuclein is associated with the emergence of PD, whereas Huntington and other CAG triplet diseases are typified by the accumulation of polyglutamine-containing aggregates. This also includes prion diseases such as Creutzfeldt–Jakob disease with accumulation of misfolded prion protein, type II diabetes with accumulation of islet amyloid polypeptide, and amyotrophic lateral sclerosis with aggregated superoxide dismutase-1 (186). One of the most characteristic features present in most brain oxidant disorders is the occurrence of extra- or intracellular fibrillar aggregates containing misfolded proteins with beta-sheet conformation. These aggregates are composed of distinct proteins in each neurodegenerative disease. As already mentioned, toxicity deriving from misfolded proteins or peptides are collectively termed proteotoxicity, and it has become evident that protein aggregation is tightly linked to the emergence and development of neurodegenerative diseases. Moreover, the mechanisms that have been found to counter toxic protein aggregation in different cell types and different organisms are highly conserved. Thus, it is likely that proteotoxicity that is associated the expression of neurodegeneration-linked aggregation-prone proteins in any tissue can provide insights into the neuronal defence mechanisms. Many of the insights that have been obtained from invertebrate-based or nonneuronal studies await confirmation in mammalian neuronal systems and clinical observations (132). Recent data suggest that small oligomeric aggregating structures, termed also protofibrils, are the underlying cause, rather than high-molecular-mass aggregates as previously assumed. In vitro studies on fragments of amyloidogenic proteins and synthetic peptides have established that the tendency for a protein to form amyloid is often limited to a short sequence of the full protein, known as an SRE (selfrecognition element). SREs form the core of amyloid fibrils. These amyloidogenic sequences constitute hotspots for aggregation of the native protein into amyloid fibrils and are often the sites of mutations leading to early-onset amyloidosis. In the case of tau-paired helical filaments, which accumulate in the neurofibrillary tangles characteristic of AD and other neurodegenerative diseases, it has been shown that only three residues (Val-Tyr-Lys) are sufficient for fibril formation. Similarly, short sequences forming the core domain of various amyloid fibrils have been identified for Aβ (amyloid β-peptide), calcitonin, IAPP (islet amyloid polypeptide), insulin, and α-synuclein. It may be possible to delete residues freely on either side of an SRE while retaining the ability to form amyloid (11). Genetic and age-related factors, as it is known, act as vicious cycle increasing the amounts of pathogenic proteins in AD, where the increase in Aβ42 levels is caused by (a) mutations in amyloid precursor protein or presenilins (e.g., γ-secretase), (b) by reactive oxygen species, and (c) by reductions in Aβ-degrading enzymes (AβDE), such as neprilysin and insulin-degrading enzyme, as well as increases in tau concentrations are influenced by oxidant damage, phosphorylation, and calcium (278); in PD, where increased levels of α-synuclein caused by triplication of its gene or mutations in parkin, DJ1, ubiquitin carboxy-terminal hydrolase 1 (UCHL1), phosphatase and tensin homolog induced kinase 1 (PINK1), or leucine-rich repeat kinase 2 (LRKK2) are associated with proteasome impairment and oxidative stress; and in HD, with polyglutamine expansions in huntingtin (HTT). The protein aggregation process itself is enhanced by increasing protein concentration; the action of transglutaminases; protein chaperone insufficiency; mutations in α-synuclein (PD) and polyglutamine expansions in huntingtin (HD); and/or posttranslational modifications, such as oxidations induced by, for example, hydrogen peroxide (H2O2), Fe2+, and Cu+, and phosphorylation. Although the proteins involved can differ, there is considerable overlap in the mechanisms by which they damage and kill neurons. Oligomers of Aβ, α-synuclein, and HTT might damage and kill neurons by inducing membrane-associated oxidative stress (MAOS), thereby impairing mitochondrial function and thus causing degenerative cell death (287).
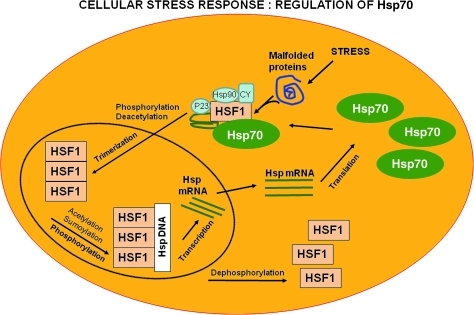
Cellular stress response is the ability of a cell to counteract stressful conditions (Fig. 4). This phenomenon, which includes heat shock response (HSR), represents an ancient and highly conserved cytoprotective mechanism (270, 312, 416). Production of Hsps, including protein chaperones, is essential for the folding and repair of damaged proteins, serving thus to promote cell survival conditions that would otherwise result in apoptosis (20). The term “molecular chaperone” denotes a large family of ubiquitous proteins that function as part of an ancient defense system in our cells. Chaperones promote cell survival by sequestering damaged proteins and preventing their aggregation. During stressful conditions, such as elevated temperature, they prevent protein aggregation by facilitating the refolding or elimination of misfolded proteins. The stress-induced response to damaged proteins is helped by a sophisticated regulatory system, which shuts down most cellular functions and, in parallel, induces the synthesis of several chaperones and other survival-promoting proteins. Therefore, many of the chaperones are also called stress or heat shock proteins in reference to the archetype of cellular stress, heat shock. Besides their role during stress, chaperones have multiple roles under normal conditions. They promote the transport of macromolecules (e.g., proteins and RNA) and participate in remodeling events involving larger protein complexes, including signaling, transcription, cell division, migration, and differentiation. Molecular chaperones both in the cytosol (heat-shock proteins, crystallins, prefoldin, and Hsc70) and in the ER (Bip, Grp94, calnexin, and calreticulin) form large complexes and have a large number of cochaperones to regulate their activity, binding properties, and function (380). These chaperone complexes regulate local protein networks, such as the mitochondrial protein transport apparatus and the assembly and substrate specificity of the major cytoplasmic proteolytic system, the proteasome (380). For instance, mood and anxiety disorders are considered stress-related diseases characterized by an impaired function of mineralocorticoid and glucocorticoid receptors (MR and GR, respectively), the major regulatory elements of the hypothalamus–pituitary–adrenocortical (HPA) axis. Hence, a number of chaperone proteins moderate the function of these receptors. Genetic variations in one of these chaperones, FKBP5, have been associated with antidepressant treatment response and with a major risk factor for the development of posttraumatic stress disorder (395). Chaperones are well known to protect the cell nucleus after stress. Consistent with this, Hsp70 was shown to drive damaged nuclear proteins to the nucleolus, clearing other nuclear components of misfolded proteins and decreasing the danger of their widespread aggregation (139). In agreement with these findings, chaperones promote the transport of ribosomal subunits and the mobility of steroid receptors inside the nucleus (139). Molecular chaperones regulate both the activation and the disassembly of numerous transcriptional complexes (380). Thus, chaperones emerge as regulators of the transcriptional network (139, 272). Stress-induced nuclear translocation of chaperones may preserve nuclear remodeling capacity during environmental damage, and thus protect the integrity of DNA. Consistently, there is significant interest in the discovery and development of small molecules that modulate HSRs and parallel stress response pathways for therapeutic purposes (99, 106, 108, 309, 452).
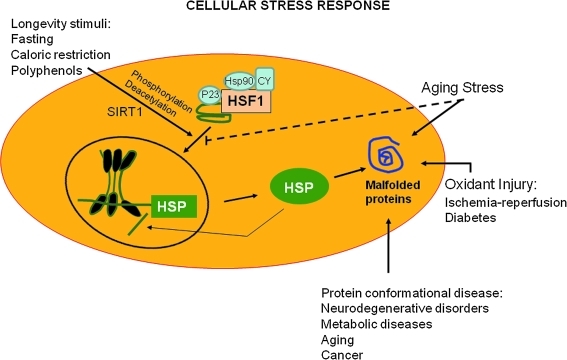
FIG. 4.
Cell stress responses. Expression of HS genes including chaperones and components of the clearance machinery is induced in response to physiological and environmental stress conditions, including longevity stimuli, such as fasting, caloric restriction, or polyphenol antioxidants, and protein conformational diseases. HSF1 can also be directly stimulated by longevity stimuli such as the histone deacetylase SIRT1 that directly activates HSF1 by deacetylation, thus fostering longevity. The increased flux of damaged or misfolded proteins in response to proteotoxic environmental conditions (stress) is the trigger for the induction of the cellular stress response. Aging, however, is associated with a gradual decline in potency of the heat shock response and this may prevent repair of protein damage, leading to degeneration and cell death. HSF, heat shock transcription factor; SIRT, silent information regulator two.
Cellular stress response (Fig. 4) requires the activation of pro-survival pathways that, under control of protective genes called vitagenes (99, 100, 106–108), result in the production of molecules (Hsps, glutathione, and bilirubin) endowed with antioxidant and antiapoptotic activities. Generally, molecular chaperones help hundreds of signaling molecules to keep their activation-competent state, and regulate various signaling processes ranging from signaling at the plasma membrane to transcription. In addition to these specific regulatory roles, recent studies have revealed that chaperones act as genetic buffers stabilizing the phenotypes of various cells and organisms (43). Protein function is regulated by the proteostasis network (20), an integrated biological system that generates and protects the protein fold. Proteostasis refers to controlling the concentration, conformation, binding interactions (quaternary structure), and location of individual proteins making up the proteome by readapting the innate biology of the cell, often through transcriptional and translational changes. Proteostasis thus influences specific cellular functions and enables differentiated cells to change their physiology for successful organismal development and aging in the face of constant intrinsic and environmental challenges to prevent disease onset. Proteostasis is influenced by the chemistry of protein folding/misfolding and by numerous regulated networks of interacting and competing biological pathways that influence protein synthesis, folding, trafficking, disaggregation, and degradation. The composition of the proteostasis network is regulated by signaling pathways, including the unfolded protein response (UPR), the HSR, the ubiquitin proteasome system (UPS), together with epigenetic programs (185). The same networks are preferentially remodeled in various diseases and aging, which may help us to design novel therapeutic and antiaging strategies (312). Among the cellular pathways involved in the so-called programmed cell life conferring protection against oxidative stress, a key role is played by the products of vitagenes (97–100, 105–108, 281). These include members of the Hsp family, such as heme oxygenase-1 and Hsp72, sirtuins, and the thioredoxin/thioredoxin reductase system (103, 106, 107). Recent studies have shown that the HSR contributes to establishing a cytoprotective state in a wide variety of human diseases, including inflammation, cancer, aging, and neurodegenerative disorders. Given the broad cytoprotective properties of the HSR, there is now strong interest in discovering and developing pharmacological agents capable of inducing the HSR (99). Molecular chaperones are known to disrupt aggregates actually promoted active aggregation when the concentration of the aggregating protein is high. Consistent with this notion, it has been proposed that, although protein aggregation is hazardous under certain circumstances, the creation of apparently less-toxic large aggregates is protective (335). This hypothesis is the basis of the therapeutic potential of Hsps, which prevent protein misfolding and aggregation. Transgenic animal models of the diseases have demonstrated that induction or overexpression of Hsps can suppress neuronal dysfunction and degeneration. Hsp70 can reduce the amount of misfolded and aggregated a-Syn species in vivo and in vitro, and protect neuronal cells from a-Syn-dependent neurotoxicity (120). Hsp104 reduced the phosphorylation of a-Syn inclusions and prevented the nigrostriatal dopaminergic neurodegeneration induced by mutant a-Syn (A30P) (235). Recent findings regarding the pathogenic species generated during fibril formation have highlighted some of the beneficial and problematic aspects of Hsp-based therapy.
X. Sirtuins and the Integration of Adaptive Stress Responses in Neurons
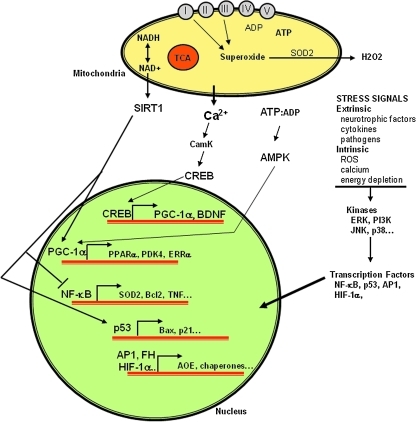
Sirtuins are a family of histone deacetylases that, in humans, includes at least seven members (silent information regulator two [SIRT]1–7) that exhibit different cellular and subcellular localizations and substrate specificities [see ref. (144) for review]. The best studied sirtuin is SIRT1, an NAD+-dependent enzyme that deacetylates several different protein substrates involved in the regulation of cellular energy metabolism and redox state, thereby influencing cell survival and plasticity (Fig. 5). In yeast, worms, and flies, the SIRT1 homolog (Sir2) has been shown to play a major role in lifespan determination and stress resistance [see ref. (34) for review]. Similarly, emerging evidence suggests important roles for SIRT1 in hormetic responses of cells to a range of metabolic and oxidative stressors. In addition to roles in chromatin remodeling effected by deacetylating histones, SIRT1 has been shown to deacetylate and thereby activate a transcriptional regulator called peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α). PGC-1α is believed to play a pivotal role in adaptive responses of cells to dietary energy restriction and exercise (30, 183), effectively increasing cellular stress resistance by upregulating expression of a set of genes that encode proteins (peroxisome proliferators activated receptor-γ, pyruvate dehydrogenase kinase isoform 4, and estrogen-related receptor-γ) that control the production of antioxidant and detoxifying enzymes (Fig. 5). PGC-1α has also been shown to increase the proliferation of mitochondria in neurons (362). In comparison with SIRT1, very little is known concerning the functions of SIRTs 2–7, but their differential subcellular localization is providing interesting clues; for example, SIRT3 is associated with mitochondria, where it may deacetylate protein substrates involved in energy metabolism (3).
FIG. 5.
SIRT1-based and related hormetic signaling pathways in neurons are coupled to transcriptional regulators that control expression of genes involved in neuronal plasticity and cell death. AMPK, adenosine monophosphate-activated protein kinase; AOE, antioxidant enzyme; BDNF, brain-derived neurotrophic factor; CREB, cyclic AMP response element binding protein; ERK, extracellular signal regulated kinase; ERR, estrogen-related receptor; FH, forkhead transcription factor; HIF, hypoxia inducible factor; JNK, jun N-terminal kinase; PDK, pyruvate dehydrogenase kinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1α; PPAR, peroxisome proliferator activated receptor; SOD, superoxide dismutase.
Together with DNA methylation, acetylation of histones and transcription factors plays roles in regulating gene expression in neurons [see ref. (285) for review]. Through these activities, sirtuins are shown to regulate important biological processes, such as apoptosis, cell differentiation, energy transduction, and glucose homeostasis (262, 289, 361). In cultured mammalian cells, Sirt1 is activated in response to growth factor deprivation and increased oxidative stress. Changes in the cellular redox state as reflected by pyridine nucleotide homeostasis, specifically concentrations of NAD+ or the ratio of the concentration of NAD+ and its reduced form NADH, control the deacetylase activity of Sir2 and its homologs. During hypoxic stress, redox changes in cellular metabolism occur that activate Sirt1 and integrate also with hypoxia-inducible factors (HIFs) which are transcriptional regulators that control genes induced during hypoxia and other stresses (Fig. 5). Activation of the founding HIF member, HIF-1a, is increased when oxygen concentrations are reduced. The second HIF alpha member, endothelial known as HIF-2a, is closely related to HIF-1a in structure and is likewise activated during hypoxia. HIF-2a target genes identified from mouse knockout studies include Sod2 encoding the mitochondrial-localized manganese superoxide dismutase, VegfA encoding the proangiogenic regulator vascular endothelial growth factor A, and Epo encoding the cytokine erythropoietin. Sirt1 augmentation of HIF-2 signaling is conferred through formation of a Sirt1/HIF-2a complex as well as through Sirt1-mediated deacetylation of acetylated HIF-2a (161). Some studies have provided evidence that activation of SIRT1 can protect neurons against degeneration, whereas others suggest that SIRT1 promotes neuronal death. In a mouse model of peripheral nerve degeneration, nicotinamide prevents axonal degeneration and SIRT1 is a pivotal downstream mediator axonal protection (15). Overexpression of nicotinamidase in flies increases their lifespan in a Sir2-dependent manner, and nicotinamidase overexpression in cultured human neurons increases their resistance to oxidative stress, also in a sirtuin-dependent manner (19). The function of Sir2 deacetylases is negatively regulated by nicotinamide, a by-product feedback inhibitor in deacetylation reactions involving NAD+-dependent deacetylases and also a metabolite in the nucleotide salvage pathway. Reactions performed by these enzymes can rapidly deplete cellular NAD and generate nicotinamide, which acts as a potent feedback inhibitor of the NAD+-dependent deacetylases (199). Thus, in the absence of nicotinamide, sirtuin protein activity is enhanced. Salvage enzymes, such as nicotinamidase that deaminates nicotinamide into metabolites that can be recycled back to NAD, increase activity of Sir2 deacetylases, thus exerting, in yeast, effects on life span. All this is corroborated in mammals, by the finding that increased nicotinamide clearance in cells provides positive effects on organism life span and cellular response to oxidative stress. This supports the hypothesis that manipulation of nicotinamide metabolism through genetic approaches or pharmacological agents in vertebrates could yield similar beneficial results (125). D'Mello and his colleagues showed that SIRT1 can protect cultured cerebellar neurons against apoptosis induced by potassium deprivation, apparently by a mechanism independent of its deacetylase activity (352). In contrast, SIRTs 2, 3, and 6 induced apoptosis, suggesting opposing actions of different sirtuins, at least in this particular cell culture model.
Interestingly, emerging evidence suggests that sirtuins may be involved in the regulation of synaptic plasticity (22, 303), and adaptive responses of brain cells to a range of stressors (Figs. 4 and 5). For example, decreased activities of histone deacetylases (HDAC1 and HDAC2), whose activity is not dependent on NAD+, during early synaptic development enhance excitatory synapse maturation associated with increased synapse numbers, whereas in mature neurons a decrease in HDAC2 levels attenuates basal excitatory neurotransmission without a change in the numbers of synapses (4). SIRT1 may also play important roles in the regulation of neural progenitor cell fate decisions because SIRT1 is upregulated in neural progenitor cells in response to mild oxidation, and suppression of SIRT1 expression in neural progenitors prevents oxidative stress-induced suppression of neurogenesis (360). Because of their roles in cellular stress responses, sirtuins would be expected to play particularly important roles in adaptive responses of neural cells to stress and, presumably, the enhancement of synaptic plasticity and neurogenesis in response to exercise, dietary energy restriction, and other hormetic environmental factors (291). In support of this notion, SIRT1 has been shown to interact either directly or indirectly with several pathways known to be involved in adaptive neural plasticity, including the Ca2+-calmodulin-cyclic adenosine monophosphate (AMP) response element binding protein (409) and NF-κB (382) systems (Fig. 5).
It has also been reported that the SIRT1 activator resveratrol can protect neurons against degeneration in experimental models of Alzheimer's disease and amyotrophic lateral sclerosis (229). Similarly, resveratrol treatment protected neurons against the toxic effects of polyglutamine proteins in animal models of Huntington's disease (348). Dietary energy restriction can protect neurons against dysfunction in mouse models of Alzheimer's disease (197), and recent evidence suggests a role for SIRT1 in this neuroprotective action of caloric restriction (363).
Resveratrol was recently shown to affect the activity of SIRT1 in vitro although its effects seem to depend on the nature of the substrate for deacetylation (6). However, in vivo, resveratrol has been shown to exert effects dependent on sirtuin orthologs—extension of lifespan in yeast, C. elegans, and Drosophila, and metabolic effects on mammalian cells (378). In addition, resveratrol protects C. elegans neurons expressing a fragment of the Huntington-disease-associated protein huntingtin and mammalian neurons from mutant polyglutamine cytotoxicity in an HdhQ111 knock-in mouse model of Huntington disease (144). Two recent studies have also shown that deleterious effects of high-fat, high-caloric diets in mice were mitigated by resveratrol feeding. In one study, the shortening of lifespan by the high-fat diet was reversed (305). In a second study, resveratrol increased SIRT1 activation, PGC-1α deacetylation, and mitochondrial biogenesis in muscle (247). Although resveratrol has been claimed to be a bona fide SIRT1 activator, whereby offering a promising approach for treating metabolic disorders, with a potential to change the practice of medicine, recent reports indicate that this finding might be an experimental artifact and need to be clarified (26). It has been suggested that metabolism of the redox couple NAD/NADH provides a link between sirtuin activity and the control of cell senescence and organism life-span: NAD-dependent protein deacetylation helps maintain the juvenile phenotype, whereas inhibition of deacetylation activity by NADH or nicotinamide, or by NAD unavailability, promotes the onset of cellular aging and decrease organism lifespan (6, 100). Raising NAD levels, or lowering NADH levels by increasing its oxidation also promote sirtuin activation, with concomitant beneficial effects on cell survival (201). There exists an interrelationship and overlap between sirtuin regulation, generation of altered proteins, and mitochondrial activity, exerted by metabolic effects on NAD and NADH levels (Fig. 5). It has been proposed that when protein synthesis is strongly upregulated (e.g., during growth and sirtuin-enhanced mitogenesis) there is a concomitant stimulation in chaperone protein synthesis and proteolytic potential, to ensure rapid removal of erroneously synthesized/misfolded polypeptide chains. This hypothesis is sustained by recent finding that heat-shock protein expression (466) and autophagy (383) are enhanced by NAD+-activated SIRT1 activity, thereby facilitating recognition and elimination of altered proteins. Further, sirtuin-mediated stimulation of aerobic mitochondrial activity, for example, in response to caloric restriction (14), will also facilitate NADH oxidation back to NAD+, thereby improving NAD+ availability and decreasing the potential for generation and accumulation of altered proteins (202). In the Wallerian degeneration slow (Wlds) mouse model, SIRT1 activation protects axons against neuronal injury (144). This Wlds mouse bears in fact a dominant mutation producing an overexpressed chimeric Wlds protein composed of the ubiquitin assembly protein Ufd2a and the NAD+ salvage pathway enzyme NMNAT1. Decreasing SIRT1 activity reduces the axonal protection originally observed, whereas SIRT1 activation by resveratrol decreases the axonal degeneration after neuronal injury (430). This suggests that the neuroprotection in the Wlds mouse model is achieved by increasing the neuronal NAD+ reserve and/or SIRT1 activity (144). In addition, SIRT1 activation significantly decreases neuronal cell death induced by amyloid-beta (Aβ) peptides through inhibition of NFκB signaling (144). Specific brain hSIRT1 overexpression in transgenic animals induces a significant increase in the α-secretase activity, an enzyme that cleaves the amyloid precursor peptide (APP) within the Aβ peptide, thereby mediating the nonamyloidogenic pathway of the APP processing (144). These studied animal models have indicated that SIRT1 could contribute to the pathogenesis of some complex diseases. In line with this hypothesis, genetic variants (single nucleotide polymorphisms) in the human SIRT1 gene have been shown to be tightly associated with energy expenditure (247).
While SIRT1 may promote neuronal survival and plasticity in some settings, it can contribute to the death of neurons in experimental models of some neurodegenerative disorders (353). For example, sirtuin inhibitors protect neurons against α-synuclein-mediated toxicity in models of Parkinson's disease (309). Others have reported that nicotinamide treatment restores cognitive performance in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and reduction of levels of hyperphosphorylated tau (194). Another study provided evidence that SIRT1 may promote neuronal death by activating a insuline-like growth factor-1 signaling pathway (258), although other findings suggest that insuline-like growth factor-1 signaling is itself neuroprotective (123). Our own findings suggest that SIRT1 can promote neuronal survival under conditions where cellular energy levels are sufficient, but may endanger neurons by depleting NAD+ under conditions of marginal cellular energy levels (262, 263). This biphasic action of SIRT1 depending upon cellular NAD+ levels is similar to that previously described for PARP [poly-ADP(ribose) polymerase], which can protect neurons against apoptosis when energy levels are sufficient, but contributes to energy failure in conditions such as ischemia and hypoxia (169). While the roles of SIRT1 in neurodegenerative conditions therefore appear complex, the development of agents that selectively activate or inhibit SIRT1 is being actively pursued with the expectation of novel therapeutic treat ents for a range of neurological disorders (248). Moreover, it has been recently shown that SIRT2 inhibition, through nicotinamide (NAM), O-acetyl-ADP-ribose (O-AA-ribose), and AGK, prevented α-synuclein cytotoxicity and modulated its aggregation in cultured cells; ameliorated mutant α-synuclein neurotoxicity in rat primary dopamine-positive neurons; and rescued degeneration of dopaminergic neurons from α-synuclein toxicity in a Drosophila animal PD model (341). These results suggested that modulation of α-synuclein aggregation pathway could be one of the sirtuin neuroprotective mechanisms (340). The exact mechanism whereby SIRT2 inhibition affects α-Synuclein aggregation remains uncertain. Increased α-tubulin acetylation is associated with microtubule stabilization, and has been reported to interact with α-tubulin as well as the microtubule-binding proteins MABP1 and tau. One possibility is that the increase in acetylated α-tubulin resulting from SIRT2 inhibition may stimulate aggregation of α-Synuclein through its affinity to microtubules. Moreover, microtubule stabilization itself could be an important factor contributing to neuroprotection (341). Sirtuins hold a great potential as therapeutic targets in neurodegeneration; however, the development of therapeutic activators and inhibitors against the various sirtuin isoforms is necessary to assess the therapeutic potential of these targets in rodent models of neurodegenerative diseases, so that the development of lead-candidate compounds for human clinical trials can be expedited.
XI. The Kelch-Like ECH-Associated Protein 1/Nrf2/Antioxidant Response Element Pathway
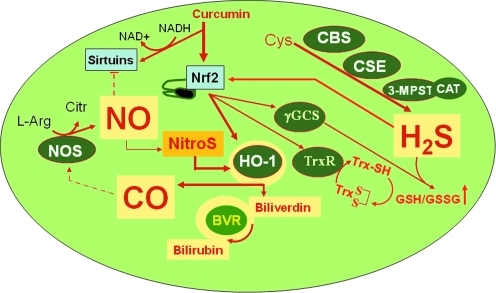
As mentioned above, a central regulator in gene expression of Hsps is heat shock transcription factor 1 (HSF1) (310). In addition, some of the vitagenes are also upregulated as part of the phase 2 response, also known as the electrophile counterattack response, a cytoprotective response that protects against various electrophiles and oxidants (97, 104–106, 146) (Figs. 6 and 7). Examples include heme oxygenase 1, thioredoxin, and thioredoxin reductase, all of which can be upregulated by the transcription factor Nrf2 (Nuclear factor-erythroid 2 p45-related factor 2) coordinately with a battery of cytoprotective proteins, such as glutathione S-transferases (GSTs), UDP-glucuronosyltransferase, NAD(P)H quinone oxidoreductase 1 (NQO1), epoxide hydrolase, ferritin, γ-glutamylcysteine synthetase, glutathione reductase, aldo-keto reductases, and glutathione conjugate efflux pumps (106). This elaborate network of protective mechanisms allows eukaryotic organisms to counteract the damaging effects of oxidants and electrophiles, major agents involved in the pathogenesis of cancer, atherosclerosis, neurodegeneration, and aging (227). Gene expression of this group of functionally diverse proteins is regulated by the Kelch-like ECH-associated protein 1 (Keap1)/Nrf2/antioxidant response element (ARE) pathway (Fig. 7). Long before this pathway was discovered, it had been reported that exposure to low doses of carcinogens protects against the toxicity of a subsequent encounter with high doses of carcinogens [reviewed in ref. (205)], illustrating the phenomenon of hormesis, or preconditioning. Thus, as far back as the late 1920s Berenblum showed that topical application of dichlorodiethyl sulfide reduced skin tumor incidence in mice painted with tar (31). Similar were the findings of Lacasagne and colleagues and Riegel and colleagues, who reported that weakly carcinogenic hydrocarbons (e.g., 1,2,5,6-dibenzofluorene and 1,2,5,6-dibenzoacridine) protected against the carcinogenicity of highly active carcinogenic hydrocarbons 1,2,5,6-dibenzo(a)anthracene and 3-methylcholanthrene (246, 371). Importantly, the protection was much more effective with pretreatment and simultaneous treatment compared to simultaneous treatment alone. The studies of Richardson and Cunningham, who showed that intravaginal administration of 3-methylcholanthrene could inhibit liver carcinogenesis caused by orally administered 3′-methyl-4-dimethyl-aminoazobenzene, demonstrated that protection could be achieved even when the protector and the carcinogen were administered by two different routes (370) and thus excluded the possibility of a direct interaction between the protector and the carcinogen. The first insights into the protective mechanism(s) were provided by James and Elizabeth Miller and their colleagues, who demonstrated that 3-methylcholanthrene was an inducer of enzymes that catalyze the conversion of carcinogens to inactive (detoxification) products (134, 304).
FIG. 6.
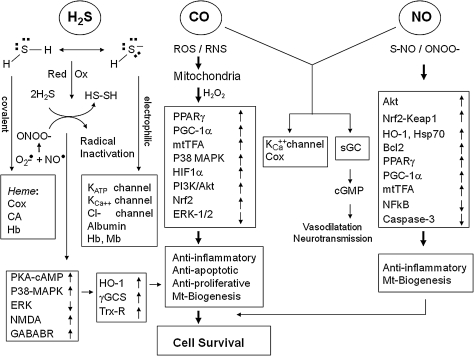
Endogenous biosynthetic pathways of (a) NO, involving NOSs, (b) CO, involving HO isoforms (HO-1, HO-2), and (c) H2S, involving CBS, CSE, and MPST. Methionine, which is derived from alimentary sources, is converted to S-adenosylmethionine by methionine adenosyltransferase. S-adenosylmethionine is subsequently hydrolyzed to homocysteine by glycine N-methyltransferase. Cystathionine β-synthase catalyses the production of cystathionine by transferring serine to homocysteine. Cystathionine γ-lyase, a pyridoxal 5′-phosphate-dependent enzyme, subsequently converts cystathionine to cysteine. In the mitochondria, cysteine can get converted to 3-mercaptopyruvate by aspartate aminotransferase, which can then be converted to H2S by MPST. CBS, cystathionine β-synthase; CO, carbon monoxide; CSE, cystathionine γ-lyase; HO, Heme oxygenase; H2S, hydrogen sulfide; MPST, 3-mercaptopyruvate sulfur transferase; NO, nitric oxide; NOS, NO synthase.
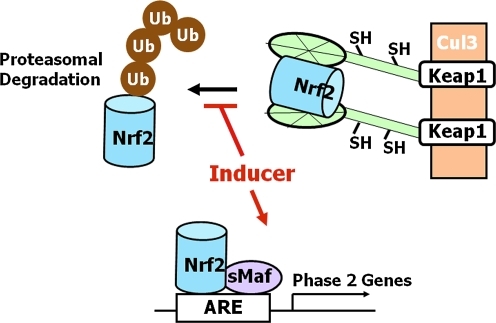
FIG. 7.
The Keap1/Nrf2/ARE pathway. Under basal conditions transcription factor Nrf2 is bound to a cytoplasmic repressor Keap1, which targets Nrf2 for ubiquitination and proteasomal degradation via association with the Cullin 3-based E3 ubiquitin ligase complex. Small molecule inducers modify highly reactive (sensor) cysteine residues of Keap1, which loses its ability to target Nrf2 for degradation. This results in stabilization of Nrf2, binding to the ARE (in heterodimeric combinations with a small Maf transcription factor), and activation of the transcription of cytoprotective (phase 2) genes. ARE, antioxidant response element; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor-erythroid 2 p45-related factor 2.
In the 1960s Charles Huggins and his colleagues reported that small doses of various aromatic hydrocarbons protected against the toxicity and carcinogenicity of subsequent exposure to large doses of 7,12-dimethylbenz(a)anthracene (208, 209). The phenomenon was called induced protection. Importantly, protection only occurred when the protector was given before the carcinogen and protection was long-lasting (in some cases up to 6 days), indicating that some synthetic process was of critical importance. This hypothesis was further strengthened by the finding that protein synthesis was required for protection; protection was abolished by the protein synthesis inhibitor ethionine and could be specifically restored only by methionine, but not by any other amino acid (208). This study led to the discovery that NQO1 was induced by the protective agents in several tissues, that is, in liver, lung, adrenal, and mammary gland (208). It is now widely recognized that NQO1 is a cytoprotective enzyme (152, 439, 440). A highly quantitative and robust bioassay based on the ability to induce NQO1 in murine hepatoma (Hepa1c1c7) cells is used to screen for potent small molecule inducers. Importantly, inducers identified using this screen have subsequently been confirmed to protect against tumor development, and to confer cardiovascular and neuroprotective effects in various animal models (155, 170, 222, 357).
In the 1960s Frankfurt (180) and Wattenberg (463) found independently that the phenolic antioxidants BHA and BHT that are widely used as food preservatives and therefore consumed by humans protected experimental animals against the toxicity and carcinogenicity of 7,12-dimethylbenz(a)anthracene and other carcinogens. The findings of Talalay, Bueding, and their colleagues that supplementation of the diet of rodents with BHA resulted in dramatic reduction of the mutagenic metabolites of benzo(a)pyrene, which was accompanied by selective induction (by enhanced transcription) of several cytoprotective proteins (i.e., GSTs, uridine diphosphate [UDP]-glucuronosyltransferases, and NQO1) without significant effects on enzymes that catalyze the activation of this pro-carcinogen (e.g., cytochrome P450 enzymes), provided a mechanistic explanation for the observed protection [reviewed in refs. (435, 436)]. Subsequent studies involving many inducers some of which are related to BHA, but many of which belong to a wide variety of structurally unrelated chemical classes, led to two crucial discoveries: (a) all inducers share a common chemical property, namely, the ability to react with sulfhydryl groups (437); (b) inducers activate the transcription of cytoprotective genes that occurs in the liver and many peripheral tissues [reviewed in ref. (356)] ensuring fitness for meeting subsequent challenges, and ultimately survival. Again, these discoveries represented an illustration of the hormesis phenomenon and the designation of the Electrophile Counterattack response was proposed (356). It is now recognized that due to their functional versatility (Table 1) the transcriptional induction of cytoprotective genes is a highly effective strategy for achieving protection against a variety of chronic conditions, such as neoplasia, atherosclerosis, hypertension, and neurodegeneration (205, 219, 228, 329, 438).
Table 1.
Examples of the Versatility of Functions of Cytoprotective Proteins
| Conjugation and export |
| Glutathione transferases |
| UDP-glucuronosyltransferases |
| Multidrug resistance proteins |
| Glutathione homeostasis |
| γ-Glutamatecysteine ligase |
| Glutathione reductase |
| Thioredoxin |
| Thioredoxin reductase |
| Antioxidant |
| Heme oxygenase 1 |
| Ferritin |
| Catalase |
| Antiinflammatory |
| Leukotriene B4 dehydrogenase |
| Synthesis of reducing equivalents: |
| Glucose-6-phosphate dehydrogenase |
| Maleate dehydrogenase |
| Repair and removal of damaged macromolecules |
| Heat shock protein 40 |
| Heat shock protein 70 |
| 26S proteosome subunits |
| O6-methylguanine-DNA methyltransferase |
UDP, uridine diphosphate.
Gene expression of these cytoprotective proteins is coordinately regulated by a common molecular mechanism that involves the Keap1/Nrf2/ARE pathway (Fig. 7). The upstream regulatory regions of these genes contain single or multiple copies of the antioxidant/electrophile response elements (ARE and EpRE) with the consensus sequence 5′-A/CTGAC/GNNNGCA/G-3′) (182, 324, 375, 376). The major transcription factor that binds to the ARE is Nrf2, a basic leucine zipper transcription factor. Activation of gene expression requires that Nrf2 binds to the ARE in heterodimeric combinations with members of the small Maf family of transcription factors (314). Under basal conditions the pathway operates at low levels due to the repressor function of the cytosolic protein Keap1, which binds to the E3 ubiquitin ligase Cullin3-RING box1 and presents Nrf2 for ubiquitination and subsequent proteosomal degradation (200, 236, 324).
Inducers of the Keap1/Nrf2/ARE pathway belong to at least 10 distinct chemical classes: (a) oxidizable diphenols, phenylenediamines, and quinones; (b) Michael acceptors (olefins or acetylenes conjugated to electron-withdrawing groups); (c) isothiocyanates; (d) thiocarbamates; (e) trivalent arsenicals; (f ) dithiolethiones; (g) hydroperoxides; (h) vicinal dimercaptans; (i) heavy metals; and (j) polyenes. The only common property among them is their chemical reactivity with sulfhydryl groups by oxido-reduction, alkylation, or disulfide interchange. (153, 155, 356, 358, 421, 438). This common property led Talalay and his colleagues to propose that the sensor for inducers must be a molecule (perhaps a protein) endowed with exquisitely reactive cysteine residues (160, 438). Indeed, there is now compelling experimental evidence that exogenous and endogenous inducers chemically modify specific and highly reactive cysteine residues of Keap1, which, in addition to being a repressor for Nrf2, also functions as the intracellular sensor for inducers (157, 216). This reaction leads to conformational changes in Keap1 that abrogate its ability to repress Nrf2, ultimately resulting in Nrf2 stabilization, binding to the ARE and recruitment, the basal transcriptional machinery to activate transcription of cytoprotective genes (200, 237, 315). Whereas many aspects of the model are still controversial and the intricate details of the regulation of the pathway are not fully understood (200, 324, 345), there is voluminous experimental evidence for a mechanism of regulation of Keap1 activity through its reactive cysteine residues. Of note, even though Nrf2 is regulated primarily at the level of protein stability, the consequences of its stabilization are due to its downstream target genes that have relatively long half-lives (on the order of days). Thus, the evaluation of the effects of inducers on the levels of the Nrf2-target gene expression or activity rather than on Nrf2 levels themselves represents a much more biologically meaningful measure of cellular protection against damage.
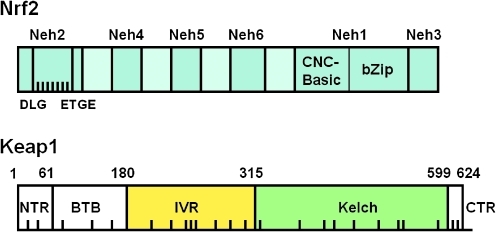
Murine Nrf2 is composed of 597 amino acids and has a molecular weight of ∼60 kD (Fig. 8). Nrf2 is a Cap ’n’ Collar (CNC) transcription factor that belongs to the class of basic leucine zipper (b-Zip) proteins. These proteins form heterodimers with members of the Maf family of transcription factors. Keap1 is a multidomain homodimeric protein that comprises 624 amino acids and has a molecular weight of ∼69 kD. Murine Keap1 contains 25 cysteine residues (its human homologue has 27), 9 of which (i.e., C23, C38, C151, C241, C273, C288, C297, C319, and C613) are flanked by basic amino acids, which in turn are known to lower the pKa values of the cysteines, favoring the formation of the thiolate anions at neutral pH, thus making them potentially highly reactive. We and others have reported that C151, C273, and C288 are of particular importance in the function of Keap1 as a sensor for inducers and as a repressor of Nrf2 (154, 253, 461, 477).
FIG. 8.
Overall structures of Nrf2 and Keap1 showing the different domains. In Nrf2, the DLG motif (amino acids 27–32) and the ETGE motif (amino acids 77–82) of the Neh2 domain comprising the two Keap1-binding sites are indicated and the black bars represent the lysine residues that are ubiquitinated by Cul3-Rbx. The Neh1 and Neh3 domains form the DNA-binding site of the transcription factor. In Keap1, the BTB domain is the dimerization domain and the site of interaction with Cul3. The Kelch domain is the Nrf2-binding domain. The black bars indicate the distribution of the cysteine residues of Keap1.
The BTB domain of Keap1 represents a protein–protein interaction motif (Fig. 8). Indeed, Keap1 exists as a homodimer through the BTB domains of its subunits. Two groups have reported the crystal structure of the Kelch domain showing the formation of a 6-bladed β-propeller structure, with residues at the C-terminus forming the first strand in the first blade (257, 344, 449). It is through the Kelch domain that Keap1 binds to the Neh2 domain of Nrf2. For binding between Keap1 and Nrf2 to occur, two motifs within Nrf2 are critically important: the ETGE and the DLG. On the basis of a series of elegant studies, McMahon et al. (299, 450, 451) proposed that, first, the ETGE motif of Nrf2 binds to an arginine triad on one of the Keap1 subunits. This binding then positions the DLG motif of Nrf2 in the vicinity of the triad on the second subunit of Keap1. This so-called fixed-ends or hinge-and-latch model explains the observation that the binding affinity of Keap1 for the ETGE motif is much higher than its affinity for the DLG motif and predicts that the lysine residues in Nrf2 targeted for ubuquitination must be positioned between the two Keap1-binding sites. Indeed, all seven lysine residues of Nrf2 (out of a total of 27) that have been implicated in ubiquitination are located between the ETGE and DLG motifs, a portion of the protein that is largely helical (451).
Whereas Keap1 is the major repressor of Nrf2 and the sensor for inducers of Nrf2-dependent genes, DJ-1 has been recently implicated as a necessary factor required for Nrf2 stability and activity (130), even though up to date no evidence for a physical interaction between DJ-1 and either Nrf2, Keap1, or Cullin 3 has been reported. Nonetheless, the ability of DJ-1 to stabilize Nrf2 may be an essential contributor to the importance of DJ-1 for cellular protection against oxidative and ER stress. Conversely, the loss of DJ-1 may represent an important factor in the decreased inducibility of Nrf2-dependent genes that has been observed under certain disease conditions (275) and with aging (133). Thus, loss-of-function mutations in PARK7, the gene encoding DJ-1, are associated with autosomal recessive early onset Parkinsonism (39).
Another protein that interacts with Keap1 resulting in Nrf2 activation is p62 (238). Both genetic and pharmacological activation of Nrf2 leads to accumulation of p62, which can then further activate Nrf2. Conversely, impaired p62 expression could lead to decreased Nrf2 activity and may further explain the association between low p62 levels, a common occurrence in many neurodegenerative disorders (165), and decreased Nrf2-dependent inducibility. Importantly, mice that lack p62 have increased oxidative DNA damage in brain as well as biochemical and cognitive defects that resemble Alzheimer's disease (342, 365).
The generation of the nrf2-knockout mouse (117, 215) provided an unequivocal demonstration of the cytoprotective role of Nrf2-dependent gene battery [see ref. (227) for a scholarly review]. Under standard laboratory conditions, nrf2-knockout mice do not display any obvious phenotypic abnormalities. In contrast to wild-type mice, however, they fail to adapt to environmental challenges and are much more sensitive to the toxicity and carcinogenicity of electrophiles and oxidants. Unlike their wild-type littermates, nrf2-knockout mice have low basal levels of cytoprotective proteins and cannot be protected by inducers. Conversely, liver-specific keap1-knockout mice and keap1-knockdown mice have constitutively high basal levels of cytoprotective proteins and are much more resistant to acute drug toxicity (332, 333, 336, 369, 370, 471).
The neuroprotective effects of upregulation of the Keap1/Nrf2/ARE pathway have been demonstrated in several in vitro and in vivo models. Thus, adenovirus-mediated overexpression of Nrf2 was shown to upregulate ARE-dependent genes in primary cultures of neurons and astrocytes that were derived from the cortex of the rat (407) or the mouse (249) brain. Importantly, this upregulation of the Keap1/Nrf2/ARE pathway protected against the toxicity of glutamate and H2O2. Similar protection was also observed when the small molecule inducers tert-butyl hydroquinone (tBHQ) or sulforaphane were used instead of Nrf2 overexpression (240, 407). Strikingly, the protective effects of induction of the Keap1/Nrf2/ARE pathway in astrocytes extend beyond this cell type and also provide protection to neurons. Even though the intricate mechanisms are not fully understood, one very important player is undoubtedly reduced glutathione, the secretion of which from the astrocytes increases after Nrf2 activation. It has been hypothesized that this effect is the primary factor leading to neuroprotection of both cortical and motor neurons in culture (219). In addition, the upregulation of proteins that are concerned with the synthesis, use, and transport of reduced glutathione (GSH), including xCT cystine/glutamate antiporter, γ-glutamatecysteine ligase, glutathione S-transferases, glutathione reductase, and the multidrug resistance protein 1, in the astrocyte were shown to be critical for neuronal protection. However, contrary to the expectations based on the in vitro experiments, nrf2-knockout mice did not differ from their wild-type littermates in terms of basal activity of the xCT cystine/glutamate antiporter in the ventral striatum, cocaine-induced behaviors either after acute or repeated exposure, or dopamine depletion after methamphetamine-induced oxidative stress (343).
Compared to their wild-type counterparts, neurons isolated form nrf2-knockout mice were much more sensitive to the cytotoxic effects of the mitochondrial toxins 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine and rotenone (249). Oligonucleotide microarray analysis on primary neuronal cultures isolated form wild-type and nrf2-knockout mice revealed differences in several functional categories of genes: (a) detoxification/antioxidant/reducing potential; (b) calcium homeostasis; (c) receptor/channel/carrier proteins; (d) growth factors; (e) cell signaling; (f ) defense/immune/inflammation; and (g) neuron-specific proteins (249). Of special interest are the genes playing roles in calcium homeostasis. Indeed, neuronal cultures isolated from nrf2-knockout mice were more sensitive to increases in intracellular Ca2+ levels caused by ionomycin- and 2,5-di-(t-butyl)-1,4-hydroquinone (249). Importantly, adenoviral-vector-mediated overexpression of Nrf2 recovered ARE-dependent gene expression in nrf2-knockout neuronal cultures and protected nrf2-knockout neurons from rotenone- or ionomycin-induced cell death.
The exciting findings using mixed primary cultures of neurons and astrocytes showing that activation of the Keap1/Nrf2/ARE pathway in astrocytes provides protection to neurons prompted subsequent in vivo studies. A large number of in vivo experiments have demonstrated the neuroprotective role of the Keap1/Nrf2/ARE pathway and suggested the potential use of Nrf2 inducers for achieving protection against neurodegenerative diseases. Thus, in comparison to wild-type mice, nrf2-knockout mice are hypersensitive to the mitochondrial complex II inhibitors 3-nitropropionic acid and malonate, which induce lesions in the striatum (111, 406). Hypersensitivity was both time and dose dependent such that motor deficits and striatal lesions developed more rapidly in homozygous null mice than in animals that are either homozygous or heterozygous for nrf2 (406). Importantly, striatal succinate dehydrogenase activity, the target of 3-nitropropionic acid, was inhibited to the same extent in all genotypes, indicating that the absence of Nrf2 did not lead to alterations in metabolism and brain concentration of the drug. The toxicity of 3-nitropropionic acid was lower when the diet of wild-type but not nrf2-knockout mice was supplemented with the Nrf2 inducer tBHQ, suggesting that protection was dependent on Nrf2. Indeed, similar protective effects were achieved via intrastriatal adenovirus-mediated Nrf2 overexpression, which significantly reduced lesion size compared with green fluorescent protein-overexpressing controls. tBHQ, administered intracerebroventricularly or intraperitoneally, was also protective against the occurrence of cortical damage and sensory and motor deficits at 24 h and even at 30 days after ischemia-reperfusion in rats (408). In mice, tBHQ attenuated neuronal death caused by intracortical endothelin-1 microinjection (a penumbral model of stroke), but was ineffective in nrf2-knockout animals (408). Nrf2-knockout mice are also more susceptible to seizures, neuronal damage, and microglial infiltration in hippocampus induced by kainic acid exposure (241). Nrf2-dependent protection against excess release of dopamine was also recently reported (405).
Transplanted Nrf2-overexpressing astrocytes into the mouse striatum before challenge with malonate provided dramatic protection against malonate-induced neurotoxicity; remarkably, brain hemispheres receiving Nrf2-producing astrocyte transplants were essentially resistant to malonate toxicity, whereas hemispheres receiving control astrocytes were no different from untransplanted controls (111). Recently, transgenic mice overexpressing Nrf2 selectively in astrocytes have been generated using the glial fibrillary acidic protein (GFAP) promoter (456). These mice were crossed with two ALS-mouse models. It was found that overexpression of Nrf2 in astrocytes significantly delayed disease onset and extended survival. In a model of Parkinson's disease, nrf2-knockout mice were found to be more sensitive than wild-type mice to the toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (48, 122). Further, whereas MPTP challenge led to upregulation of the Keap1/Nrf2/ARE pathway in substantia nigra of wild-type mice (indicative of a counterattack in response to the toxic challenge), such upregulation was absent in nrf2-knockout mice, demonstrating that the absence of Nrf2 results in failure of an adaptive stress response (122). Importantly, activation of the Keap1/Nrf2/ARE pathway by oral administration of the Nrf2 inducer 3H-1,2-dithiole-3-thione to wild-type mice resulted in partial protection against MPTP-induced neurotoxicity, but had no effect when administered to nrf2-knockout mice (48). Strikingly, the toxicity of MPTP was abolished in GFAP-Nrf2 transgenic mice (overexpressing Nrf2) on both nrf2-wild-type and nrf2-knockout backgrounds. Because the GFAP-Nrf2 transgenic mice on an nrf2-knockout background express Nrf2 only in astrocytes, this finding confirmed the previous observations that Nrf2 expression restricted to astrocytes is sufficient to protect the brain against MPTP toxicity. In a model of permanent cerebral ischemia it was shown that whereas absence of Nrf2 appears to have no significant effect on cortical infarction at 24 h, it exacerbates tissue injury at 7 days after permanent ischemia, as demonstrated by the large increase (twofold) in infarct volume at the 7-day time point (408). In contrast, there was no increase in infarct size between 24 h and 7 days in wild-type animals. On the basis of this observation, the authors proposed a model in which Nrf2-dependent gene expression in surviving glial cells restricts infarct expansion in wild-type, but is unable to do so in nrf2-knockout mice (408). It was recently reported that nrf2-knockout mice develop spongiform leukoencephalopathy characterized by degeneration of myelin in the white tracts of the brain accompanied by widespread reactive gliosis (206). In this study, the mean age of the mice with vacuolar leukoencephalopathy was 8.3 ± 2.6 months (mean ± SE), and the prevalence at the age of 1 year or older was 100% (11 of 11 animals). In the age- and gender-matched wild-type controls, only one animal had a locally extensive area of mild vacuolar degenerative changes in the cerebellar white tracts. These findings show the importance of Nrf2 in supporting myelin integrity and suggest that antioxidant responses mediated through the Keap1/Nrf2/ARE pathway are essential to prevent oxidative injury within the myelin sheath. Taken together, these studies convincingly demonstrate the neuroprotective effect of the Keap1/Nrf2/ARE pathway and it was recently proposed that it represents a novel neuroprotective pathway that confers resistance to a large variety of oxidative-stress-related and neurodegenerative insults (112, 219, 327).
It is noteworthy that the levels of markers of the Keap1/Nrf2/ARE pathway such as NQO1, GST, and GSH in brain do not differ as dramatically as they do in liver of wild-type and nrf2-knockout mice (406). Thus, nrf2-knockout mice have only ∼25% of the liver NQO1 activity of wild-type controls. In sharp contrast, the brain NQO1 activity in nrf2-knockout mice is ∼85% of the wild type. Liver glutathione levels in nrf2-knockout mice are ∼30% lower than in wild-type mice, whereas there is no significant difference between the two genotypes in terms of total glutathione content in the brain. The implications of these finding are at least twofold. First, there must be other Nrf2-independent pathways of regulation of cytoprotective genes in brain. Curiously, the roles of the other members of the Nrf family of transcription factors (Nrf1 and Nrf3) have not been investigated in the brain even though the expression levels of brain Nrf1 are much higher than those of Nrf2 (298). Second, because nrf2-knockout mice are clearly much more sensitive than their wild-type littermates to all of the above mentioned challenges, differences in expression of cytoprotective genes as small as 10%–20% seem to have a very significant impact on the sensitivity of the brain to damage, and especially on its ability to cope with the long-term consequences of such damage. Because the battery of cytoprotective genes is so large (>100) and their functions so diverse, it is perhaps not surprising that very small differences in a large number of proteins could have a big overall impact.
XII. Hormetic Phytochemicals and the Neuroprotective Effects of Pharmacological Activators of the Keap1/Nrf2/ARE Pathway
Phytochemicals include compounds with various biological properties (i.e., antimicrobial, antifungal, antioxidant, and antiproliferative) that have presumably evolved, in part, to allow plants to cope with environmental challenges, including exposure to radiation and toxins, and defense against pests and infectious agents. These chemicals, or their metabolic precursors, are particularly concentrated in the seeds, the growing buds, and the skin of fruits and function as natural pesticides. From an evolutionary perspective, the noxious properties of such phytochemicals play an important role in dissuading insects and other pests from eating the plants. However, at the relatively small doses ingested by humans who consume the plants, the phytochemicals are not toxic and instead induce mild cellular stress responses (319). The now compelling evidence for biphasic dose–response effects of environmental toxins in biological systems suggests the possibility that chemicals in foodstuffs, particularly from plants, might also exert biphasic dose responses with health benefits resulting from ingestion of the chemicals in doses within the hormetic range. Whereas at high concentrations phytochemicals can be toxic to mammalian cells, subtoxic doses may induce adaptive stress responses. Thus, there is voluminous evidence on the protective effects of isothocyanates, curcumin, and resveratrol, all present in edible plants. In spite of this, however, high doses of these phytochemicals can also be toxic to some types of cells (289, 291, 293). Although in the past it was assumed that the direct free radical scavenging activity of phytochemicals might be responsible for their direct health benefits, to date it is unclear, if not unlikely, whether humans consume fruits and vegetables to an extent sufficient to achieve the high (micromolar) concentrations of the phytochemicals required to scavenge free radicals directly. Indeed, epidemiological studies and clinical trials have failed to demonstrate benefits of dietary supplementation with antioxidants such as vitamin E and vitamin C. Within this context, emerging evidence suggests that hormetic mechanisms may account for the health benefit of phytochemicals (330). One general mechanism of action of phytochemicals is that they activate adaptive cellular stress response pathways, including kinases and transcription factors that induce the expression of genes that encode antioxidant enzymes, protein chaperones, phase 2 enzymes, neurotrophic factors, and other cytoprotective proteins (294). Sulforaphane, curcumin, and resveratrol are inducers of the Keap1/Nrf2/ARE pathway. Resveratrol activates the sirtuin–FOXO pathway, resulting in increased expression of antioxidant enzymes and cell survival-promoting proteins (294). Ingestion of other phytochemicals may activate the hormetic transcription factors cyclic AMP response element binding protein, resulting in the induction of genes encoding growth factors and antiapoptotic proteins (289, 293).
A. Tert-butylhydroquinone
Several pharmacological activators (inducers) of the Keap1/Nrf2/ARE pathway have been shown to be neuroprotective (Fig. 9). Most of them are either natural products or synthetic compounds that are present in the human diet. As already discussed, tBHQ is one of the most widely used inducers of the Keap1/Nrf2/ARE pathway in both in vitro and in vivo studies. tBHQ is also one of the very early inducers that was shown to elevate cytoprotective enzymes (NQO1 and GST) in several tissues of CD-1 mice and Hepa1c1c7 murine hepatoma cells (145, 359, 360) and to reduce the number of gastric tumors in female ICR/Ha mice treated with benzo[a]pyrene (464) in a series of elegant studies carried out more than a decade before the discovery of the Keap1/Nrf2/ARE pathway. Among several phenolic antioxidants, tBHQ is more potent as an inducer compared to compounds in which the phenolic groups are alkylated. Further, its oxidation-reduction lability was identified as an essential feature for its inducer ability (359). It was recently suggested that compounds with hydroquinone and catechol moieties could constitute novel neuroprotective agents based on their ability to activate the Keap1/Nrf2/ARE pathway (388). It was pointed out that such molecules have an advantage over classical antioxidants (such as ascorbic acid) because their action is sustained and amplified by transcription-mediated signaling pathways (385). Further, because hydroquinones and catechols are not electrophilic themselves, they could be viewed as pro-drugs that are converted to the ultimate inducers by oxidation reactions (29). Critically, because redox imbalance is a recognized component of many neurodegenerative states, a novel strategy against neurodegenerative disorders has been proposed, namely, activation of such pro-drugs via the pathological activity that they are intended to combat (260). Thus, the catechol-containing carnosic acid is a compound abundant in rosemary (Rosmarinus officinalis) that accounts for 5% of the dry weight of the leaves (239). Carnosic acid induces Nrf2-dependent genes by binding to specific cysteine residues of Keap1 located in its BTB and/or IVR domains (386). Further, carnosic acid crosses the blood–brain barrier, increases the level of reduced glutathione in the brain, and protects against ischemia/reperfusion (386). Electrophilic neurite outgrowth-promoting prostaglandin derivatives have been shown to be taken up preferentially into neurons, where they bind to Keap1 leading to Nrf2 activation, binding to the ARE and upregulation of ARE-dependent genes (387). These electrophilic neurite outgrowth-promoting prostaglandin are neuroprotective in vitro against glutamate-related excitotoxicity as well as in vivo in a model of cerebral ischemia/reperfusion injury. Because of their neuroprotective effects, there are currently efforts toward the targeted development of neuroprotective electrophilic drugs, including prostaglandin derivatives and hydroquinones that exert their activity through activating the Keap1/Nrf2/ARE pathway (385).
FIG. 9.
Chemical structures of inducers of the Keap1/Nrf2/ARE pathway for which neuroprotective activities have been demonstrated.
B. Sulforaphane
One of the most potent naturally occurring inducers of the Keap1/Nrf2/ARE pathway is the isothiocyanate sulforaphane, and it is therefore not surprising that the interest in its development as a protective agent in many chronic conditions, including neurodegeneration, is growing rapidly. The protective activities of sulforaphane in various animal models of carcinogenesis have been recently reviewed (159). The focus here is on its neuroprotective activities. Sulforaphane (Fig. 9) was isolated from extracts of the common cruciferous vegetable broccoli (Brassica oleracea) via an activity-guided fractionation using the Prochaska test (see above), a bioassay based on induction of the prototypical ARE-dependent gene NQO1 (480, 481). In the brain of male Long–Evans rats, the levels of heme oxygenase-1 (HO-1) mRNA were elevated after intraperitoneal administration of sulforaphane (5 mg/kg) (482). The protein expression of HO-1 was increased by ∼1.5-fold in both neurons and astrocytes. In a model of brain ischemia/reperfusion, administration of sulforaphane 15 min after the onset of ischemia reduced the infarct volume (which was evaluated 3 days later) by ∼50%. The same dose of sulforaphane administered to Sprague-Dawley rats 6 h after traumatic brain injury attenuated the loss of the water channel aquaporin-4 in the injury core, and additionally, it also increased the levels of aquaporin-4 in the penumbra region (483). The protective effects were long-lasting, and even 3 days postinjury there was a significant, although modest, reduction in brain edema. The mRNA levels of several cytoprotective genes (i.e., HO-1, NQO1, and GST) in the parietal cortex and brain microvessels were increased 24 h after dosing (484). Postinjury (6 h later) administration of sulforaphane reduced the loss of endothelial cell markers and tight junction proteins and preserved the blood brain barrier function. The protective effects were evident only in wild-type but not in nrf2-knockout mice. Moreover, compared to their wild-type counterparts, nrf2-knockout mice were hypersensitive to injury-induced blood–brain barrier permeability.
In a model of intracerebral hemorrhage in Sprague-Dawley rats, sulforaphane administration caused induction of several Nrf2-dependent genes (catalase, superoxide dismutase, GST, and NQO1) in brain 3 h later, concomitantly with reduction in markers of oxidative damage (3′-nitrotyrosine and 4-hydroxynonenal) in the perihematoma area (485). Three days after intracerebral hemorrhage, the perihematoma neutrophil count was reduced by ∼60%, and the total intracerebral hemorrhage-affected striatum neutrophil count was reduced by 33%. Again, the protective effects were long-lasting; 10 days after intracerebral hemorrhage, the neurologic deficits (a composite score from several behavioral tests, including foot fault, forelimb placing, postural reflex, cylinder, and circling) were substantially reduced. Conversely, Nrf2 deficiency resulted in exacerbated neurologic deficits and loss of the protection. In addition, treatment of organotypic nigrostriatal cocultures with tBHQ or sulforaphane protected dopaminergic neurons against 6-hydroxydopamine-induced toxicity (410).
Interestingly, sulforaphane as well as many other inducers of the Keap1/Nrf2/ARE pathway also have antiinflammatory properties. Further, there is a linear correlation spanning more than 6 orders of magnitude between Nrf2 inducer potency and potency in inhibiting pro-inflammatory responses among small molecules that belong to structurally diverse chemical classes (158, 264). In cultured murine macrophages sulforaphane induces NQO1 and inhibits the upregulation of inducible NO synthase (iNOS) and Cox-2 caused by lipopolysaccharide (LPS) or interferon (IFN)-γ and TNFα (158, 264). Similarly, in primary cocultures of rat microglial and astroglial cells, sulforaphane induces Nrf2-dependent genes and attenuates LPS-stimulated production of TNFα, interleukin (IL)-1β, IL-6, and NO (466). The protective effects of sulforaphane against brain inflammation were recently reported in C57BL/6 mice that received endotoxin injection (213). Sulforaphane treatment decreased microglial activation and the upregulation of inflammatory markers (inducible NO synthase, IL-6, and TNFα) in response to LPS. The same study also showed that compared to wild-type mice, nrf2-knockout mice were hypersensitive to LPS-induced neuroinflammation. Although the mechanistic relationship between Nrf2 inducer and antiinflammatory activities of sulforaphane (and other Nrf2 inducers) are not clear at present, there is no doubt that both of these activities contribute to the cytoprotective effects of these small molecules (441).
C. Dimethyl fumarate
Fumaric acid is the active principal of shepherd's purse (Capsella bursa-pastoris), a cruciferous weed that has been widely used as a traditional herbal medicine. In the 1940s Crabtree (137, 138) demonstrated that fumaric acid and several related compounds delay or prevent the development of skin tumors caused by benzo(a)pyrene in mice. Fumaric acid is also protective against the carcinogenic effects of a nitrofuran on the forestomach and lung in mice and against the toxicity of mitomycin C (244, 245). It was shown nearly 20 years ago that addition of dimethyl fumarate to the diet of female CD-1 mice and female Sprague-Dawley rats at 0.2%–0.5% concentrations elevated cytosolic GST and NQO1 activities in many organs (420). In primary cocultures of rat microglial and astroglial cells, dimethyl fumarate attenuated LPS-induced production of TNFα, IL-1β, IL-6, and NO, in addition to inducing Nrf2-dependent genes (468). In a model of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice, treatment with dimethyl fumarate strongly reduced macrophage inflammation in the spinal cord and increased blood levels of IL-10 (391). In humans, fumaric acid salts and esters are already used as therapeutic agents, for example, ferrous fumarate for iron deficiency (487) and alkyl esters for psoriasis (10, 324). In human keratinocytes and peripheral blood mononuclear cells stimulated with IFN-γ or LPS, dimethyl fumarate (Fig. 9) inhibits the production of chemokines that may be critically involved in the development and persistance of psoriatic lesions (426). In patients with relapsing-remitting multiple sclerosis, dimethyl fumarate reduced the formation of new inflammatory lesions in both a pilot study (392) and a multicenter, randomized, double-blind, placebo-controlled, dose-escalation, Phase IIb study (224).
D. Diallyl trisulfide
The volatile organosulfur compounds from Allium (garlic) plants are also inducers of the Keap1/Nrf2/ARE pathway (155). The unsaturated allyl or propenyl functionalities in their structures (Fig. 9) are critical determinants of inducer potency (318, 438). In the 1980s such compounds were found to elevate glutathione S-transferase activity in the forestomach and to protect against benzo[a]pyrene-induced carcinogenesis (419). Very recently using rat spinal cord explants, it was shown that pretreatment for 48 h with diallyl trisulfide elevates NQO1 and protects motor neurons against glutamate-induced excitotoxicity (429).
E. Celastrol
The quinone methide triterpene celastrol (Fig. 9) is derived from the traditional Chinese medicinal plant known as Thunder of God Vine (Tripterygium wilfordii). Celastrol is an inducer of NQO1 (Albena T. Dinkova-Kostova and Paul Talalay, unpublished observations) and a potent inhibitor of NFκB (136, 400). Celastrol decreases induced expression of class II MHC molecules by microglia (9). Moreover, administration of celastrol to rats significantly improved their performance in memory, learning, and psychomotor activity tests. In addition, celastrol activates heat shock gene transcription synergistically with other stresses and exhibits cytoprotection against subsequent exposures to lethal heat stress (465). Celastrol administration significantly attenuated the loss of dopaminergic neurons and the depletion in dopamine concentration after MPTP treatment (131). In a model of Huntington's disease, celastrol decreased the striatal lesion volume induced by 3-nitropropionic acid (131). Hsp70 within dopaminergic neurons was induced by celastrol, whereas TNF-α and NFκB and astrogliosis were suppressed. Structure-activity studies revealed a remarkable specificity in activating HSF1 with kinetics resembling those of heat shock, as determined by induction of HSF1 binding to DNA and increased transcription of heat shock genes (466, 467). Moreover, celastrol induced HSR synergistically with other stressors and this induction was protective against subsequent exposure to otherwise lethal forms of stress.
F. Curcumin
The neuroprotective role of curcumin (Fig. 9), another naturally occurring inducer of the Keap1/Nrf2/ARE pathway (151, 153), is also a subject of intense investigations (101, 102, 106). Curcumin (diferuloylmethane) is a component of turmeric, a yellow spice extracted from the rhizome of the East Indian herb Curcuma longa L., that is widely used as a food flavoring and coloring agent (e.g., in curry). Thus, treatment of astrocytes with curcumin induced the cytoprotective proteins HO-1, NQO1, and GST and provided protection against the damaging effects of glucose oxidase-mediated toxicity (390). Further, curcumin was reported to bind to amyloid plaques, and to reduce amyloid levels and plaque burden in aged Tg2576 mice (a model of familial Alzheimer's disease) with advanced amyloid accumulation (469). Extensive research within the past two decades has shown that curcumin mediates its antiinflammatory effects through the downregulation of inflammatory transcription factors (such as nuclear factor-kB [NFkB]), enzymes (such as cyclooxygenase 2 and 5 lipoxygenase), and cytokines (such as TNF, IL-1β and IL-6) (12, 444), as well as upregulation of cyprotective vitagenes (102, 106, 107). Curcumin has antiinflammatory, antioxidant, and anticancer activities. One of the most challenging tasks concerning cancer is to induce apoptosis in malignant cells; thus, more focus has been given on natural products to modulate apoptotic signaling pathways. Curcumin has chemopreventive properties, which are mainly due to its ability to arrest cell cycle and to induce apoptosis. The anticarcinogenic properties of curcumin in animals have been demonstrated by its inhibition of tumor initiation and tumor promotion (12). The number of signaling pathways and molecular targets involved is continuously growing and consequently the picture is becoming more and more complex, not least because the results often appear to be cell type specific and dose dependent (12).
Curcumin is quite stable at acidic pH, and upon ingestion almost 40%–80% of this compound remains in the gastrointestinal tract. However, curcumin undergoes a marked first-pass metabolism that limits its systemic bioavailability (∼60%) as demonstrated in humans and rodents (102, 109). Interestingly, to increase its bioavailability, the coadministration of curcumin with piperine or its complexation with phospholipids to form a curcumin-phospholipids complex has been proposed (12). Preclinical studies have shown that administration of 1 g/kg of curcumin to the rat allows the polyphenol to reach plasma concentrations around 1.3 μM. Early studies have shown that curcumin has strong antioxidant activity. In fact, the beta-diketone moiety, especially the phenolic hydroxyl group, is critical for the antioxidant activity of curcumin and its analogs (109, 151, 206). Very recently, many articles have appeared in the literature demonstrating that curcumin and its metabolites interact with several intracellular systems such as transcription factor NFκB, iNOS, and HIF-1 and members of the vitagene family (see below). This complex array of interactions is in agreement with the well-known ability of curcumin to serve not only as an antioxidant but also as antiinflammatory and anticarcinogenic molecule (264). In lung epithelial cells, curcumin exerted anticarcinogenic activity and prevented the cigarette-smoke-induced NFκB activation through inhibition of IkBα kinase activation, IkBα phosphorylation, and degradation (264). The inhibition of the NFκB activation was paralleled by the suppression of many NFκB-related genes, including cyclin D1, cyclooxygenase-2, and matrix metalloproteinase-9 (264). Particularly interesting is the interaction of curcumin with the vitagene system. Notably, curcumin increased expression of HO-1 in human cardiac myoblasts, hepatocytes, monocytes, and endothelial cells (316), as well as rat neurons and astrocytes (390). In several rodents and human cells, the curcumin-induced HO-1 overexpression was correlated with production of mitochondrial ROS, activation of transcription factors Nrf2 and NFkB, induction of mitogen-activated protein kinase (MAPK) p38, and inhibition of phosphatase activity (300). Moreover, curcumin upregulated Hsp70 in human colorectal carcinoma cells, proximal tubule cells, and leukemia cells (443). Quite different is the effect of curcumin on thioredoxin reductase, as it has been shown that curcumin irreversibly inhibits thioredoxin reductase activity. As a consequence, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is stimulated with overproduction of ROS (171). This latter paradoxical effect may explain, at least in part, the cancer chemopreventive activity of curcumin (171).
G. Ferulic acid
Ferulic acid is another phenolic compound (Fig. 9) and a major constituent of fruit and vegetables with strong antioxidant and antinfammatory properties. Recently, it has been demonstrated that ferulic acid ethyl ester, a more hydrophobic form of ferulic acid, protects synaptosomal membrane system and neuronal cell culture systems against hydroxyl and peroxyl radical oxidation, as well as mice against Aβ-induced microglial activation (97, 351). In addition to this direct antioxidant property, ferulic acid ethyl ester has been shown to increase HO activity in rat astrocytes and neurons (106, 389). Recent finding indicates that ferulic acid promotes nuclear translocation of Nrf2, whereby increasing the transcription of HO-1 together with other antioxidant related genes, such as GCLC (glutamate-cysteine ligase catalytic subunit), GCLM (glutamate-cysteine ligase regulatory subunit), and NQO1. These changes involve increase of the intracellular GSH and activation of PI3K and extracellular signal regulated kinase signaling pathways (268). All this corroborates the hypothesis that HO activation is a common pathway through which phenolic compounds can exert neuroprotective effects (106).
XIII. Heme Oxygenases
The heme oxygenases have been recognized as dynamic sensors of cellular oxidative stress and modulators of redox homeostasis throughout the phylogenetic spectrum. Heme oxygenases are located within the ER where they act in association with NADPH cytochrome P450 reductase to oxidize heme to biliverdin, free ferrous iron, and carbon monoxide (CO) (Fig. 6). Biliverdin reductase further catabolizes biliverdin to the bile pigment, bilirubin (104, 105, 108, 274), a linear tetrapyrrole that has been shown to effectively counteract nitrosative stress due to its ability to interact with NO and reactive nitrogen species (RNS) (99, 106–108). Bilirubin is then conjugated with glucuronic acid and excreted (393). In the last 20 years, many research articles have demonstrated that bilirubin is an endogenous cytoprotective molecule. The first evidence about the antioxidant activity of bilirubin was provided by Stocker and colleagues in 1987, who suggested that bilirubin may act as chain-breaker because of its ability to scavenge peroxyl radicals transforming itself in a stable carbon centered radical (425). Later, Snyder and colleagues based on the evidence that bilirubin, in the nanomolar range, may protect cortical neurons from the toxicity elicited by ∼10,000 times higher levels of hydrogen peroxide (163), proposed a novel mechanism based on an amplification cycle whereby bilirubin is oxidized to bilirubin by reactive oxygen species and then recycled by biliverdin reductase back to bilirubin. However, only a small fraction of the bile pigments undergo this redox cycle; therefore, the relevance of this mechanism of action is still debated. Finally, bilirubin has been shown to serve as an endogenous scavenger for both NO and reactive nitrogen species, which may alter the redox status of the cell and originate nitrosative stress (276). Despite this important antioxidant properties, if produced in excess, as in the case of haemolytic anaemia or sepsis, unconjugated bilirubin becomes neurotoxic through multiple mechanisms involving the disruption of cell membrane structure, the reduction of mitochondrial transmembrane potential, and the activation of the apoptotic cascade.
Mammalian cells express at least two isoforms of HO, HO-1 and HO-2. A third protein, HO-3, determined to be a retrotransposition of the HO-2 gene (pseudogene), has been found unique to rats (390). Heme oxygenase-1, also referred as Hsp32, is induced by various stimuli, including oxidative and nitrosative stress, ischemia, heat shock, LPS, hemin, phenolic compounds, and the neuroprotective agent Neotrofin. Further, in cultured human and rodent cells, HO-1 expression can be repressed by hypoxia, cigarette smoke, or by treatment with IFN-γ or desferrioxamine. On the other hand, HO-2, the constitutive form, is responsive to developmental factors and adrenal glucocorticoids. Although HO-1 and HO-2 catalyze the same reaction, they play different roles in protecting tissues against injury. A convincing hypothesis suggests that HO-1 induction is one of the earlier cellular response to tissue damage and is responsible for the rapid clearance of the intracellular pro-oxidant heme and its transformation into CO and biliverdin, the latter being the precursor of the antioxidant bilirubin. On the contrary, constitutively expressed HO-2 is primarily involved in maintaining cell heme homeostasis and in the sensing of intracellular levels of gaseous compounds including NO and CO.
Although HO-1 and HO-2 exhibit identical substrate and cofactor specificities, the isoforms are encoded by distinct genes, which share only 43% amino acid sequence homology in humans, and exhibit significant differences with regard to molecular weight, electrophoretic mobility, tissue distribution, regulation, and antigenicity (393). HO-1 contains a destabilizing proline-glutamic acid-serine-threonine sequence at the carboxy terminus that renders the peptide sensitive to rapid proteolysis, a characteristic not exhibited by HO-2. The half-lives of HO-1 mRNA and protein have been estimated to be 3 and 15–21 h, respectively (148). The HO-1 gene is now well defined in its redox regulation as it contains the ARE in its promoter region, similar to other antioxidant enzymes (223, 273). HO-1, in fact, can be induced by several stimuli associated with oxidative and/or nitrosative stress, such as heme, Aβ, dopamine analogues, H2O2, hyperoxia, UV light, heavy metals, prostaglandins, NO, peroxynitrite, Th1 cytokines, oxidized lipid products, and LPS, as well as certain growth factors (148, 273, 393). HO-1 gene expression is regulated by a variety of factors such as pro-oxidant states or inflammation (273). The molecular mechanism that confers inducible expression of ho-1 in response to numerous and diverse conditions has remained elusive. One important clue has recently emerged from a detailed analysis of the transcriptional regulatory mechanisms controlling the mouse and human ho-1 genes, which in humans is located on chromosome 22q12. The induction of ho-1 is regulated principally by two upstream enhancers, E1 and E2 (5, 106, 273). Both enhancer regions contain multiple stress (or antioxidant) responsive elements (StRE, also called ARE) that also conform to the sequence of the Maf recognition element (5, 325, 393) with a consensus sequence (GCnnnGTA) similar to that of other antioxidant enzymes (107) (Fig. 6). Polymorphisms in the lengths of GT repeats [11–40] within the ho-1 gene (HMOX1) promoter appear to be an important determinant of HO-1 expression and function in humans. Long GT sequences code for relatively unstable (Z-conformational) DNA with attenuated transcriptional activity and diminished baseline and stimulated HO-1 protein expression profiles. Significantly higher HO-1 activity is associated with the short-GT polymorphisms, which may protect against atherosclerosis-linked conditions (e.g., coronary artery disease), whereas the malignant behavior of various neoplasms was fairly consistently enhanced by the short-GT form (393). Due to its strong antioxidant properties and wide distribution within the CNS, HO-1 has been proposed as a key enzyme in the prevention of brain damage (106, 107). The neuroprotective effects of overexpressed HO-1 can be attributed to (a) increase in cGMP and bcl-2 levels in neurons; (b) inactivation of p53, a protein involved in promoting cell death; (c) increase in antioxidant sources, and (d) increase in the iron sequestering protein, ferritin (346). Specifically, the interaction between HO-1, p53, and Bcl2 could involve the heme-regulating motifs of HO-2 (297), which could modulate gene expression by promoting oxygen radical formation and acting as a sink for NO. When HO-1 levels are increased, as in Tg mice, a decreased level of heme for binding to HO-2 heme-regulating motifs may alter heme/oxygen gene-regulating events. bcl-2 and p53 may well be among those genes whose expression would be affected as the consequence of the change in the oxidoreductase state of the cell brought about by altered heme-regulating motif interactions with oxygen and/or NO.
Particularly interesting is the role played by HO-1 in AD, a neurodegenerative disorder that involves a chronic inflammatory response associated with both oxidative brain injury and Aβ-associated pathology. Significant increases in the levels of HO-1 have been observed in AD brains in association with neurofibrillary tangles and also HO-1 mRNA was found increased in AD neocortex and cerebral vessels; the HO-1 increase also colocalized with senile plaques and glial fibrillary acidic protein-positive astrocytes in AD brains (393).
It is plausible that the dramatic increase in HO-1 in AD may be a direct response to an increase in free heme concentrations, associated with neurodegeneration, and can be considered as an attempt of brain cells to convert the highly toxic heme into the antioxidants CO and bilirubin (100). The protective role played by HO-1 and its products in AD raised new possibilities regarding the possible use of natural substances, which are able to increase HO-1 levels, as potential drugs for the prevention and treatment of AD. In this light very promising are the polyphenolic compounds contained in some herbs and spices, for example, curcumin (102, 106, 109). Curcumin is the active antioxidant principle in Curcuma longa, a coloring agent and food additive commonly used in Indian culinary preparations. This polyphenolic substance has the potential to inhibit lipid peroxidation and to effectively intercept and neutralize ROS and RNS (102, 105, 109, 390). In addition, curcumin has been shown to significantly increase HO-1 in astrocytes and vascular endothelial cells (316, 317). This latter effect on HO-1 can explain, at least in part, the antioxidant properties of curcumin, in particular keeping in mind that HO-1-derived bilirubin has the ability to scavenge both ROS and RNS (316). Epidemiological studies suggested that curcumin, as one of the most prevalent nutritional and medicinal compounds used by the Indian population, is responsible for the significantly reduced (4.4-fold) prevalence of AD in India compared to United States (102, 259, 469). On the basis of these findings, Lim and colleagues have provided convincing evidence that dietary curcumin given to an AD transgenic APPSw mouse model (Tg2576) for 6 months resulted in a suppression of indices of inflammation and oxidative damage in the brain of these mice (258). Further, increasing evidence indicates that curcumin inhibits NFκB activation, efficiently preventing cell death (12, 384).
In the absence of elevated intracellular heme or oxidative stress, the basic region leucine zipper transcriptional regulator BACH1 binds HMOX1 AREs and represses transcription. Conversely, increased intracellular heme or sulfhydryl oxidation inactivates BACH1, permitting transcriptional induction of HMOX1. Although it is generally agreed that increased HO-1 expression is a common feature during oxidative stress, recent articles demonstrated that HO-1 can be repressed during oxidative stress conditions. In particular, human cells exposed to hypoxia, thermal stress, and IFN-γ showed a marked HO-1 repression, and this effect seems to be peculiar for human, because rodent cells increased HO-1 expression when exposed to the same stimuli (232). The importance of HO-1 repression has been corroborated by the discovery of Bach1/Bach2 as heme-regulated transcription factors for HO-1 gene (232). In fact, Bach1 is broadly expressed in mice and human tissues; in human cells, it is induced by the same stimuli that are able to repress HO-1 gene (5, 325). Current hypothesis suggests that HO-1 repression is useful for the cell because it (a) decreases the energy costs necessary for heme degradation, (b) reduces the accumulation of CO and bilirubin, which can become toxic if produced in excess, and (c) increases the intracellular content of heme necessary for the preservation of vital functions such as respiration (5, 126, 232). Delineation of the diverse roles of HO-1 in brain senescence, aging-related human neurodegenerative disorders, and other CNS conditions has progressed substantially in these last years. Whereas the acute induction of this enzyme in neural and other tissues is predominantly cytoprotective in nature, protracted or repeated upregulation of the Hmox1 gene in astrocytes, oligodendroglia, and possibly neurons may perpetuate cellular dysfunction and demise in many chronic degenerative and neuroinflammatory conditions long after provocative stimuli have dissipated. In the case of normal brain aging, AD, PD, and other late-life neurodegenerations, there is converging evidence that the associated tissue iron sequestration, intracellular oxidative stress, and mitochondrial dysfunction may constitute a single pathogenic momentum acting downstream from the sustained action of HO-1 within the astrocytic compartment. In AD and mild cognitive impairment, immunoreactive HO-1 protein is overexpressed in neurons and astrocytes of the cerebral cortex and hippocampus relative to age-matched, cognitively intact controls and colocalizes to senile plaques, neurofibrillary tangles, and corpora amylacea. In PD, HO-1 is markedly overexpressed in astrocytes of the substantia nigra and Lewy bodies in affected dopaminergic neurons. HMOX1 is also upregulated in glial cells located around human cerebral infarcts, hemorrhages, and contusions, within multiple sclerosis plaques, and in other oxidant degenerative and inflammatory human CNS disorders. Within this context, heme-derived free ferrous iron, CO, and biliverdin/bilirubin are all biologically active substances that can either ameliorate or exacerbate neural injury contingently to the specific disease conditions, such as intensity and duration of HO-1 expression and/or the nature of the resulting redox milieu. In stressed astroglia, HO-1 hyperactivity promotes mitochondrial sequestration of nontransferrin iron and macroautophagy and may thereby contribute to the pathological iron deposition and bioenergetic failure found in most age-related oxidant neurodegenerative disorders. Glial HO-1 expression may impact also cell survival and neuroplasticity by modulating brain sterol metabolism and proteosomal degradation of neurotoxic protein aggregates (394).
XIV. Adaptive ER Stress Responses: Calcium and Protein Chaperones
Two major functions of the ER are the biosynthesis and quality control of proteins, and the regulation of cellular calcium homeostasis (302, 478). Several key proteins that control these two ER functions are responsive to cellular stress, including metabolic and oxidative stress. One of the first ER stress-responsive proteins discovered is glucose-regulated protein 78 (GRP78 or Bip), which is a protein chaperone that protects nascent proteins from misfolding and damage (326). The expression of GRP78 is highly sensitive to metabolic stress, and its upregulation under such adverse conditions has been shown to protect cells against death (326). Several other such chaperones, with complementary functions, have been identified, including GRP94, GRP170, and calreticulin. Interestingly, even relatively mild physiological stressors can upregulate ER chaperones, including dietary energy restriction (17) and exercise (454). ER Ca2+-regulating proteins, including IP3 receptors, ryanodine receptor, and the sarco-/endo-plasmic reticulum Ca2+-ATPase, are also influenced by and, in turn, modify cellular stress. For example, activation of IP3 receptor channels in the ER of nerve cells leads to activation of the transcription factor NFκB, which, in turn, induces expression of proteins that protect the neurons against stress, including Mn-superoxide dismutase and Bcl-2 (188).
A very important stereotyped sequence of events is now known as the UPR (320). Eukaryotic cells respond to unfolded proteins in their ER stress, amino acid starvation, or oxidants by phosphorylating the alpha subunit of translation initiation factor 2 (eIF2α). This adaptation inhibits general protein synthesis while promoting translation and expression of the transcription factor ATF4. Atf4(−/−) cells are impaired in expressing genes involved in amino acid import, glutathione biosynthesis, and resistance to oxidative stress (198). Under low stress conditions, GRP78 is associated with several different ER proteins, including protein kinase–like ER kinase (PERK), inositol requiring element-1 (IRE-1), and activating transcription factor 6 (ATF6). When cells are under oxidative and metabolic stress, GRP78 is recruited to oxidatively damaged and misfolded proteins, and PERK, IRE-1 and ATF-6 are mobilized to serve the complementary roles in the UPR. PERK suppresses protein translation by phosphorylating eIR2α, and also phosphorylates and thereby activates the transcription factor Nrf2, resulting in the production of antioxidant and phase 2 enzymes. IRE-1 activation results in the production and nuclear translocation of the transcriptional regulator XBP-1, which, in turn, induces expression of GRP78 and GRP94. During the UPR, ATF-6 is cleaved to generate an active transcription factor that translocates into the nucleus and induces expression of several cytoprotective proteins. Examples of stimuli that activate the UPR include nutrient deprivation, altered protein glycosylation, oxidative stress, and increased intralumenal Ca2+ levels.
Until recently, the ER was viewed as a protein synthesis factory that was not involved in signal transduction processes or cellular stress responses. We now know that the ER plays pivotal roles in regulating cellular Ca2+ homeostasis and transduces Ca2+-mediated signals from extracellular signals (excitatory neurotransmitters and growth factors) and signals emanating from internal organelles, including mitochondria and the nucleus (456). Indeed, the ER is believed to be involved in the integration of signaling events within axon terminals and dendrites of neurons (283, 347, 413). When stimulated by IP3 or Ca2+ itself, Ca2+ is released from the ER through opened IP3 receptor and ryanodine receptor channels, respectively (Fig. 10). An ER membrane Ca2+ ATPase is responsible for transporting Ca2+ into the ER. Excessive ER stress and dysregulation of ER Ca2+ homeostasis is believed to contribute to the dysfunction and death of neurons in ischemic stroke (412), AD (32, 283, 290), and Parkinson's disease (233). The ER is also an important sensor of cellular stress. In response to energetic, oxidative, and ionic (particularly Ca2+) stress, protein synthesis may be decreased, and signals are sent from the ER to the nucleus that result in the upregulation of expression of genes encoding ER protein chaperones such as GRP78 and GRP94, and antioxidant enzymes (320). Recently, an ER membrane-associated protein called Herp was shown to stabilize cellular Ca2+ homeostasis in neurons during ER stress (118).
FIG. 10.
Regulatory systems in mitochondria and the endoplasmic reticulum involved in hormetic responses of neurons to a range of environmental factors. Modified from Mattson et al. (292). CytC, cytochrome c; ER, endoplasmic reticulum; GRP, glucose regulated protein; PTP, permeability transition pore; RyR, ryanodine receptor; SERCA, sarco endoplasmic reticulum calcium ATPase; TCA, tricarboxylic acid cycle; UCP, uncoupling protein; VDAC, voltage-dependent anion channel.
The ER plays important roles in hormetic responses to stress in neurons. Thapsigargin is an agent that inhibits the ER Ca2+-ATPase resulting in depletion of ER Ca2+ stores and activation of the UPR. Treatment of cultured cerebellar neurons with thapsigargin increased their survival under culture conditions (low extracellular K+ concentration) in which they would otherwise undergo apoptosis (470). The mechanism by which ER Ca2+ mobilization can promote neuron survival is poorly understood, but emerging data suggest the involvement of both the released Ca2+ itself and a protein(s) released from the ER. In either case, it appears that transcription factors are activated, resulting in the upregulation of cell-survival-promoting proteins. Glazner et al. (188) showed that a cell survival signal is released from the ER in response to emptying of IP3-sensitive Ca2+ stores; the signal, which may be a protein, activates NFκB. Another study showed that the immediate early gene pip92 and mitogen-activated protein kinase are activated in response to ER Ca2+ mobilization (129). Interestingly, Bcl-2 can decrease ER Ca2+ uptake, resulting in enhanced Ca2+ signaling and activation of cyclic AMP response element binding protein, thereby promoting the regeneration of injured axons (218). Moreover, Mendes et al. (301) showed that an ER-mediated signal associated with the UPR enhances antioxidant defenses and inhibits caspase-dependent cell death. Thus, mild ER stress can elicit multiple hormetic responses in neurons.
A better understanding of adaptive responses to ER stress may lead to novel therapeutic approaches for both acute and chronic neurodegenerative conditions. As evidence, it has been reported that, by activating ryanodine receptors in the ER, paraxanthine (the major metabolite of caffeine) can prevent the degeneration of dopaminergic neurons in a model of Parkinson's disease (196). Induction of ER protein chaperones is another therapeutic approach that is supported by experimental data showing that GRP78 (474), GRP94 (21), and ORP150 (234) can protect neurons against excitotoxic and ischemic death. Mild ER stress responses may be elicited by factors that are known to be neuroprotective including mild energetic stress (dietary energy restriction and 2-deoxyglucose treatment) (166, 475) and exercise (454).
XV. Hsps and Neuroprotection
The HSR is one possible cellular stress response where a set of Hsps are induced, playing important roles in cellular repair and protective mechanisms (311, 312). HSR is regulated at the transcriptional, translational, and posttranslational levels by a family of heat HSFs that are expressed and maintained in an inactive state under nonstress conditions. Among three functionally different HSFs in humans, HSF1 represents the major regulator of the HSR genes, mediating signaling of stress-induced stimuli, such as elevated temperatures, as well as those involved in development and in many pathophysiological conditions, including cancer, ischemia-reperfusion injury, diabetes, and aging (Figs. 4 and 11) (452, 466). HSF1 is generally found in the cytoplasm as an inert monomer lacking transcriptional activity; both DNA-binding and transcriptional transactivation domains are repressed through intramolecular interactions and constitutive serine phosphorylation (308). Upon exposure to heat shock and other types of stresses, which cause protein damage, HSF1 is derepressed in a stepwise process that involves oligomerization of HSF1 monomers to a trimeric state, localization to the nucleus, inducible phosphorylation and sumoylation, binding of nuclear-localized trimers to DNA, and transcription of HS genes [reviewed in ref. (13)]. The main targets for HSF1 are specific promoter elements composed of repeats of the pentameric sequence nGAAn (heat shock elements [HSE]) located upstream of HS genes. High rates of transcription are maintained only when HSF1 trimers remain bound to the HSE; when either the stress signal is removed or damaged proteins are no longer generated, the HSR attenuates rapidly, with subsequent conversion of HSF1 back to the monomeric state (310). Inducible acetylation has also been recently shown to negatively regulate DNA binding activity (42, 311).
FIG. 11.
Damaged or misfolded proteins titrate away heat shock proteins that are bound to HSF1 and maintain it in a repressed state before stress, resulting in its activation. Multistep activation of HSF1 involves posttranslational modifications, such as hyperphosphorylation and deacetylation, which allow HSF1 to trimerize and translocate into the nucleus, where inducible acetylation, phosphorylation, and sumoylation occur before binding of nuclear-localized trimers to DNA, and HS genes are transcripted. In particular, upon activation, HSF1 is transiently sumoylated on lysine 298, which requires the phosphorylation of serine 303 adjacent to the consensus site (13). Hence, small ubiquitin-related modifier modification is elaborately regulated, and the small ubiquitin-related modifier substrate specificity can be determined by regulatory elements outside the consensus site. Hsp, heat shock protein.
Mammalian genomes encode three homologs of HSF (HSF1, HSF2, and HSF4) regulating Hsp expression. Among these HSF1 is considered to be the paralog responsible for regulating the heat-induced transcriptional response (42, 311, 312). HSF2 has also been reported to contribute to inducible expression of heat shock genes through interplay with HSF1 (373). Whereas it is well established that HSF1 regulates inducible Hsp expression, recently it has become evident that the regulation of the mammalian HSR is a more complex phenomenon than previously thought. Microarray and chromatin immunoprecipitation analysis, while confirming that many known heat-inducible genes have HSF1-binding sites in their promoters, have defined another class of genes that recruit HSF1 to their HSE promoter elements but that nevertheless are not induced by heat shock (373). On the other hand, these studies have provided evidence that, in some cases, HSF1 may also control expression of genes with nonchaperone function (373), such as superoxide dismutase, the multiple-drug resistance genes, lactate dehydrogenase, and the T-cell death-associated gene 51 (373). Neurons appear to be deficient in the HSR while retaining the ability to express such HSF proteins (312). Further, HSF1 fails to be activated in motor neurons even when microinjected with plasmids encoding an HSF1 expression vector, suggesting a block to the HSF1 signal transduction pathways in these cells (24). HSF1 is repressed under nonstress conditions by a complex containing Hsp90 and other proteins (Fig. 11). In this inactive state, HSF1 is a monomer that lacks the ability to bind cis-acting HSE in the promoters of Hsp genes (110). During protein stress and the consequent generation of misfolded proteins that displace HSF1 form the inhibitory chaperone complex, HSF1 trimerizes, become phosphorylated, and is translocated to the nucleus, where it is acetylated and sumoylated before being able to bind to the heat shock element of Hsp genes (310, 466). Activation of HSF1 by heat shock is a multistep process, involving multiple inducible phosphorylation, dephosphorylation, acetylation, deacetylation, and sumoylation steps, the sum of which results in the transcription of Hsp genes (Fig. 11). Extracellular signal input during heat shock involves tyrosine phosphorylation upstream of HSF1, involving the receptor tyrosine kinase HER2 and launching downstream signaling cascades through intracellular kinase Akt (110). (Fig. 11) Akt regulates HSF1 at least in part through modulating its association with the phosphoserine binding scaffold protein (110).
In addition to thermal stress, the inducible expression of Hsps is also triggered by environmental redox changes or exposure to electrophiles, which cause trimerization and DNA binding of HSF1 (310, 452), pointing to the importance of the cysteine redox state for the activation of this transcription factor. Thus, an intermolecular disulfide bond formation between C36 and C103 within HSF1 causes trimerization and DNA binding, whereas an intramolecular disulfide bond formation (in which C153, C373, and C378 participate) is inhibitory for the activity of the transcription factor (266).
Manipulation of HSR may offer strategies to protect brain cells from damage that is encountered after cerebral ischemia or during the progression of neurodegenerative diseases (309). Hsps are evolutionarily conserved and present in all cellular compartments (417, 418). Some of the major chaperones (Hsc70, Hsp90, and small Hsps) are present at high concentrations in nonstressed cells reaching 1%–5% of total cellular protein, consistent with an important role for chaperones in cellular homeostasis. Hsps are classified according to their molecular weight (270). Hsp70, the 70 kDa family of stress proteins, is one of the most extensively studied. Included in this family are Hsc70 (heat shock cognate, the constitutive form), Hsp72 (the inducible form, also referred to as Hsp72), and GRP75 (a constitutively expressed glucose-regulated protein found in the ER) (269). Hsp70s function in co- and posttranslational folding and the quality control of misfolded proteins (139). More specifically, Hsp70s participate in folding and assembly of newly synthesized proteins into macromolecular complexes, aggregation prevention, dissolution and refolding of aggregated proteins, as well as protein degradation (43). Hsp70s have an N-terminal ATP-binding domain (NBD) and a C-terminal substrate-binding domain (SBD), which are both critical for chaperone function. Nonnative substrates with exposed hydrophobic stretches within an accessible polypeptide backbone associate transiently with Hsp70 via its SBD. ATP binding to the NBD triggers opening of the SBD binding pocket, decreasing affinity for polypeptide substrates, thereby accelerating both on and off rates. Reciprocally, substrate binding induces ATP hydrolysis, closing the SBD and thus stabilizing the substrate-Hsp70 complex (43). It is this cycle of rapid but controlled binding and release of the substrate that fosters folding and assembly with partner proteins while preventing aggregation of substrates; however, detailed mechanistic understanding of how Hsp70 accomplishes these feats is not yet available (43). Numerous hypotheses have been put forth to explain the molecular mechanism of Hsp70-induced structural conversion of substrate proteins. For example, an entropic pulling mechanism has been proposed, whereby Hsp70 binding stabilizes peptide segments in an unfolded state, causing local unfolding, thereby facilitating disaggregation and allowing refolding upon Hsp70 release (189). Cofactors, such as the nucleotide exchange factors and cochaperones, are crucial regulatory components of the Hsp70 cycle that confer versatility and specificity to the Hsp70 chaperone machine (43). The Hsp40 cochaperone targets substrates to Hsp70 while stimulating ATP hydrolysis; nucleotide exchange factors like Bag-1 (BCL2- associated athanogene 1) and Hsp110 reinitiate the Hsp70 cycle by facilitating ADP release and rebinding of ATP (380). Moreover, Bag-1 has the additional ability to bind to the 26S proteasome, and another BAG isoform, the Bag-3 cochaperone, links Hsp70 to the macroautophagic degradation pathway during aging (110). Carboxy terminus of HSC70-interacting protein, a cochaperone of Hsp70 that also has E3 ubiquitin ligase activity, cooperates with Bag-1, and possibly Bag-3, to facilitate degradation of terminally misfolded substrate proteins. Notably, mutations in Hsp70 cofactors are lethal (271) or may be associated with neurodegenerative disease (269, 271).
Recent studies indicate that the HSR declines in aging cells and becomes weaker as organisms live beyond the mature adult stage (104). Cells lose the capacity to activate the transcriptional pathways leading to Hsp synthesis (Fig. 4). In neuronal tissues, decline in protein quality control has been widely predicted, as the etiology of a number of diseases involves aggregation-prone proteins that form inclusion bodies whose occurrence is linked to pathology. Hsp70 has been extensively implicated in the pathogenesis of misfolding disease (110). Numerous studies indicate that Hsp70 and components of the ubiquitin–proteasome system associate with inclusion bodies/plaques characteristic of misfolding diseases, indicating a general activation of the cellular quality control machinery in an attempt to circumvent the accumulation of misfolded species (201). In these conditions the Hsp70 system is unable to refold disease-related proteins, causing perturbation of protein homeostasis associated with disease onset. Several hypotheses account for this apparent disruption of the balance between the production of misfolded proteins and Hsp70 activity. As misfolded disease proteins accumulate, these can overwhelm the capacity of the Hsp70 system to control the cellular folding milieu (312). Progressive reduction in protein levels and/or activity of Hsp70 and other components of the quality control network may exacerbate this imbalance, permitting further accumulation of toxic misfolded proteins. Such reduction could be due to the aging process, as transcription of Hsp70 decreases during aging of the human brain (43). Alternatively, disease processes themselves might cause, or worsen, chaperone deficiency. Inclusions have been proposed to sequester Hsp70 and other proteins in a nonfunctional state, inhibiting their essential function in cellular processes (43).
The availability of transgenic animals and gene transfer allowing overexpression of the gene encoding for Hsp70 has revealed that overproduction of this protein leads to protection in several different models of nervous system pathology (97). Overexpression of Hsp70 and/or its cochaperones suppresses huntingtin aggregation and toxicity in yeast and mammalian cell models of misfolding disease (380). Increased Hsp70 levels caused reduced aggregation and toxicity of tau and Aβ, respectively, two components associated with Alzheimer's disease (269). Similarly, overexpression of Hsp70 reduces toxicity and accumulation of α-synuclein in high-molecular-weight and detergent-insoluble deposits (269). Increased expression of Hsp70 has been reported to be associated with a decrease in apoptotic cell death, an increase in expression of the antiapoptotic protein Bcl-2, a suppression of microglial/monocyte activation, and a reduction in matrix metalloproteinases. Upregulation of Hsp70 likewise reduced apoptosis and the formation of coaggregates of the prion disease protein, PrP (104). Numerous studies have also shown that Hsp70 overexpression reduces polyQ toxicity. Results obtained in vitro have elucidated the mechanism of action of Hsp70 against misfolding and thus toxicity of disease proteins. Purified Hsp70 acts preferentially on monomers or oligomers, rather than fibrillar aggregates of Aβ, huntingtin, and α-synuclein species modulating the aggregation process (104). Hsp70 inhibits the aggregation of Aβ and α-synuclein species even at substoichiometric levels, suggesting that Hsp70 can recognize multimeric protein assemblies (43). As mentioned before, the effect of Hsp70 on aggregation, which requires its ATPase activity, is enhanced by the cochaperone Hsp40. Thus, Hsp70, together with Hsp40, stabilizes huntingtin in a monomeric conformation and prevents accumulation of spherical oligomers, which are the toxic species for fibril formation (403). As a result, mutant huntingtin is deviated from the potentially toxic, fibrillar aggregation pathway and instead accumulates in amorphous aggregates, or other benign conformers. Sequestered in these conformers, mutant huntingtin may no longer participate in heterotypic interactions known to inactivate essential cellular machinery, such as polyQ-containing transcription factors (311, 403).
After focal cerebral ischemia, Hsp70 mRNA is synthesized in most ischemic cells except in areas of very low blood flow, due to scarce ATP levels. Hsp70 proteins are produced mainly in endothelial cells, in the core of infarcts in the cells that are most resistant to ischemia, in glial cells at the edges of infarcts, and in neurons outside the areas of infarction (479). It has been suggested that this neuronal expression of Hsp70 outside an infarct can be used to define the ischemic penumbras, which means the zone of protein denaturation in the ischemic areas; consistently in in vivo transgenic mice overexpressing Hsp70, compared to wild-type mice in a middle cerebral artery occlusion model of permanent cerebral ischaemia, it has been demonstrated that overexpression of Hsp70 reduces the overall lesion size and also limits the tissue damage within the lesion (479). Hsp72 overexpression has been documented in postmortem cortical tissue of AD patients (95, 270). Consistently, the use of agents that limit microglial activation and inflammation in AD has recently emerged as an attractive therapeutic strategy for this disease. For instance, the vasoactive intestinal peptide has been shown to prevent Aβ-induced neurodegeneration, through inhibition of major pathways involved in the production of inflammatory mediators, such as the p38 MAPK, p42/p44 MAPK, and NFκB cascades, in activated microglial cells (147, 221).
A large body of evidence now suggests a correlation between mechanisms of nitrosative stress and Hsp induction. We have demonstrated in vitro and in vivo that cytokine-induced nitrosative stress is associated with an increased brain synthesis of Hsp70 stress proteins. The molecular mechanisms regulating the NO-induced activation of heat-shock signal seem to involve cellular oxidant/antioxidant balance, mainly represented by the glutathione status and the antioxidant enzymes (27, 98, 99).
A. Neuroprotective effects of extracellular Hsps
Hsps are transferred between cell types in the nervous system. Thus, stress tolerance in neurons is not solely dependent on their own Hsps, but can be supplemented by additional Hsps transferred from adjacent glial cells. Therefore, supplying exogenous Hsps at neural injury sites could be an effective strategy to maintain neuronal viability. This idea has been tested in a number of model systems. Injection of Hsc/Hsp70 into the vitreous chamber of the eye protected retinal photoreceptors from photodamage. Application of exogenous Hsc/Hsp70 to the cut end of the sciatic nerve reduced cell death in sensory and motor neurons. Extracellular Hsp70 protected spinal cord motor neurons deprived of trophic support in vitro or undergoing cell death in vivo. Thus, exogenous application of Hsps has potential as a therapeutic strategy for acute injury in the nervous system. Hsps are released into the blood stream after stressful stimuli and this may represent an important feature of the stress response. Exercise stress has been reported to induce the release of Hsp70 from the human brain into the blood stream in vivo. The biological significance of this neural release is yet to be determined (44).
XVI. Neuro Gas Biology and the Roles of CO, NO, and Hydrogen Sulfide in Brain Physiopathology
A. Carbon monoxide
The first detection of a combustible gas in the blood occurred in 1894 by Grehant (195). This gas was supposed by de Saint Martin and Nicloux to be CO. However, it was not until 1949 that Sjorstrand discovered that endogenously produced CO arose from the degradation of hemoglobin released from senescing erythrocytes (414). Greater than 75% of CO produced in humans arises from erythrocyte turnover generated as a by-product of haem metabolism. In 1969, the source of endogenous CO was discovered, as Tenhunen and collaborators described and characterized heme oxygenase as the enzyme responsible for breaking down heme in the body, demonstrating that heme catalysis resulted in the subsequent release of CO and free iron as by-products (445) (Fig. 6). Since then, a large body of experimental evidence has demonstrated that CO is an extraordinary signaling molecule playing an important role in the regulation of cellular homeostasis, especially in the brain, where CO can influence physiological and pathological processes both in the central and peripheral nervous systems (Fig. 12). Due to its capacity to freely diffuse from cell to cell, together to the fact that is not stored in synaptic vesicles from which is released through membrane depolarization processes, it is a nonconventional neurotransmitter capable of influencing signal transduction mechanisms. CO binds to heme in guanylyl cyclase to activate cGMP, a process responsible for maintaining endogenous levels of cGMP (354). This effect is blocked by potent HO inhibitors but not NO inhibitors (274). Consistent with endogenous distribution of HO in the brain, it has been suggested that CO can influence neurotransmission like NO (457). CO is involved as a retrograde messenger in LTP as well as in mediating glutamate effectson metabotropic receptors (193). This is corroborated by the finding that HO inhibitors block the conductance of specific ions channels associated with metabotropic receptor activation in the brain regulates via a cGMP-dependent mechanism (187, 255). Experimental evidence indicates that CO plays a role similar to that of NO in signal transduction processes. HO resembles NOS in that the electrons for CO synthesis are donated by cytochrome P450 reductase, which is 60% homologous at the amino acid level to the carboxy-terminal half of NOS (96, 458). Like NO, CO binds to iron in the heme moiety of guanylyl cyclase. However, while NO mediates glutamate's effects primarily at N-methyl-d-aspartate (NMDA) receptors, CO is involved in the effects of glutamate at a level of metabotropic receptors. As CO and NO play important roles in the regulation of CNS function, impairment of CO and NO metabolism results in abnormal brain function (Fig. 12) (97, 99, 337).
FIG. 12.
Schematic signal transduction pathways underlying the interfunctional role of CO, NO, and H2S in the modulation of cell survival. NO exerts neuroprotection through stimulation of the sGC/cGMP/PKG system as well as through S-nitrosylation. As a consequence of S-nitrosylating reactions, inhibition of NFκB and caspase-3 activity and a decrease in cell death occur. NO-mediated transcriptional activation of the Keap1/Nrf2/ARE pathway, associated with activation of KCa++ channel and inhibition of mitochondrial energy transductions at level of complex IV (Cox), activates mitochondrial-dependent H2O2-mediated redox signaling, leading to upregulation of pro-survival mechanisms, such as vitagenes HO-1 and heat shock protein 70, Bcl2, and mitochondrial biogenesis. The latter results from activation of coactivator PGC-1α and mtTFA. CO-mediated activation of mitochondrial redox signaling results in antiinflammatory, antiproliferative, and antiapoptotic effects, which confer neuroprotection. This occurs through activation of multiple pathways, including PPARγ, PGC-1α, and mtTFA, which induce mitochondrial biogenesis; P38 MAPK, HIF1α, KCa++ channel, PI3K-Akt, and Nrf2, as well as inhibition of ERK-1/2. H2S is a highly reactive, strong reducing molecule that easily reacts with ROS and RNS, thus providing antioxidant activity and, in addition, activates ATP-sensitive potassium channels. Known cellular targets of H2S include cytochrome c oxidase (complex IV, Cox) and carbonic anhydrase (CA). This gas H2S has also been demonstrated to regulate cellular signal transduction pathways, including thioredoxin reductase, HO-1, and glutamyl-cysteine synthetase, resulting in cytoprotection. KATP, ATP-dependent K+; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase.
CO, is a primordial gas, produced and metabolized in almost all organisms from prokaryotes to mammals, and serves as an important signaling molecule owing to a its capability to bind to reduced transition metal centers in heme proteins (354). Both exogenous CO and endogenous CO produced by heme oxygenase generate a pro-oxidant milieu in aerobic mammalian cells that initiates signaling via ROS formation. Signaling promoted by CO and mediated by ROS presumably is dependent on the CO concentration and the time of exposure, on the localization of heme proteins (e.g., mitochondrial or nonmitochondrial sites), or on the specificity of redox reactions. Activation of redox-sensitive transcription factors or stress-activated kinases, which induce expression of cytoprotective enzymes, thus conferring adaptations and tolerance to oxidative stress, represent a fundamental response to CO. However, elevated or protracted CO exposures elicit responses that generally are nonspecific, favoring biological oxidations to an extent that disrupt cellular homeostasis (354). It is generally recognized that CO exposures that avoid macromolecular damage should stand in a range of tissue CO concentrations that are below those produced at 20%–25% carboxyhemoglobin, the conventional threshold in mammals for serious poisoning. Beneficial for health are slow CO-releasing agents that do not exceed a concentration of 50 micromolar within a cell, as well as inhaled CO concentrations that acutely do not exceed 500 ppm for 1 h or 50 ppm continuously, in rodents. In humans, exposure limits of 50 ppm for 8 h and exposure concentration maximum of 200 ppm appear safe and may induce physiological effects (354).
The normal rate of endogenous CO production results in cellular CO concentrations, ranging from picomolar to nanomolar levels. This physiological concentration, however, can increase several fold depending on the heme turnover, whereas cellular CO content can increase 20–50-fold after exogenous CO administration. At high CO levels, the physiology is dominated by a range of adaptive stress redox-related responses, overlapping those observed during hypoxia. In the past, mitochondrial CO effects in living tissues were considered minor because the a3 heme is primarily in the oxidized state. However, even at physiological PO2, CO does bind cytochrome oxidase, as some a3 does remain in the reduced state, especially in metabolically active tissues like the brain and the heart. In this mileu, bound CO reduces the cytochrome bc1 region of the chain respiratory conplexes, thereby promoting significant mitochondrial ROS production (Fig. 13), which disrupts mitochondrial glutathione redox status. CO-dependent mitochondrial ROS generation has also been demonstrated for endogenous CO (427). Recently, signalization through mitochondrial ROS production by CO has become an emerging area and mitochondrial ROS are now implicated in various cellular regulatory processes, including cell proliferation, expression of angiogenesis factor, metalloproteinase mitochondrial biogenesis (328), and stabilization of hypoxia-inducible factor-1 (HIF-1). Low physiological levels of CO such as those generated by HO-1 induction have been demonstrated to elicit strong proliferative and antiapoptotic effects mediated by various signaling pathways, including PI3K-Akt, signal transducer and activator of transcription, and MAPK-Erk (33) (Figs. 10 and 13). Consistent to the metal-binding properties of CO, all heme-containing proteins are possible candidates with biological significance as CO sensors. These include proteins with heme as prosthetic group, such as hemoglobin, myoglobin, sGC, cyclooxygenase, cytochrome p450, cytochrome c oxidase, NOS, NADPH oxidase (Nox), transcription factors, neuronal PAS domain protein 2, and Bach-1 and Bach-2 (33).
FIG. 13.
The ubiquinone (Q) cycle is initiated when one electron from ubiquinol (QH2) is donated to the Rieske-iron sulfur protein and the second electron is donated to cytochrome b. The intermediate moiety is the free radical ubisemiquinone (Qo), which can donate electrons to molecular oxygen to generate superoxide. Mitochondrial electron transport chain generates superoxide at complexes I, II, and III. Complexes I and II generate superoxide within the mitochondrial matrix. Complex III can generate superoxide in both the intermembrane space and the matrix. Release of superoxide from complex III into the cytosol is followed by conversion to H2O2 and subsequent activation of oxidant-dependent (redox) signaling pathways, which results in preconditioning. R-FeS, Rieske-iron sulfur.
Although CO at high concentrations is toxic, generation of lower amounts of ROS in response to exogenous low-dose CO might affect cellular respiration eventually resulting in adaptation (33). This is consistent with the general notion that the mitochondrial respiratory chain complexes I and III are the main sites of superoxide production (177). In this context, any impairment in the electron transfer process determines that upstream carriers become more reduced, thus filling Q cycle electron pool. The cytochrome (cyt)2 bc1 complex (EC 1.10.2.2) (cyt bc1 complex), localized in the inner membrane of mitochondria, couples the oxidation of a substrate quinol (QH2) with the generating proton motive force across the energy transducing membrane system, where the energy is ultimately stored in the form of ATP (Fig. 13). Cytochrome bc1 complexes contain four redox-active metal centers, arranged in two separate chains. The high potential chain consists of the Rieske iron-sulfur [2Fe2S] cluster (Fig. 13) and cyt c1. The low potential chain, which binds to the cyt b subunit of ubiquinol oxidizing complexes, consists of two b-type hemes, cyt bL and bH, labeled for their relatively lower and higher electrochemical potentials (177). Three enzymatic binding sites participate in catalysis on the cyt bc1 complex: the quinol oxidase (Qo) site, quinol reductase (Qi) site, and a docking site for soluble cyt c on cyt c1. The Qo site is located toward the positively charged side of the membrane, where protons are released during turnover of the enzyme (Fig. 13). The Qi site is located toward the negatively charged surface of the membrane, where protons are taken up during catalysis. The water-soluble cyt c docking site is located on the c-type cyt representing the terminal electron carrier within the cyt bc1 complex on the positive side of the membrane. Cyt bc1 complex catalysis is thought to occur by a Q-cycle mechanism (Fig. 13). Conceivably, a key reaction in the Q-cycle is the bifurcated electron transfer at the Qo site, although the exact mechanism is still controversial (177). In the bifurcated reaction, QH2 is oxidized at the Qo site, with one electron being transferred through the high potential chain to reduce cyt c, and the other electron is transferred through the low potential chain, to reduce a quinoid species (Q or SQ, depending on the state of the two-electron gate) at the Qi site. Two turnovers of the Qo site are required to reduce a Qi site Q to a QH2 (Fig. 13). Under some conditions, the Q-cycle can be short-circuited by various bypass reactions, some of which yield the physiologically deleterious superoxide (177). The bypass reactions are typically observed in vitro under partially inhibited conditions, for example, in the presence of antimycin A, or under high proton motive force, where it is possible that the [2Fe2S] cluster can oxidize QH2 to a semiquinone (SQ), but processing of electrons by the low potential chain is hindered, resulting in the accumulation of an SQ intermediate, which in turn can reduce O2 to superoxide (427).
Physiologically, electron transport chain consumes up to 90% of the oxygen taken up by a cell, of which ∼1% is transformed in superoxide under normal physiological conditions (33). The rate of mitochondrial electron transfer from semireduced Q to molecular O2 to form superoxide is proportional to the product of the concentrations of semireduced Q and O2. It is estimated that under pathophysiological conditions, superoxide production can occur at 2%–10% of the uninhibited rates. Superoxide dismutases, in turn, generate the more stable hydrogen peroxide before it is transformed by catalase in water or, depending on the presence of metals, into highly reactive hydroxyl radicals. Thus, SOD2, which directs H2O2 out of the mitochondrion, accomplishes two functions: the classical scavenging of superoxide, as well as its signalization as ROS-dependent signaling pathways. The binding of CO to cytochromes localized within complex IV (i.e., cytochrome a3) promotes reduction in the respiratory carriers in the cytochrome bc1 region of complex III, associated with increased leakage of superoxide and H2O2 production (Fig. 13).
ROS-dependent signaling mediated by CO may involve other pathways, such as xanthine oxidase and Nox. The latter is assembled at the membrane only after stimulation. This confers biological significance to the cytosolic compartment, where the NADPH membrane-dependent system generating radicals represent the primary site of action for the CO molecule, rather than oxidases, which are located in the more distal mitochondrial milieu. Increasing evidence indicates that CO modulates mitochondrial ROS production. Mitochondrial H2O2 production as consequence of CO binding to Cox leads to activation of PI3K-Akt pathway with subsequent activation of mitochondrial biogenesis (33). Further, CO increases RNA expression of Nrf1, Nrf2, and peroxisome proliferator-activated receptor-gamma coactivator-1α, the transcription factors involved in the regulation and activation of mitochondrial biogenesis (33). In addition, CO upregulates mitochondrial transcription factor A, SOD2, Bad, as well as activates KCa channels and modulates vascular relaxation through inhibition of endothelin-1 (422). Of note, in Rhodospirillum rubrum, a photosynthetic bacterium, CO binds to CooA, which, upon exposure to CO, acquiring DNA-binding transcriptional activity for the CO dehydrogenase gene, promotes the oxidative conversion of CO to CO2. Consistently, heart cytochrome c oxidase is able to metabolize CO to CO2, via its oxygenase activity (473), However, whether this occurs also in brain mitochondria remains to be determined.
CO was also found to be protective through an antiinflammatory action, as demonstrated initially in a model of endotoxic shock, as well as mouse cardiac xenotransplantation (336). Accordingly, mouse hearts transplanted into rats treated with cyclosporine A and cobra venom factor as immunosyppressants survive indefinitely. However, in these experimental conditions, inhibition of HO activity or ablation of gene expression for HO-1 causes acute rejection, unless the donor and recipient received CO at a concentration of 250 ppm, similar to that found in heavy smokers, in which case grafts survive indefinitely (338). In this experimental paradigm, CO appears to be able to substitute for HO-1 in suppressing the pro-inflammatory response that causes graft rejection (339). These seminal experiments launched CO as a powerful antiinflammatory agent acting at a level of macrophages, to control the balance of pro-inflammatory and antiinflammatory molecular events. Inflammation is a complex dynamic process initiated by the body in response to tissue injury or infective agents. In this context macrophages are central for initiation, maintenance, and resolution of inflammatory process, through a series of steps involving antigen presentation, phagocytosis, and immunomodulation via cytokines and growth factors production (338). Activation signals include cytokines, such as IFNs, granulocyte-macrophage colony-stimulating factor, and TNFα as well as bacterial LPS, extracellular matrix proteins, and other chemical mediators. Macrophages recognize distinct pathogen-associated molecular patterns by Toll-like receptors (TLRs) that activate signaling pathways inducing expression of pro-inflammatory genes (33, 334). Consequently, inhibition of inflammatory events is a necessary process when the noxious stimulus promoted by invading cells and pathogens has been removed, so that survival of host cells is ensured. During the inflammatory process, secretion of antiinflammatory cytokines such as IL-10 and transforming growth factor β promoted by CO leads to neutralization of activated macrophages, as demonstrated in vivo and in vitro after LPS administration (121). Notably, IL-10 itself induces HO-1 expression, thus generating a feedback mechanism whereby a persistent antiinflammatory effect promoted by CO is maintained (33). In conclusion, CO modulates inflammatory processes through the (a) prevention of platelet aggregation, thus exerting antithrombotic affects, (b) downregulation of plasminogen activator inhibitor type 1 expression, and (c) antiapoptotic action in several cell types, including endothelial cells, fibroblasts, hepatocytes, and β-cells of the pancreas (25). In addition, CO inhibits smooth muscle cell proliferation associated with neointimal thickening in inflammatory lesions in vivo (339). Many of the observed effects of CO have been obtained by exposing cells or animals to gaseous CO by inhalation; interestingly, the recently discovered CO-releasing molecules appear to afford similar protective action, thereby providing an alternative therapeutic approach for those pathophysiological conditions where CO treatment is warranted (7, 121).
B. NO and NO synthases
NO plays multiple roles in the nervous system (18, 108, 323). In addition to regulating proliferation, survival, and differentiation of neurons, NO is also involved in synaptic activity, neural plasticity, and memory function. NO exerts long-lasting effects through regulation of transcription factors and gene, thus modulating cell differentiation and survival processes in the brain. Signaling by NO is also mediated by reactive nitrogen species through targeted modifications of critical cysteine residues in proteins, including S-nitrosylation and S-oxidation, as well as by lipid nitration. NO is involved neuroinflammation and neurodegeneration, such as in AD, amyothrophic lateral sclerosis, Parkinson's disease, multiple sclerosis, Friedreich's ataxia, and Huntington's disease. Susceptibility to NO and its reactive nitrogen species is modulated by intracellular reducing potential, mainly reduced glutathione with its related antioxidant enzymes as well as redox-dependent cellular stress resistance signaling pathways. In view of this, neurons, in contrast to astrocytes, appear particularly vulnerable to the effects of nitrosative stress (323).
NO synthesis is catalyzed by the NOS family of enzymes, which convert arginine to citrulline and NO• (Fig. 6). NOS, localized in the CNS and in the periphery (323), is present in three well-characterized isoforms: (a) neuronal NOS (nNOS, type I), (b) endothelial NOS (eNOS; type III), and (c) inducible NOS (iNOS, type II). Activation of different isoforms of NOS requires various factors and cofactors. In addition to a supply of arginine and oxygen, an increase in intracellular calcium leads to activation of eNOS and nNOS, and formation of calcium/calmodulin complexes is a prerequisite before the functional active dimer exhibits NOS activity, which depends also on cofactors such as tetrahydrobiopterin, flavin adenine dinucleotide, flavin mononucleotide, and NADPH (99). nNOS has a predominant cytosolic localization, whereas the eNOS is bound to the plasma membrane by N-terminal myristylation (18). In contrast to nNOS and eNOS, iNOS can bind to calmodulin even at very low concentration of intracellular calcium; thus, iNOS can exert its activity in a calcium-independent manner. iNOS, usually present only in the cytosol, also requires NADPH, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin for full activity. eNOS expressed in cerebral endothelial cells critically regulates cerebral blood flow. However, a small population of neurons in the pyramidal cells of CA1, CA2, and CA3 subfields of the hippocampus and granule cells of the dentate gyrus express eNOS. nNOS, which is expressed in neurons, is critically involved in synaptic plasticity, neuronal signaling, and neurotoxicity. Activation of nNOS forms part of the cascade pathway triggered by glutamate-receptor activation that leads to intracellular cyclic GMP elevation. The levels of iNOS expression in the CNS are generally very low. However, after noxious stimuli, such as viral infection or trauma, astrocytes and microglia express high levels of induced iNOS (350). Activation of iNOS requires gene transcription, and the induction can be promoted by endotoxin and cytokines (IL-1, IL-2, IFN-γ, and TNF). iNOS induction can be blocked by antiinflammatory drugs (dexamethasone), inhibitory cytokines (IL-4 and IL-10), prostaglandins, tissue growth factors, or inhibitors of protein synthesis, for example, cycloheximide (350).
The discovery of the role of NO as a messenger molecule has revolutionized the concept of neuronal communication in the CNS. NO is a gas freely permeable to the plasma membrane. Thus, NO does not need a biological receptor to influence the intracellular communication or signaling transduction mechanisms (108). Once generated, the cell cannot regulate the local concentration of NO; therefore, the other way to influence NO activity is to control its synthesis. The activity of NO also terminates when it chemically reacts with a target substrate. NO when produced in small quantities can regulate cerebral blood flow and local brain metabolism (8, 99, 149), neurotransmitter release, and gene expression, and play a key role in morphogenesis and synaptic plasticity (181, 288). It is also generally accepted that NO is a major component in signaling transduction pathways controlling smooth muscle tone, platelet aggregation, host response to infection, and a wide array of other physiological and pathophysiological processes (317, 398, 453). Under conditions of excessive formation, NO is emerging as an important mediator of neurotoxicity in a variety of disorders of the nervous system (8, 486).
A large body of evidence shows cytoprotection by NO in various models of cellular toxicity and death: (a) direct scavenging of superoxide, as well as indirect action on the release of iron from ferritin stores through formation of iron-nitrosyl (399); (b) interaction of NO with cysteine residues on the NMDA receptor and inhibition of calcium influx (321); (c) inactivation of caspases (486); (d) activation of a cyclic GMP-dependent survival pathways (181); and (e) signal for the induction of cytoprotective proteins, such as Hsps (108). Current opinion holds that the intracellular redox state is the critical factor determining whether in brain cells NO is toxic or protective (323). The mechanistic involvement of NO as pro-inflammatory or antiinflammatory agent is dictated by the complexity of NO chemistry when applied to biological systems (190) (Fig. 12). As described in complete mechanistic details by Stamler, the reactivity of the NO depends on the oxidation state of the nitrogen atom, which makes this molecule prone to acquire different redox-active forms (190). In contrast to NO, which contains one unpaired electron in the outer orbital, nitrosonium cation and nitroxyl anion are charged molecules being, respectively, the one-electron oxidation and reduction products of NO. Whereas nitrosonium cation can be transferred reversibly between cysteine residues (transnitrosation), nitroxyl anion can be formed by hemoglobin, nNOS, and S-nitrosothiols (RSNO). Central to the biochemistry of NO is the reaction with sulphydryl moieties to form S-nitrosyl derivatives or RSNO, a process termed S-nitrosation (28). Conceivably, RSNO, such as S-nitroso-glutathione or nitroso-cysteine, represents a chemical process for storage, stabilization, and preservation of bioavalilable NO in vivo (179). In addition, due to its relatively aboundant presence, GSH, as the main intracellular free thiol, represents a critical determinant of the reactivity and fate of NO. Thus, through S-nitrosation NO modulates activity and function of several enzymes and proteins (28). However, oxidative modification in protein structure and function may occur when reactive nitrogen species reach a critical threshold, and hence nitrosative stress may ensue (179). At the cellular level, nitrosative stress inhibits cell growth and causes apoptosis, mediating a wide array of pathogenic effects elicited by NO (179). The intriguing aspect of the parallels between the effects mediated by increased RNS and ROS is the ability of cells to respond to these two types of stress, and depending on the severity of the nitrosative/oxidative insult, this response may result in adaptation and resistance to toxicity (108, 179, 281, 292, 317).
C. Hydrogen sulfide
Hydrogen sulfide (H2S), a gas with the characteristic odor of rotten eggs, is known for its toxicity and as an environmental hazard, inhibition of mitochondrial respiration resulting from blockade of cytochrome c oxidase being the main toxic mechanism. Recently, however, H2S has been recognized as a signaling molecule of the cardiovascular, inflammatory, and nervous systems; therefore, alongside NO and CO, H2S is referred to as the third endogenous gaseous transmitter (404, 434, 460) (Fig. 12).
H2S is an endogenously produced gas in the central nervous system (442) as well as in various peripheral tissues and is present in the blood at an estimated concentration of approximately 1 μM, which can rise up one order of magnitude in pathological states such as diabetes or hepatic encephalopathy (230, 434). There is some lack of clarity about the various biological forms of H2S. Chemically, it can be distinguished as free H2S, acid-labile sulfide (ALS), and dithiothreitol-labile sulfide (DLS) forms, each probably having quite different physiological significance. Free H2S is the form also known as inorganic sulfide, which can be present as H2S, HS− (hydrosulfide anion), or S2− (sulfide anion) in the aqueous milieu and as H2S in the gas phase (breath and flatus). The pKa values for the first and second dissociation steps of H2S are 7.04 and 11.96, respectively. Therefore, in the aqueous state, such as within a cell or in plasma at physiologic pH 7.4, approximately one-third exists in the undissociated volatile form (H2S) and the remainder largely as the hydrosulfide anion (HS−). Very small amounts of (S2−) are present. However, there is considerable ambiguity in literature regarding the terms describing free H2S. Besides inorganic sulfide, terms such as H2S, sulfide, and total sulfide are used. We here refer to the sum of H2S and HS− as total sulfide. Also convenient is to distinguish these forms of H2S from acid-labile sulfide (ALS) and dithiothreitol-labile sulfide (DLS). ALS is the form from which H2S is liberated when a biological matrix is treated under acidic conditions, and is contained in iron–sulfur clusters of nonheme iron–sulfur proteins (e.g., ferredoxins). DLS is the form from which H2S is liberated when a biological matrix is treated with dithiothreitol (DTT). This form belongs to the class of biological sulfane sulfur. Sulfane sulfur atoms are defined as divalent sulfur atoms bonded only to other sulfur, except that they may also bear ionizable hydrogen at some pH values. Labile sulfane compounds are compounds where elemental sulfur is associated with protein, either in the form of persulfides (R–SSH) or polysulfides such as (R–Sn–R) where n ≥ 3, polythionates (−O3S–Sn–SO3−), thiosulfate (SSO3 2−), and thiosulfonates (R–S(O2)S−) (443).
H2S is produced by three enzymes in mammalian tissues, two pyridoxal phosphate–dependent enzymes (cystathionine β-synthase [CBS] and cystathionine γ-lyase [CSE]), and a newly described enzyme, 3-mercaptopyruvate sulfurtransferase (460) (Figs. 6 and 12). CBS utilizes pyridoxal phosphate as a cofactor (Hishigami 2009) and it catalyzes the production of H2S from cysteine by β-elimination (214) (L-cys + L-Hcy → L-cystathionine + H2S) or β-replacement reactions (L-cys + 2-mercaptoethanol → S-hydroxyl-L-cysteine + H2S) (442). In addition, nonenzymatic H2S production from organic polysulfides derived from garlic has been suggested as a potential source in the circulation (434).
There are three known pathways of H2S degradation: mitochondrial oxidation to thiosulfate, which is further converted to sulfite and sulfate; cytosolic methylation to dimethylsulfide; and sulfhemoglobin formation after binding to hemoglobin (434). Similar to NO and CO, H2S can also bind to hemoglobin—which represents the biochemical sink for these gaseous transmitters. Consequently, saturation with one of these gases might lead to enhanced plasma concentrations and, subsequently, to biological effects of the other gases. H2S is oxidized into thiosulfate (S2O32−), sulfite (SO32−), and sulfate (SO42−) (113) and excreted from the kidney (434).
In the central nervous system, H2S functions not only as a neuromodulator, but also a neuroprotectant against oxidative stress (455). In the cardiovascular system, H2S relaxes vascular smooth muscles by the activation of ATP-dependent K+ channels and inhibits smooth muscle cell proliferation via the mitogen-activated protein kinase signaling pathway. These effects are important for maintaining blood pressure and preventing vessel structural remodeling, and identifies H2S as an important factor in the development of some vascular diseases, such as hypertension. H2S also shows cardioprotective effects in ischemic myocardium and septic and endotoxin shock (119).
In the CNS, CBS is highly expressed in the hippocampus and the cerebellum (230). CBS is mainly localized to astrocytes (442). CSE is mainly expressed in cardiovascular system, but was also found in microglial cells, spinal cord, and cerebellar granule neurons. 3-Mercaptopyruvate sulfurtransferase is localized to neurons (442). H2S production in astrocytes is approximately 10-fold higher than in cultured microglial cells, in NT-2 cells, or in SH-SY5Y cells (251). This finding suggests that astrocytes are the main brain cells producing H2S. H2S selectively enhances the NMDA receptor-mediated responses and facilitates the induction of hippocampal long-term potentiation (LTP), a synaptic model of memory and learning (433). H2S may activate NMDA receptor by virtue of its reducing property. One plausible redox modulatory site is the cysteine (Cys) pair (Cys744 and Cys798) located on the extracellular domains of the NR1 subunit (442). Intracellularly, H2S enhances NMDA receptor-mediated response via cAMP production. Exogenous H2S increases the production of cAMP in primary cultures of rat cerebral and cerebellar neurons, and in some neuronal and glial cell lines (230). cAMP activates cAMP-dependent protein kinase during the initiation and late phase of LTP; consequently, activated cAMP-dependent protein kinase may, in turn, phosphorylate NMDA receptor subunits NR1, NR2A, and NR2B at specific sites and thus enhance NMDA currents, which is essential for LTP induction (442). H2S also modulates the synaptic responses of serotonergic neurons and regulates the release of corticotropin-releasing hormone from the hypothalamus (364). H2S upregulates γ-aminobutyric acid B receptor, a G protein-coupled receptor located at pre- and postsynaptic sites (364). In addition, H2S has been shown to hyperpolarize neurons in the CA1 and dorsal raphe nucleus by increasing K+ efflux probably via ATP-dependent K+ channels (442). H2S influences tyrosine and mitogen kinases; receptor tyrosine kinases as well as MAPKs regulate fundamental cellular activities such as apoptosis, differentiation, metabolism, motility, cell division, and survival (442).
In addition to its role in signal transduction, H2S can protect neuron cells from oxidative stress, not only by increasing the levels of antioxidant glutathione (113), but also by activating ATP-dependent K+ channels and Cl− channels (442). H2S appears to increase GSH levels by activating γ-glutamyl-cysteine synthetase (114). In addition, increased intracellular levels of cysteine were found as consequence of glutamate-independent activation of cystine/glutamate antiporter. This leads to increased cystine uptake that promotes formation of cysteine and glutathione (231). Another important mechanism for the effect of H2S on GSH may be via enhancing glutamate uptake (265). In human cultured neuron cells, H2S inhibits peroxynitrite, acting as an endogenous peroxynitrite scavenger (119). Thus, H2S may act as a neuroprotectant against oxidative stress (Fig. 6). Changes in the level of H2S in the brain have been demonstrated in various CNS pathologies. A 55% decrease in the brains of AD patients has been documented for both H2S content and CBS activity, which was associated with a parallel loss of AdoMet content, a CBS activator. The CBS activity is decreased in homocysteinuria, febrile seizure frequently occurring in children. Conversely, CBS levels in Down's syndrome brains were approximately three times greater than those found in normal controls (119).
D. H2S and suspended animation
Suspended animation is a hibernation-like metabolic status characterized by a marked, still reversible, reduction of energy expenditure, which allows nonhibernating species to sustain environmental stress, such as extreme changes in temperature or oxygen deprivation (460). The first line of experiments show that in animals that do not normally hibernate, an hibernation-like state can be obtained, as shown by the Roth laboratory (36, 331, 374). In this work, nematode embryos exposed to anoxic conditions were shown to survive by entering into a suspended animation-like state, which was associated with specific changes in gene expression. This was observed below and above a 10-fold range of hypoxic oxygen concentrations from 0.01% to 0.1%. While embryos exposed outside this range survived completely and progressed to normal development, exposure within this range was associated with loss of protection and cellular damage and death after 24 hours. In these conditions exposure to CO resulted in survival, an effect mediated by a competition with cytochrome oxidase as a target for gas binding resulting in a scavenging of all available oxygen (434). Subsequently, the Roth group provided further evidence that inhaled H2S can induce a suspended animation-like state in mice. In this condition, animals breathing 80 ppm H2S exhibited a dose-dependent reduction of respiratory and heart rates, oxygen uptake, and CO2 production, associated with a drop in body core temperature to levels ∼2°C above ambient temperature (460). All these effects were totally reversible after H2S washout, with animals showing a complete recovery and normal behavior (363). Relevant to the finding that a controlled reduction in cellular energetic expenditure is consistent with maintenance of ATP homoeostasis is the notion of an improved outcome during shock states. Notably, pretreatment with inhaled H2S (150 ppm) for only 20 minutes significantly prolonged survival without apparent damage for mice exposed to lethal hypoxia (5% oxygen), as well as in rats receiving a single intravenous bolus of Na2S (460). Thus, H2S can induce adaptive (hormetic) stress responses that protect cells against a range of adverse conditions.
It is generally recognized that hibernation confers cytoprotection, so that tissues from hibernating animals are particularly resistant to various hypoxic and ischaemic insults (460). This is relevant in organ transplantation where, similarly to what was shown for CO, H2S effects may provide better preservation of transplantable organs. It is noteworthy that, owing to a larger surface area-to-mass ratio than humans, rodents are much more capable of a rapid reduction in core temperature when challenged with a toxic insult than are humans (434). This makes the clinical relevance of some murine models questionable. Mice, in fact, present a body mass 3,000-times less than that of adult humans, and a heart rate 14-times greater and respiratory rates 10-times faster than in humans. In addition, at rest, O2 consumption is 10 times less in humans than in rodents, so that much of the oxygen consumed in a mouse is used for heat generation. It is possible, therefore, that in mice H2S-induced reduction of O2 consumption may largely account for specific ATP-requiring processes, such as mitochondrial uncoupling, whereas ion homeostasis is not drastically compromised. As a consequence, the human window of compromised oxidative phosphorylation is likely to be much smaller than in rodents (460).
E. Functional interrelation of NO, CO, and H2S gases and their relevance to hormesis
Both CO and NO activate the GC system by binding the active heme and enhancing cGMP production. Although NO is definitely more potent than CO in activating guanylyl cyclase, CO generally operates in the system under specific physiological conditions, as in the case of regulation of vascular smooth muscle cell proliferation during hypoxic conditions (355). Of the two gases, NO is the more reactive, having the same effective size and polarity as the O2 molecule, and, unlike CO, binds to both Fe2+ and Fe3+ heme. Moreover, low CO concentrations stimulate NO release and are associated with peroxynitrite generation in vascular cells. The chemistry of CO-mediated NO release depends on the initial distributions of NO and CO in the cell and the differing equilibrium constants for transition-metal binding of the two gases. The association constants for NO binding to Fe2+ heme proteins such as myoglobin and hemoglobin are greater than those for CO (355). For hemoglobin, the affinity of Fe2+ for NO is at least a thousand times greater than that for CO; however, CO dissociation is much slower than for NO or O2. Therefore, CO gradually displaces NO from Fe2+ heme proteins, requiring minutes or longer for equilibrium even with an excess of CO. NO displacement from Fe2+ by CO may also be enhanced in proximity to reduced thiols that serve as a sink for SNO protein formation. (355). HO-1 and/or CO, and NOS2 and/or NO are two systems functionally interrelated. In some situations, CO can induce expression of NOS2 and, in others, inhibits expression of NOS2 and consequently NO. NO upregulates HO-1 with production of CO (316). We have recently found evidence for a functional relationship between CO and NO. In endotoxic shock, the salutary action of CO in rat brain appears to depend on the activation of NFκB, which triggers transcription of NOS2 with production of NO, and subsequently results in the upregulation of HO-1 (96). In the absence of any of these steps, the beneficial effect of CO is lost (33). To what extent CO and NO act interdependently in other physiological and pathological conditions that involve CO and/or NO is unknown. Endogenous CO has been suggested to control constitutive NOS activity. Moreover, CO may interfere with NO binding to guanylyl cyclase, and this in addition to the important role of HO in regulating NO generation, owing to its function in the control of heme intracellular levels, as part of the normal protein turnover (33). This hypothesis is sustained by recent findings showing that HO inhibition increases NO production in mouse macrophages exposed to endotoxin (128).
H2S belongs to a family of labile biological mediators termed gasotransmitters, which share many similarities. As a gasotransmitter, H2S rapidly travels through cell membranes without using specific transporters (434). H2S can induce an upregulation of heme oxygenase 1 and cytochrome c oxidase subunit V. By upregulating HO1, H2S can trigger the production of CO, thus providing cytoprotective and antiinflammatory effects. These effects are also mediated by inhibition of the nuclear factor-κB (NFκB) pathway and downregulation of inducible NO synthase (iNOS) expression and NO production by inflammatory stimuli. Expression of HO1 is probably the result of the activation by sulfide of the extracellular signal regulated kinase pathway (434). H2S can also inhibit cellular respiration, at a level of cytochrome c oxidase via a reaction with its copper center. Cytochrome c oxidase is a key regulator of cellular respiration; all three gasotransmitters (NO, CO, and H2S) have competitive effects on this enzyme. Inhibition of cytochrome c oxidase is central to the regulation of cellular oxygen consumption by H2S, and has been implicated in the pharmacological effects of H2S, together with induction of suspended animation (see above).
Moreover, evidence is accumulating to demonstrate that H2S, as opposed to NO and CO, induces vasorelaxation that is not mediated by the cGMP signaling pathway. This indicates that H2S is a novel endogenous gaseous modulator of vascular contractility. At the same time, similar to NO and CO, H2S can inhibit VSMC proliferation and induce apoptosis in vitro (23). Using cultured VSMC, exogenous H2S could dose dependently suppress the proliferation of VSMC through the MAPK signaling pathway. Studies using molecular means to overexpress CSE in cultured VSMC found that endogenous H2S could also attenuate the rate of cell proliferation and increase the rate of cell apoptosis. The latter effect is via the activation of MAPK and caspase-3. This may be relevant to the pathogenesis of atherosclerosis, as well as vascular graft occlusion and restenosis after angioplasty (23). However, the precise role of smooth muscle cell proliferation and apoptosis in the development of diseases, particularly the molecular mechanisms underlying the relationship between the diseases and cell proliferation, remains to be characterized.
As a key early event in the cellular phenotypic changes, smooth muscle cells that are in a quiescent state (G0) in normal uninjured vessels transit through the G1 phase of the cell cycle and enter into the S phase to undergo replication (472). Cell cycle progression is under the control of cyclin-dependent kinases, which phosphorylate different specific target proteins when the cells enter different phases of cell cycle. Synchronized (G0) smooth muscle cells treated with S-diclofenac, a recently discovered H2S donor, recover at the early cell cycle alteration, a finding associated with a decreased p53 and p21 activity. Interestingly, it has been suggested that both vascular diseases and cancer similarly develop from a clonal proliferation at the sites of local tissue injury and inflammation, with underlying genomic alterations (23).
NO activity in the CNS provides an example of hormesis, being extremely toxic at high concentrations yet neurotrophic at lower concentrations. NO may be released by a variety of cell types (i.e., astrocytes, activated microglia, and macrophages) after injury or disease, becoming 10-fold greater than normal physiological concentrations (35, 322). At such elevated concentrations, NO can facilitate massive cell death via apoptotic mechanisms, contributing to the progression of a variety of neurodegenerative diseases, including Alzeheimer's disease and ALS. Of significance is that when low doses of NO are administered before a high and neurotoxic dose of NO, the prior preconditioning dose induces a marked protective effect. The dose–response characteristics of preconditioning doses conform to those of an hormetic dose response, suggesting that preconditioning is a manifestation of hormesis (87). The mechanism inducing the protective response appears to be mediated by the induction of H0-1 and is supported by multiple lines of research, including the use of H01 inhibitors and H01 null mice. H0-1 activation generates CO, bilirubin, and free iron via its actions on heme, which is released from NO-damaged heme proteins (35). It is likely that the protective effects induced by NO may be mediated, in part, by low doses of CO, which can induce the synthesis of MnSOD, and facilitate the activation of mitogen-activation protein kinase (MAKP) pathways, including the p38 MAPK pathway, which then activates NFκB, thereby inhibiting apoptosis (401, 447). These and other related findings have linked CO-induced effects at low and high concentrations with those of NO in an integrated, yet complex series of responses that are hormetic.
As is the case with NO and CO, H2S also has been reported to induce hormetic dose responses for a broad range endpoints of potential biomedical importance. In fact, the hormetic concept has integratively resolved numerous apparent disputes in the literature in which different groups of researchers reported opposite types of responses when using either high or low concentrations of H2S. However, when subsequent experiments were conducted that included a far broader dose–response continuum, the hormetic-biphasic dose response was typically found. This has been now widely reported for infarct volume in stroke-related experimental studies, investigations concerning various types of inflammatory processes, vasorelaxation/tension in aortic tissue, cell proliferation/apoptosis, as well as in the case of tumor promotion and inhibition. Since the concentration of H2S can vary considerably both within and between tissues and under differing conditions, it is likely that H2S could affect a complex array of biological responses that may challenge the capacity to make definitive biomedical and/or clinical interpretations or predictions. Li and Moore (256) closed their recent insightful review of the role of H2S in health and disease with a statement probing their articulated biological conundrum of how does one molecule display such widely differing effects and could it be due to different effects at differing concentrations. The answer to this seminal question is found within the framework of an hormetic dose–response interaction that is independent of biological model, tissue, and endpoint.
XVII. Future Directions
The burden of chronic neurodegenerative diseases on our society is expected to continue to increase, largely due to the aging of the population. Although no satisfactory treatments are yet available, it is timely to think about implementing prevention measures that harness the intrinsic cytoprotective mechanisms described in this review. Exploiting the ability of small molecules, many of which are dietary agents and therefore of presumed low toxicity, to mimic various stimuli that lead to the upregulation of these intracellular defenses is an exciting area for future development. As described in this review, oxidizable diphenols, sulforaphane, dimethyl fumarate, celastrol, curcumin, and FA induce the Keap1/Nrf2/ARE pathway and the HSR, which collectively control expression of more that 100 genes that are concerned with cellular protection and survival under various conditions of oxidative and electrophilic stresses. Importantly, this induction correlates with protection against chronic diseases, including neurodegenerative conditions, in many animal models. Since redox regulation and transcription factor cysteine modifications are central to both the Keap1/Nrf2/ARE pathway and HSR, such multitarget agents, all of which are reactive with sulfhydryl groups, are ideal for simultaneously manipulating these pathways and achieving synergistic protective effects.
Although there are many specific technical questions that could be identified and prioritized for follow-up research activities in the area of neurodegenerative disorders, we believe that the most significant future direction in this area is conceptual. That is, we believe that it is essential that the biomedical community become more informed about the robustness of the data underlying the hormetic dose response and the generalizability of this concept across biological models, endpoints, and chemical classes and its many biomedical and therapeutic implications. We believe that major concepts such as pre- and postconditioning are specific manifestations of the general hormesis concept. The hormesis concept has the capacity to expand the range of biological questions and testable hypotheses, as well as affect how studies are designed, including how results will be analyzed and modeled. The hormesis concept also suggests that therapeutic interventions will be constrained by the limits of biological plasticity that conforms to the quantitative features of the hormetic dose response. This framework would affect the expectations of pharmaceutical companies in their development of new treatment modalities across the broad spectrum of therapeutic activities. Thus, we believe that the hormetic concept is a basic biological concept that needs to be better studied and understood to more successfully treat human diseases and to improve human performance for numerous endpoints. In the future we would strongly encourage the many professional societies that are concerned with issues related to dose response to routinely include technical sessions at annual meetings and specialty conferences on hormesis. We would also recommend that the concept of hormesis be incorporated into the formal biomedical courses in graduate and medical schools. These suggestions are just the start, but these are necessary if the biomedical and clinical fields, including the neurosciences, are going to get the dose right, improving the health and well-being of those individuals we serve.
Abbreviations Used
- AD
Alzheimer's disease
- AMPK
AMP-activated protein kinase
- AOE
antioxidant enzyme
- ARE
antioxidant response element
- BDNF
brain-derived neurotrophic factor
- CBS
cystathionine β-synthase
- CO
carbon monoxide
- CoQ
coenzyme Q10
- CREB
cyclic AMP response element binding protein
- CSE
cystathionine γ-lyase
- cyclic GMP
cyclic guanosin monophospate
- Cys
cysteine
- CytC
cytochrome c
- eNOS
endothelial NOS
- ER
endoplasmic reticulum
- ERR
estrogen-related receptor
- ETS
electron transport system
- FA
ferulic acid
- FH
forkhead transcription factor
- GFAP
glial fibrillary acidic protein
- GRP
glucose regulated protein
- GSH
reduced glutathione
- GST
glutathione S-transferase
- H2S
hydrogen sulfide
- HD
Huntington's disease
- HDAC
histone deacetylase
- HIF-1
hypoxia-inducible factor 1
- HMOX1
ho-1 gene
- HNE
4-hydroxynonenal
- HO-1
Heme oxygenase-1
- HSE
heat shock elements
- HSF
heat shock transcription factor
- Hsp
heat shock protein
- HSR
heat shock response
- IFN
interferon
- IL
interleukin
- iNOS
inducible NOS
- JNK
jun N-terminal kinase
- KATP
ATP-dependent K+
- Keap1
Kelch-like ECH-associated protein 1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MPST
3-mercaptopyruvate sulfur transferase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAD+
oxidized nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAD(P)H
reduced nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor-κB
- NMDA
N-methyl-d-aspartate
- nNOS
neuronal NOS
- NO
nitric oxide
- NOS
NO synthase
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
Nuclear factor-erythroid 2 p45-related factor 2
- PD
Parkinson's disease
- PDK
pyruvate dehydrogenase kinase
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1α
- PI3K
phosphatidylinositol 3-kinase
- PMRS
plasma membrane redox system
- PPARγ
peroxisome proliferator-activated receptor γ
- PTP
permeability transition pore
- R-FeS
Rieske-iron sulfur
- ROS
reactive oxygen species
- RSNO
S-nitrosothiols
- RyR
ryanodine receptor
- SERCA
sarco endoplasmic reticulum calcium ATPase
- SIRT
silent information regulator two
- SMase
sphingomyelinase
- SOD
superoxide dismutase
- SPT
serine palmitoyl CoA transferase
- tBHQ
tert-butyl hydroquinone
- TCA
tricarboxylic acid cycle
- TNF
tumor necrosis factor
- UCP
uncoupling protein
- UPR
unfolded protein response
- VDAC
voltage-dependent anion channel
Footnotes
Reviewing Editors: Narayan Bhat, Jin-Song Bian, Susan Browne, Enrique Cadenas, Paola Chiarugi, Jeffrey Keller, Daniel Linseman, Pamela Maher, Mark Smith, Russell Swerdlow, and Bobby Thomas
Acknowledgments
Work from the authors' laboratories was supported by grants from MIUR, FIRB RBRN07BMCT, I.N.B.B., RCUK, the American Cancer Society (RSG-07-157-01-CNE), Cancer Research UK (C20953/A10270), Tenovus, the Royal Society, the Anonymous Trust, and by “Fondi Ateneo” 2007 and 2008, and the National Institute on Aging Intramural Research Program (M.P.M.). The authors acknowledge helpful discussions for the HSF posttranslational regulation with M.G. Santoro (Department of Biology, University of Rome Tor Vergata, Rome).
References
- 1.Abramowitz J. Dai C. Hirschi KK. Dmitieva RI. Doris PA. Liu L. Allen JC. Ouabain- and marinobufagenin-induced proliferation of human umbilical vein smooth muscle cells and a rat vascular smooth muscle cell lines, A7r5. Circulation. 2003;108:3048–3053. doi: 10.1161/01.CIR.0000101919.00548.86. [DOI] [PubMed] [Google Scholar]
- 2.Afonso V. Champy R. Mitrovic D. Collin P. Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Ahn BH. Kim HS. Song S. Lee IH. Liu J. Vassilopoulos A. Deng CX. Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akhtar MW. Raingo J. Nelson ED. Montgomery RL. Olson EN. Kavalali ET. Monteggia LM. Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function. J Neurosci. 2009;29:8288–8297. doi: 10.1523/JNEUROSCI.0097-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam J. Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 6.Albani D. Polito L. Batelli S. De Mauro S. Fracasso C. Martelli G. Colombo L. Manzoni C. Salmona M. Caccia S. Negro A. Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 7.Alberto R. Motterlini R. Chemistry and biological activities of CO-releasing molecules (CORMs) and transition metal complexes. Dalton Trans. 2007;7:1651–1660. doi: 10.1039/b701992k. [DOI] [PubMed] [Google Scholar]
- 8.Aliev G. Palacios HH. Lipsitt AE. Fischbach K. Lamb BT. Obrenovich ME. Morales L. Gasimov E. Bragin V. Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox Res. 2009;16:293–305. doi: 10.1007/s12640-009-9066-5. [DOI] [PubMed] [Google Scholar]
- 9.Allison AC. Cacabelos R. Lombardi VR. Alvarez XA. Vigo C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:1341–1357. doi: 10.1016/s0278-5846(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 10.Altmeyer PJ. Matthes U. Pawlak F. Hoffmann K. Frosch PJ. Ruppert P. Wassilew SW. Horn T. Kreysel HW. Lutz G. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double-blind study in 100 patients. J Am Acad Dermatol. 1994;30:977–981. doi: 10.1016/s0190-9622(94)70121-0. [DOI] [PubMed] [Google Scholar]
- 11.Amijee H. Madine J. Middleton DA. Doig AJ. Inhibitors of protein aggregation and toxicity. Biochem Soc Trans. 2009;37:692–696. doi: 10.1042/BST0370692. [DOI] [PubMed] [Google Scholar]
- 12.Anand P. Nair HB. Sung B. Kunnumakkara AB. Yadav VR. Tekmal RR. Aggarwal BB. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79:330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Anckar J. Sistonen L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv Exp Med Biol. 2007;594:78–88. doi: 10.1007/978-0-387-39975-1_8. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RM. Barger JL. Edwards MG. Braun KH. O'Connor CE. Prolla TA. Weindruch R. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in caloric restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araki T. Sasaki Y. Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 16.Argaud L. Prigent AF. Chalabreysse L. Loufouat J. Lagarde M. Ovize M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2004;286:H246–H251. doi: 10.1152/ajpheart.00638.2003. [DOI] [PubMed] [Google Scholar]
- 17.Arumugam TV. Phillips TM. Cheng A. Morrell CH. Mattson MP. Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Averna M. Stifanese R. De Tullio R. Beccaria F. Salamino F. Pontremoli S. Melloni E. Calpain-mediated activation of NO synthase in human neuroblastoma SK-N-BE cells. J Neurochem. 2009;110:412–421. doi: 10.1111/j.1471-4159.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 19.Balan V. Miller GS. Kaplun L. Balan K. Chong ZZ. Li F. Kaplun A. VanBerkum MF. Arking R. Freeman DC. Maiese K. Tzivion G. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balch WE. Morimoto RI. Dillin Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 21.Bando Y. Katayama T. Kasai K. Taniguchi M. Tamatani M. Tohyama M. GRP94 (94 kDa glucose-regulated protein) suppresses ischemic neuronal cell death against ischemia/reperfusion injury. Eur J Neurosci. 2003;18:829–840. doi: 10.1046/j.1460-9568.2003.02818.x. [DOI] [PubMed] [Google Scholar]
- 22.Barrett RM. Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baskar R. Sparatore A. Del Soldato P. Moore PK. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Batulan Z. Shinder GA. Minotti S. He BP. Doroudchi MM. Nalbantoglu J. Strong MJ. Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer I. Pannen BH. Bench-to-bedside review: carbon monoxide—from mitochondrial poisoning to therapeutic use. Crit Care. 2009;13:220. doi: 10.1186/cc7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beher D. Wu J. Cumine S. Kim KW. Lu SC. Atangan L. Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellia F. Calabrese V. Guarino F. Cavallaro M. Cornelius C. De Pinto V. Rizzarelli E. Carnosinase levels in aging brain: redox state induction and cellular stress response. Antioxid Redox Signal. 2009;11:2759–2775. doi: 10.1089/ars.2009.2738. [DOI] [PubMed] [Google Scholar]
- 28.Benhar M. Forrester MT. Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 29.Bensasson RV. Zoete V. Dinkova-Kostova AT. Talalay P. Two-step mechanism of induction of the gene expression of a prototypic cancer-protective enzyme by diphenols. Chem Res Toxicol. 2008;21:805–812. doi: 10.1021/tx7002883. [DOI] [PubMed] [Google Scholar]
- 30.Benton CR. Wright DC. Bonen A. PGC-1alpha-mediated regulation of gene expression and metabolism: implications for nutrition and exercise prescriptions. Appl Physiol Nutr Metab. 2008;33:843–862. doi: 10.1139/H08-074. [DOI] [PubMed] [Google Scholar]
- 31.Berenblum I. The modifying influence of dichloroethyl sulphide on the induction of tumours in mice by tar. J Pathol Bacteriol. 1929;32:425–434. [Google Scholar]
- 32.Bezprozvanny I. Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilban M. Haschemi A. Wegiel B. Chin BY. Wagner O. Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 34.Bishop NA. Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 35.Bishop A. Gooch R. Eguchi A. Jeffrey S. Smallwood L. Anderson J. Estevez AG. Mitigation of peroxynitrite-mediated nitric oxide (NO) toxicity as a mechanism of induced adaptive NO resistance in the CNS. J Neurochem. 2009;109:74–84. doi: 10.1111/j.1471-4159.2009.05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackstone E. Morrison M. Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 37.Blanc EM. Kelly JF. Mark RJ. Waeg G. Mattson MP. 4-Hydroxynonenal, an aldehydic product of lipid peroxidation, impairs signal transduction associated with muscarinic acetylcholine and metabotropic glutamate receptors: possible action on G alpha(q/11) J Neurochem. 1997;69:570–580. doi: 10.1046/j.1471-4159.1997.69020570.x. [DOI] [PubMed] [Google Scholar]
- 38.Bolanos JP. García-Nogales P. Almeida A. Provoking neuroprotection by peroxynitrite. Curr Pharm Des. 2004;10:867–877. doi: 10.2174/1381612043452910. [DOI] [PubMed] [Google Scholar]
- 39.Bonifati V. Rizzu P. van Baren MJ. Schaap O. Breedveld GJ. Krieger E. Dekker MC. Squitieri F. Ibanez P. Joosse M. van Dongen JW. Vanacore N. van Swieten JC. Brice A. Meco G. van Duijn CM. Oostra BA. Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 40.Borak J. Sirianni G. Hormesis: implications for cancer risk assessment. Dose Response. 2005;3:443–451. doi: 10.2203/dose-response.003.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boreham DR. Dolling J-A. Somers C. Quinn J. Mitchel REJ. The adaptive response and protection against heritable mutations and fetal malformation. Dose Response. 2006;4:317–326. doi: 10.2203/dose-response.06-104.Boreham. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brignull HR. Morley JF. Morimoto RI. The stress of misfolded proteins: C. elegans models for neurodegenerative disease and aging. Adv Exp Med Biol. 2007;594:167–189. doi: 10.1007/978-0-387-39975-1_15. [DOI] [PubMed] [Google Scholar]
- 43.Broadley SA. Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–2653. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 44.Brown IR. Heat shock proteins and protection of the nervous system. Ann NY Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- 45.Bruce AJ. Boling W. Kindy MS. Peschon J. Kraemer PJ. Carpenter MK. Holtsberg FW. Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 46.Bruce-Keller AJ. Begley JG. Fu W. Butterfield DA. Bredesen DE. Hutchins JB. Hensley K. Mattson MP. Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem. 1998;70:31–39. doi: 10.1046/j.1471-4159.1998.70010031.x. [DOI] [PubMed] [Google Scholar]
- 47.Bruce-Keller AJ. Geddes JW. Knapp PE. McFall RW. Keller JN. Holtsberg FW. Parthasarathy S. Steiner SM. Mattson MP. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J Neuroimmunol. 1999;93:53–71. doi: 10.1016/s0165-5728(98)00190-8. [DOI] [PubMed] [Google Scholar]
- 48.Burton NC. Kensler TW. Guilarte TR. In vivo modulation of the Parkinsonian phenotype by Nrf2. Neurotoxicology. 2006;27:1094–1100. doi: 10.1016/j.neuro.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 49.Calabrese EJ. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol Environ Saf. 1999;42:135–137. doi: 10.1006/eesa.1998.1729. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese EJ. Baldwin LA. Chemical hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19:2–31. doi: 10.1191/096032700678815585. [DOI] [PubMed] [Google Scholar]
- 51.Calabrese EJ. Baldwin LA. The marginalization of hormesis. Hum Exper Toxicol. 2000;19:32–40. doi: 10.1191/096032700678815594. [DOI] [PubMed] [Google Scholar]
- 52.Calabrese EJ. Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19:41–75. doi: 10.1191/096032700678815602. [DOI] [PubMed] [Google Scholar]
- 53.Calabrese EJ. Baldwin LA. Radiation hormesis: the demise of a legitimate hypothesis. Hum Exp Toxicol. 2000;19:76–84. doi: 10.1191/096032700678815611. [DOI] [PubMed] [Google Scholar]
- 54.Calabrese EJ. Baldwin LA. Tales of two similar hypotheses: the rise and fall of chemical and radiation hormeis. Hum Exp Toxicol. 2000;19:85–97. doi: 10.1191/096032700678815620. [DOI] [PubMed] [Google Scholar]
- 55.Calabrese EJ. Baldwin LA. The frequency of U-shaped dose-responses in the toxicological literature. Toxicol Sci. 2001;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 56.Calabrese EJ. Baldwin LA. Hormesis: U-shaped dose-response and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 57.Calabrese EJ. Baldwin LA. U-shaped dose responses in biology, toxicology, and public health. Ann Rev Public Health. 2001;22:15–33. doi: 10.1146/annurev.publhealth.22.1.15. [DOI] [PubMed] [Google Scholar]
- 58.Calabrese EJ. Overcompensation stimulation: a mechanism for hormetic effects. Crit Rev Toxicol. 2001;31:425–470. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- 59.Calabrese EJ. Baldwin LA. Hormesis and high risk groups. Regul Toxiclo Pharmacol. 2002;35:14–428. doi: 10.1006/rtph.2001.1529. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese EJ. Baldwin LA. The hormetic dose response model is more common than the threshold model in toxicology. Toxicol Sci. 2003;71:246–250. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- 61.Calabrese EJ. Baldwin LA. Inorganics and hormesis. Crit Rev Toxicol. 2003;33:215–304. doi: 10.1080/713611040. [DOI] [PubMed] [Google Scholar]
- 62.Calabrese EJ. Baldwin LA. Chemotherapeutics and hormesis. Crit Rev Toxicol. 2003;33:305–354. doi: 10.1080/713611041. [DOI] [PubMed] [Google Scholar]
- 63.Calabrese EJ. Baldwin LA. Peptides and hormesis. Crit Rev Toxicol. 2003;33:355–406. doi: 10.1080/713611042. [DOI] [PubMed] [Google Scholar]
- 64.Calabrese EJ. Baldwin LA. Ethanol and hormesis. Crit Rev Toxicol. 2003;33:407–424. doi: 10.1080/713611043. [DOI] [PubMed] [Google Scholar]
- 65.Calabrese EJ. Historical blunders: how toxicology got the dose-response relationship half right. Cell Mol Biol. 2005;51:643–654. [PubMed] [Google Scholar]
- 66.Calabrese EJ. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical foundations. Crit Rev Toxicol. 2005;35:89–295. doi: 10.1080/10408440590917044. [DOI] [PubMed] [Google Scholar]
- 67.Calabrese EJ. Cancer biology and hormesis: Human tumore cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol. 2005;35:463–582. doi: 10.1080/10408440591034502. [DOI] [PubMed] [Google Scholar]
- 68.Calabrese EJ. Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202:289–301. doi: 10.1016/j.taap.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 69.Calabrese EJ. Staudenmayer JW. Stanek EJ. Drug development and hormesis: changing conceptual understanding of the dose response creates new challenges and opportunities for more effective drugs. Curr Opin Drug Discov Dev. 2006;9:117–123. [PubMed] [Google Scholar]
- 70.Calabrese EJ. Staudenmayer JW. Stanek EJ. Hoffmann GR. Hormesis outperforms threshold model in NCI anti-tumor drug screening data. Toxicol Sci. 2006;94:368–378. doi: 10.1093/toxsci/kfl098. [DOI] [PubMed] [Google Scholar]
- 71.Calabrese EJ. Staudenmayer JW. Stanek III EJ. Hoffmann GR. Hormesis and high throughput studies: crump's analysis lacks credibility. Toxicol Sci. 2007;98:602–603. [Google Scholar]
- 72.Calabrese EJ. Bachmann KA. Bailer AJ. Bolger PM. Borak J. Cai L. Cedergreen N. Cherian MG. Chiueh CC. Clarkson TW. Cook RR. Diamond DM. Doolittle DJ. Dorato MA. Duke SO. Feinendegen L. Gardner DE. Hart RW. Hastings KL. Hayes AW. Hoffmann GR. Ives JA. Jaworowski Z. Johnson TE. Jonas WB. Kaminski NE. Keller JG. Klaunig JE. Knudsen TB. Kozumbo WJ. Lettieri T. Liu SZ. Maisseu A. Maynard KI. Masoro EJ. McClellan RO. Mehendale HM. Mothersill C. Newlin DB. Nigg HN. Oehme FW. Phalen RF. Philbert MA. Rattan SI. Riviere JE. Rodricks J. Sapolsky RM. Scott BR. Seymour C. Sinclair DA. Smith-Sonneborn J. Snow ET. Spear L. Stevenson DE. Thomas Y. Tubiana M. Williams GM. Mattson MP. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 73.Calabrese EJ. Neuroscience and hormesis: overview and general findings. Crit Rev Toxicol. 2008;38:249–252. doi: 10.1080/10408440801981957. [DOI] [PubMed] [Google Scholar]
- 74.Calabrese EJ. Dose-response features of neuroprotective agents: an integrative summary. Crit Rev Toxicol. 2008;38:253–348. doi: 10.1080/10408440801981965. [DOI] [PubMed] [Google Scholar]
- 75.Calabrese EJ. Pharmacological enhancement of neuronal survival. Crit Rev Toxicol. 2008;38:349–391. doi: 10.1080/10408440801981973. [DOI] [PubMed] [Google Scholar]
- 76.Calabrese EJ. Enhancing and regulating neurite outgrowth. Crit Rev Toxicol. 2008;38:391–418. doi: 10.1080/10408440801981981. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese EJ. Alzheimer's disease drugs: An application of the hormetic dose-response model. Crit Rev Toxicol. 2008;38:419–452. doi: 10.1080/10408440802003991. [DOI] [PubMed] [Google Scholar]
- 78.Calabrese EJ. Stress biology and hormesis: the Yerkes-Dodson law in psychology—a special case of the hormesis dose response. Crit Rev Toxicol. 2008;38:453–462. doi: 10.1080/10408440802004007. [DOI] [PubMed] [Google Scholar]
- 79.Calabrese EJ. Astrocytes: Adaptive responses to low doses of neurotoxins. Crit Rev Toxicol. 2008;38:463–472. doi: 10.1080/10408440802004023. [DOI] [PubMed] [Google Scholar]
- 80.Calabrese EJ. P-glycoprotein efflux transporter activity often displays biphasic dose-response relationships. Crit Rev Toxicol. 2008;38:473–487. doi: 10.1080/10408440802004049. [DOI] [PubMed] [Google Scholar]
- 81.Calabrese EJ. An assessment of anxiolytic drug screening tests: Hormetic dose responses predominate. Crit Rev Toxicol. 2008;38:489–542. doi: 10.1080/10408440802014238. [DOI] [PubMed] [Google Scholar]
- 82.Calabrese EJ. Modulation of the epileptic seizure threshold: implications of biphasic dose responses. Crit Rev Toxicol. 2008;38:543–556. doi: 10.1080/10408440802014261. [DOI] [PubMed] [Google Scholar]
- 83.Calabrese EJ. Drug therapies for stroke and traumatic brain injury often display U-shaped dose responses: occurrence, mechanisms, and clinical implications. Crit Rev Toxicol. 2008;38:557–577. doi: 10.1080/10408440802014287. [DOI] [PubMed] [Google Scholar]
- 84.Calabrese EJ. Pain and U-shaped dose responses: occurrence, mechanisms, and clinical implications. Crit Rev Toxicol. 2008;38:579–590. doi: 10.1080/10408440802026281. [DOI] [PubMed] [Google Scholar]
- 85.Calabrese EJ. U-shaped dose response in behavioral pharmacology: historical foundations. Crit Rev Toxicol. 2008;38:591–598. doi: 10.1080/10408440802026307. [DOI] [PubMed] [Google Scholar]
- 86.Calabrese EJ. Addiction and dose response: the psychomotor stimulant theory of addiction reveals that hormetic dose responses are dominant. Crit Rev Toxicol. 2008;38:599–618. doi: 10.1080/10408440802026315. [DOI] [PubMed] [Google Scholar]
- 87.Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2008;27:1451–1474. doi: 10.1897/07-541. [DOI] [PubMed] [Google Scholar]
- 88.Calabrese EJ. Hormesis and medicine. Brit J Clin Pharm. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calabrese EJ. Hormesis. In: Melnick E, editor; Everitt B, editor. Encyclopedia of Quantitative Risk Assessment and Analysis. Chichester, United Kingdom: John Wiley & Sons Ltd.; 2008. pp. 838–844. [Google Scholar]
- 90.Calabrese EJ. Stanek III EJ. Nascarella MA. Hoffmann GR. Hormesis predicts low-dose responses better than threshold models. Int J Toxicol. 2008;27:369–378. doi: 10.1080/10915810802503735. [DOI] [PubMed] [Google Scholar]
- 91.Calabrese EJ. The road to linearity: Shy linearity at low doses became the basis for carcinogen risk assessment. Arch Toxicol. 2009;83:203–225. doi: 10.1007/s00204-009-0412-4. [DOI] [PubMed] [Google Scholar]
- 92.Calabrese EJ. Getting the dose-response wrong: why hormesis became marginalized and the threshold model accepted. Arch Toxicol. 2009;83:227–247. doi: 10.1007/s00204-009-0411-5. [DOI] [PubMed] [Google Scholar]
- 93.Calabrese EJ. Blain RB. Hormesis and plant biology. Environ Poll. 2009;157:42–48. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 94.Calabrese EJ. Ricci PF. Hormesis in environmental health: How hormesis will change the risk assessment process. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Elsevier; 2010. (In press). [Google Scholar]
- 95.Calabrese V. Giuffrida Stella AM. Butterfield DA. Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- 96.Calabrese V. Butterfield DA. Scapagnini G. Stella AM. Maines MD. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid Redox Signal. 2006;8:444–477. doi: 10.1089/ars.2006.8.444. [DOI] [PubMed] [Google Scholar]
- 97.Calabrese V. Guagliano E. Sapienza M. Panebianco M. Calafato S. Puleo E. Pennisi G. Mancuso C. Butterfield AD. Giuffrida Stella AM. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Research. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 98.Calabrese V. Mancuso C. Ravagna A. Perluigi M. Cini C. De Marco C. Butterfield D. Giuffrida Stella AM. In vivo induction of heat shock proteins in the substantia nigra following L-DOPA administration is associated with increased activity of mitochondrial complex I and nitrosative stress in rats: regulation by glutathione redox state. J Neurochem. 2007;101:709–717. doi: 10.1111/j.1471-4159.2006.04367.x. [DOI] [PubMed] [Google Scholar]
- 99.Calabrese V. Mancuso C. Calvani M. Rizzarelli E. Butterfield DA. Giuffrida Stella AM. Nitric oxide in the CNS: neuroprotection versus neurotoxicity. Nat Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 100.Calabrese V. Cornelius C. Mancuso C. Pennisi G. Calafato S. Bellia F. Bates TE. Giuffrida Stella AM. Schapira T. Dinkova Kostova AT. Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 101.Calabrese V. Signorile A. Cornelius C. Mancuso C. Scapagnini G. Ventimiglia B. Ragusa N. Dinkova-Kostova A. Practical approaches to investigate redox regulation of heat shock protein expression and intracellular glutathione redox state. Methods Enzymol. 2008;441:83–110. doi: 10.1016/S0076-6879(08)01206-8. [DOI] [PubMed] [Google Scholar]
- 102.Calabrese V. Bates TE. Mancuso C. Cornelius C. Ventimiglia B. Cambria MT. Di Renzo L. De Lorenzo A. Dinkova-Kostova AT. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- 103.Calabrese V. Butterfield DA. Stella AM. Aging and oxidative stress response in the CNS. In: Abel L, editor; Regino P-PJ, editor; Steffen R, editor. Development and Aging Changes in the Nervous System. Handbook of Neurochemistry and Molecular Neurobiology. 3rd. New York: Springer; 2008. pp. 128–234. [Google Scholar]
- 104.Calabrese V. Cornelius C. Mancuso C. Ientile R. Stella AM. Butterfield DA. Redox homeostasis and cellular stress response in aging and neurodegeneration. Methods Mol. Biol. 2010;610:285–308. doi: 10.1007/978-1-60327-029-8_17. [DOI] [PubMed] [Google Scholar]
- 105.Calabrese V. Calafato S. Cornelius C. Mancuso C. Dinkova-Kostova A. Heme oxygenase: a master vitagene involved in cellular stress response. In: Eleuteri AM, editor. Enzymes and the Cellular Fight Against Oxidation. Research Signpost; 37/661 (2), Fort P.O., Trivandrum-695 023, Kerala, India: 2008. [Google Scholar]
- 106.Calabrese V. Cornelius C. Mancuso C. Barone E. Calafato S. Bates T. Rizzarelli E. Dinkova-Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 107.Calabrese V. Cornelius C. Dinkova-Kostova AT. Calabrese EJ. Vitagenes, cellular stress response and acetylcarnitine: relevance to hormesis. Biofactors. 2009;35:146–160. doi: 10.1002/biof.22. [DOI] [PubMed] [Google Scholar]
- 108.Calabrese V. Cornelius C. Rizzarelli E. Owen JB. Dinkova-Kostova AT. Butterfield DA. Nitric oxide in cell survival: a Janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 109.Calabrese V. Perluigi M. Cornelius C. Coccia R. Di Domenico F. Mancuso C. Pennini G. Dinkova-Kostova AT. Phenolics in aging and neurodegenerative disorders. In: Fraga CG, editor. Phenolic Compounds of Plant Origin and Health: The Biochemistry behind their Nutritional and Pharmacological Value. New York: Wiley & Sons; 2009. pp. 427–451. [Google Scholar]
- 110.Calderwood SK. Murshid A. Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calkins MJ. Jakel RJ. Johnson DA. Chan K. Kan YW. Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Calkins MJ. Johnson DA. Townsend JA. Vargas MR. Dowell JA. Williamson TP. Kraft AD. Lee JM. Li J. Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calvert JW. Coetzee WA. Lefer D. Novel Insights into Hydrogen Sulfide Mediated Cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Calvert JW. Jha S. Gundewar S. Elrod JW. Ramachandran A. Pattillo CB. Kevil CG. Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camandola S. Mattson MP. Pro-apoptotic action of PAR-4 involves inhibition of NF-kappaB activity and suppression of BCL-2 expression. J Neurosci Res. 2000;61:134–139. doi: 10.1002/1097-4547(20000715)61:2<134::AID-JNR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 116.Cedergreen N. Streibig JC. Kudsk P. Mathiassen SK. Duke SO. The occurrence of hormesis in plants and algae. Dose Response. 2007;5:150–162. doi: 10.2203/dose-response.06-008.Cedergreen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chan K. Lu R. Chang JC. Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan SL. Fu W. Zhang P. Cheng A. Lee J. Kokame K. Mattson MP. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J Biol Chem. 2004;279:28733–28743. doi: 10.1074/jbc.M404272200. [DOI] [PubMed] [Google Scholar]
- 119.Chen C. Xin H. Zhu Y. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007:1709–1716. doi: 10.1111/j.1745-7254.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 120.Chen L. Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a drosophila model of Parkinson's disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 121.Chen P. Sun B. Chen H. Wang G. Pan S. Kong R. Bai X. Wang S. Effects of carbon monoxide releasing molecule-liberated CO on severe acute pancreatitis in rats. Cytokine. 2010;49:15–23. doi: 10.1016/j.cyto.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 122.Chen PC. Vargas MR. Pani AK. Smeyne RJ. Johnson DA. Kan YW. Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: Critical role for the astrocyte. Proc Natl Acad Sci USA. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cheng B. Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chigurupati S. Son TG. Hyun DH. Lathia JD. Mughal MR. Savell J. Li SC. Nagaraju GP. Chan SL. Arumugam TV. Mattson MP. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–341. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chong ZZ. Lin SH. Li F. Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chou YH. Ho FM. Liu DZ. Lin SY. Tsai LH. Chen CH. Ho YS. Hung LF. Liang YC. The possible role of heat shock factor-1 in the negative regulation of heme oxygenase-1. Int J Biochem Cell Biol. 2005;37:604–615. doi: 10.1016/j.biocel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Chueh S-C. Guh J-H. Chen J. Lai M-K. Teng C-M. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166:347–353. [PubMed] [Google Scholar]
- 128.Chung HT. Choi BM. Kwon YG. Kim YM. Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymo. 2008;441:329–338. doi: 10.1016/S0076-6879(08)01218-4. [DOI] [PubMed] [Google Scholar]
- 129.Chung KC. Sung JY. Ahn W. Rhim H. Oh TH. Lee MG. Ahn YS. Intracellular calcium mobilization induces immediate early gene pip92 via Src and mitogen-activated protein kinase in immortalized hippocampal cells. J Biol Chem. 2001;276:2132–2138. doi: 10.1074/jbc.M007492200. [DOI] [PubMed] [Google Scholar]
- 130.Clements CM. McNally RS. Conti BJ. Mak TW. Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cleren C. Calingasan NY. Chen J. Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- 132.Cohen E. Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Collins AR. Lyon CJ. Xia X. Liu JZ. Tangirala RK. Yin F. Boyadjian R. Bikineyeva A. Praticò D. Harrison DG. Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 134.Conney AH. Miller EC. Miller JA. The metabolism of methylated aminoazo dyes. V. Evidence for induction of enzyme synthesis in the rat by 3-methylcholanthrene. Cancer Res. 1956;16:450–459. [PubMed] [Google Scholar]
- 135.Cook RR. Calabrese EJ. The importance of hormesis to public health. Environ Health Perspect. 2006;114:1631–1635. doi: 10.1289/ehp.8606. Reprinted in: Cienc Saude Colet 12: 955–963, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corson TW. Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crabtree HG. Anti-carcinogenesis. Br Med Bull. 1947;4:345–348. doi: 10.1093/oxfordjournals.bmb.a072823. [DOI] [PubMed] [Google Scholar]
- 138.Crabtree HG. Influence of unsaturated dibasic acids on the induction of skin tumors by chemical carcinogens. Cancer Res. 1945;5:346–351. [Google Scholar]
- 139.Csermely P. Söti C. Blatch GL. Chaperones as parts of cellular networks. Adv Exp Med Biol. 2007;594:55–63. doi: 10.1007/978-0-387-39975-1_6. [DOI] [PubMed] [Google Scholar]
- 140.Cutler RG. Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. 2001;122:895–908. doi: 10.1016/s0047-6374(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 141.Cutler RG. Pedersen WA. Camandola S. Rothstein JD. Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- 142.Cutler RG. Kelly J. Storie K. Pedersen WA. Tammara A. Hatanpaa K. Troncoso JC. Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cuttler JM. What becomes of nuclear risk assessment in light of radiation hormesis? Dose Response. 2007;5:80–90. doi: 10.2203/dose-response.06-106.Cuttler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dali-Youcef N. Lagouge M. Froelich S. Koehl C. Schoonjans K. Auwerx J. Sirtuins: the “magnificent seven,” function, metabolism and longevity. Ann Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 145.De Long MJ. Prochaska HJ. Talalay P. Tissue-specific induction patterns of cancer-protective enzymes in mice by tert-butyl-4-hydroxyanisole and related substituted phenols. Cancer Res. 1985;45:546–551. [PubMed] [Google Scholar]
- 146.De Long MJ. Prochaska HJ. Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci USA. 1986;83:787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Delgado M. Varela N. Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56:1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- 148.Dennery PA. Regulation and role of heme oxygenase in oxidative injury. Curr Top Cell Regul. 2000;36:181–199. doi: 10.1016/s0070-2137(01)80008-x. [DOI] [PubMed] [Google Scholar]
- 149.Der P. Cui J. Das DK. Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol. 2006;40:313–320. doi: 10.1016/j.yjmcc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 150.Di Stasi AM. Mallozzi C. Macchia G. Maura G. Petrucci TC. Minetti M. Peroxynitrite affects exocytosis and SNARE complex formation and induces tyrosine nitration of synaptic proteins. J Neurochem. 2002;82:420–429. doi: 10.1046/j.1471-4159.2002.00980.x. [DOI] [PubMed] [Google Scholar]
- 151.Dinkova-Kostova AT. Talalay P. Relation of structure of curcumin analogs to their potencies as inducers of phase 2 detoxification enzymes. Carcinogenesis. 1999;20:911–914. doi: 10.1093/carcin/20.5.911. [DOI] [PubMed] [Google Scholar]
- 152.Dinkova-Kostova AT. Talalay P. Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radic Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 153.Dinkova-Kostova AT. Massiah MA. Bozak RE. Hicks RJ. Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dinkova-Kostova AT. Holtzclaw WD. Cole RN. Itoh K. Wakabayashi N. Katoh Y. Yamamoto M. Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dinkova-Kostova AT. Fahey JW. Talalay P. Chemical structures of inducers of nicotinamide quinone oxidoreductase 1 (NQO1) Methods Enzymol. 2004;382:423–448. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 156.Dinkova-Kostova AT. Holtzclaw WD. Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 157.Dinkova-Kostova AT. Liby KT. Stephenson KK. Holtzclaw WD. Gao X. Suh N. Williams C. Risingsong R. Honda T. Gribble GW. Sporn MB. Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dinkova-Kostova AT. Jenkins SN. Fahey JW. Ye L. Wehage SL. Liby KT. Stephenson KK. Wade KL. Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 159.Dinkova-Kostova AT. The isothiocyanate sulforaphane induces the phase 2 response by signaling of the Keap1-Nrf2-ARE pathway: implications for dietary protection against cancer. In: Surh YJ, editor; Dong Z, editor; Cadenas E, editor; Packer L, editor. Dietary Modulation of Cell Signaling Pathways. Boca Raton, FL: CRC Press; 2008. pp. 205–229. [Google Scholar]
- 160.Dinkova-Kostova AT. Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res 52 Suppl. 2008;1:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 161.Dioum EM. Chen R. Alexander MS. Zhang Q. Hogg RT. Gerard RD. Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 162.Dirnagl U. Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 163.Doré S. Takahashi M. Ferris CD. Zakhary R. Hester LD. Guastella D. Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Douglas PM. Cyr DM. Interplay between protein homeostasis networks in protein aggregation and proteotoxicity. Biopolymers. 2010;93:229–236. doi: 10.1002/bip.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Du Y. Wooten MC. Gearing M. Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duan W. Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 167.Eaton DL. Klaassen CD. Principles of toxicology. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 6th. New York: McGraw-Hill; 2003. pp. 6–20. [Google Scholar]
- 168.Ekdahl CT. Kokaia Z. Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 169.Eliasson MJ. Sampei K. Mandir AS. Hurn PD. Traystman RJ. Bao J. Pieper A. Wang ZQ. Dawson TM. Snyder SH. Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 170.Fahey JW. Dinkova-Kostova AT. Stephenson KK. Talalay P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382:243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- 171.Fang J. Lu J. Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 172.Ferrari R. Guardigli G. Mele D. Percoco GF. Ceconi C. Curello S. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004;10:1699–16711. doi: 10.2174/1381612043384718. [DOI] [PubMed] [Google Scholar]
- 173.Flood JF. Smith GE. Cherkin A. Memory retention—enhancement by cholinergic drug-combinations in mice. Gerontologist. 1982;22:230–231. [Google Scholar]
- 174.Flood JF. Smith GE. Cherkin A. Memory retention—potentiation of cholinergic drug-combinations in mice. Neurobiol Aging. 1983;4:37–43. doi: 10.1016/0197-4580(83)90052-0. [DOI] [PubMed] [Google Scholar]
- 175.Flood JF. Smith GE. Cherkin A. Memory retention—enhancement by synergistic oral cholinergic drug-combination in mice. Gerontol. 1984;24:149. [Google Scholar]
- 176.Flood JF. Smith GE. Cherkin A. Memory enhancement—supra-additive effect of subcutaneous chlolinergic drug-combinations in mice. Psychopharmacology. 1985;86:61–67. doi: 10.1007/BF00431685. [DOI] [PubMed] [Google Scholar]
- 177.Forquer I. Covian R. Bowman MK. Trumpower BL. Kramer DM. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem. 2006;281:38459–38465. doi: 10.1074/jbc.M605119200. [DOI] [PubMed] [Google Scholar]
- 178.Foster KA. Galeffi F. Gerich FJ. Turner DA. Müller M. Optical and pharmacological tools to investigate the role of mitochondria during oxidative stress and neurodegeneration. Prog Neurobiol. 2006;79:136–171. doi: 10.1016/j.pneurobio.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Frankfurt OS. Lipchina LP. Bunto TV. Emanuel NM. Effect of 4-methyl-2,6-di-tert-butylphenol (Ionol) on induction of liver tumors in rats. Byull Eksp Biol Med USSR. 1967;64:86–88. [PubMed] [Google Scholar]
- 181.Friebe A. Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21:149–156. doi: 10.1016/j.niox.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 182.Friling RS. Bensimon A. Tichauer Y. Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci USA. 1990;87:6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gerhart-Hines Z. Rodgers JT. Bare O. Lerin C. Kim SH. Mostoslavsky R. Alt FW. Wu Z. Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gerich FJ. Funke F. Hildebrandt B. Fasshauer M. Müller M. H2O2—mediated modulation of cytosolic signaling and organelle function in rat hippocampus. Pflugers Arch. 2009;458:937–952. doi: 10.1007/s00424-009-0672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gidalevitz T. Kikis EA. Morimoto RI. A cellular perspective on conformational disease: the role of genetic background and proteostasis networks. Curr Opin Struct Biol. 2010;20:23–32. doi: 10.1016/j.sbi.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Glabe CG. Structural Classification of Toxic Amyloid Oligomers. J Biol Chem. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Glaum SR. Miller RJ. Zinc protoporphyrin-IX blocks the effects of metabotropic glutamate receptor activation in the rat nucleus tractus solitarii. Mol Pharmacol. 1993;43:965–969. [PubMed] [Google Scholar]
- 188.Glazner GW. Camandola S. Geiger JD. Mattson MP. Endoplasmic reticulum D-myo-inositol 1,4,5-trisphosphate-sensitive stores regulate nuclear factor-kappaB binding activity in a calcium-independent manner. J Biol Chem. 2001;276:22461–22467. doi: 10.1074/jbc.M101315200. [DOI] [PubMed] [Google Scholar]
- 189.Goloubinoff P. De Los Rios P. The mechanism of Hsp70 chaperones: (entropic) pulling the models together. Trends Biochem Sci. 2007;32:372–380. doi: 10.1016/j.tibs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 190.Gonzalez DR. Treuer A. Sun QA. Stamler JS. Hare JM. S-nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:188–195. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Goodman Y. Mattson MP. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults, and amyloid beta-peptide toxicity. J Neurochem. 1996;66:869–872. doi: 10.1046/j.1471-4159.1996.66020869.x. [DOI] [PubMed] [Google Scholar]
- 192.Gopalakrishna R. Gundimeda U. Schiffman JE. McNeill TH. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J Biol Chem. 2008;283:14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Graser T. Vedernikov YP. Li DS. Study on the mechanism of carbon monoxide induced endotheliumindependent relaxation in porcine coronary artery and vein. Biomed Biochim Acta. 1990;49:293–296. [PubMed] [Google Scholar]
- 194.Green KN. Steffan JS. Martinez-Coria H. Sun X. Schreiber SS. Thompson LM. LaFerla FM. Nicotinamide restores cognition in Alzheimer's disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J Neurosci. 2008;28:11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grehant N. Le gas du sang. Paris: 1894. [Google Scholar]
- 196.Guerreiro S. Toulorge D. Hirsch E. Marien M. Sokoloff P. Michel PP. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol. 2008;74:980–989. doi: 10.1124/mol.108.048207. [DOI] [PubMed] [Google Scholar]
- 197.Halagappa VK. Guo Z. Pearson M. Matsuoka Y. Cutler RG. Laferla FM. Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 198.Harding HP. Zhang Y. Zeng H. Novoa I. Lu PD. Calfon M. Sadri N. Yun C. Popko B. Paules R. Stojdl DF. Bell JC. Hettmann T. Leiden JM. Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 199.Harting K. Knöll B. SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol. 2010;89:262–269. doi: 10.1016/j.ejcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 200.Hayes JD. McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 201.Hipkiss AR. On why decreasing protein synthesis can increase lifespan. Mech Ageing Dev. 2007;128:412–414. doi: 10.1016/j.mad.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 202.Hipkiss AR. NAD + availability and proteotoxicity. Neuromol Med. 2009;11:97–100. doi: 10.1007/s12017-009-8069-y. [DOI] [PubMed] [Google Scholar]
- 203.Hishiya A. Takayama S. Molecular chaperones as regulators of cell death. Oncogene. 2008;27:6489–6506. doi: 10.1038/onc.2008.314. [DOI] [PubMed] [Google Scholar]
- 204.Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009;7:1–51. doi: 10.2203/dose-response.08-023.Hoffmann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Holtzclaw WD. Dinkova-Kostova AT. Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: the quest for the elusive sensor that responds to inducers. Adv Enzyme Regul. 2004;44:335–367. doi: 10.1016/j.advenzreg.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 206.Hubbs AF. Benkovic SA. Miller DB. O'Callaghan JP. Battelli L. Schwegler-Berry D. Ma Q. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170:2068–2076. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Huddleston AT. Tang W. Takeshima H. Hamilton SL. Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol. 2008;99:1565–1571. doi: 10.1152/jn.00659.2007. [DOI] [PubMed] [Google Scholar]
- 208.Huggins C. Fukunishi R. Induced protection of adrenal cortex against 7,12-dimethylbenz[α]anthracene. Influence of ethionine. Induction of menadione reductase. Incorporation of thymidine-H3. J Exp Med. 1964;119:923–942. doi: 10.1084/jem.119.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Huggins C. Pataki J. Aromatic azo derivatives preventing mammary cancer and adrenal injury from 7,12-dimethylbenz(a)anthracene. Proc Natl Acad Sci USA. 1965;53:791–796. doi: 10.1073/pnas.53.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hyun DH. Hernandez JO. Mattson MP. de Cabo R. The plasma membrane redox system in aging. Ageing Res Rev. 2006;5:209–220. doi: 10.1016/j.arr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 211.Hyun DH. Emerson SS. Jo DG. Mattson MP. de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hyun DH. Hunt ND. Emerson SS. Hernandez JO. Mattson MP. de Cabo R. Up-regulation of plasma membrane-associated redox activities in neuronal cells lacking functional mitochondria. J Neurochem. 2007;100:1364–1374. doi: 10.1111/j.1471-4159.2006.04411.x. [DOI] [PubMed] [Google Scholar]
- 213.Innamorato NG. Rojo AI. García-Yagüe AJ. Yamamoto M. de Ceballos ML. Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 214.Ishigami M. Hiraki K. Umemura K. Ogasawara Y. Ishii K. Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 215.Itoh K. Chiba T. Takahashi S. Ishii T. Igarashi K. Katoh Y. Oyake T. Hayashi N. Satoh K. Hatayama I. Yamamoto M. Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 216.Itoh K. Wakabayashi N. Katoh Y. Ishii T. Igarashi K. Engel JD. Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jiang F. Zhang ZG. Katakowski M. Robin AM. Faber M. Zhang F. Chopp M. Angiogenesis induced by photodynamic therapy in normal rat brains. Photochem Photobiol. 2004;79:494–498. doi: 10.1562/2003-11-19-rc.1. [DOI] [PubMed] [Google Scholar]
- 218.Jiao J. Huang X. Feit-Leithman RA. Neve RL. Snider W. Dartt DA. Chen DF. Bcl-2 enhances Ca(2+) signaling to support the intrinsic regenerative capacity of CNS axons. EMBO J. 2005;24:1068–1078. doi: 10.1038/sj.emboj.7600589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Johnson JA. Johnson DA. Kraft AD. Calkins MJ. Jakel RJ. Vargas MR. Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Johnson JB. Summer W. Cutler RG. Martin B. Hyun DH. Dixit VD. Pearson M. Nassar M. Telljohann R. Maudsley S. Carlson O. John S. Laub DR. Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kakimura J. Kitamura Y. Takata K. Umeki M. Suzuki S. Shibagaki K. Taniguchi T. Nomura Y. Gebicke-Haerter PJ. Smith MA. Perry G. Shimohama S. Microglial activation and amyloid-beta clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16:601–603. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- 222.Kang YH. Pezzuto JM. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Methods Enzymol. 2004;382:380–414. doi: 10.1016/S0076-6879(04)82021-4. [DOI] [PubMed] [Google Scholar]
- 223.Kapitulnik J. Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 224.Kappos L. Gold R. Miller DH. Macmanus DG. Havrdova E. Limmroth V. Polman CH. Schmierer K. Yousry TA. Yang M. Eraksoy M. Meluzinova E. Rektor I. Dawson KT. Sandrock AW. O'Neill GN. BG-12 phase IIb study investigators. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 225.Keller JN. Kindy MS. Holtsberg FW. St. Clair DK. Yen HC. Germeyer A. Steiner SM. Bruce-Keller AJ. Hutchins JB. Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Keller JN. Mattson MP. Roles of lipid peroxidation in modulation of cellular signaling pathways, cell dysfunction, and death in the nervous system. Rev Neurosci. 1998;9:105–116. doi: 10.1515/revneuro.1998.9.2.105. [DOI] [PubMed] [Google Scholar]
- 227.Kensler TW. Wakabayashi N. Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 228.Kensler TW. Chemoprevention by inducers of carcinogen detoxication enzymes. Environ Health Perspect. 1997;105:965–970. doi: 10.1289/ehp.97105s4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim D. Nguyen MD. Dobbin MM. Fischer A. Sananbenesi F. Rodgers JT. Delalle I. Baur JA. Sui G. Armour SM. Puigserver P. Sinclair DA. Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 231.Kimura H. Hydrogen sulfide as a biological mediator. Antioxid Redox Signal. 2005;7:778–780. doi: 10.1089/ars.2005.7.778. [DOI] [PubMed] [Google Scholar]
- 232.Kitamuro T. Takahashi K. Ogawa K. Udono-Fujimori R. Takeda K. Furuyama K. Nakayama M. Sun J. Fujita H. Hida W. Hattori T. Shirato K. Igarashi K. Shibahara S. Bach1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003;278:9125–9133. doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 233.Kitao Y. Imai Y. Ozawa K. Kataoka A. Ikeda T. Soda M. Nakimawa K. Kiyama H. Stern DM. Hori O. Wakamatsu K. Ito S. Itohara S. Takahashi R. Ogawa S. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum Mol Genet. 2007;16:50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- 234.Kitao Y. Ozawa K. Miyazaki M. Tamatani M. Kobayashi T. Yanagi H. Okabe M. Ikawa M. Yamashima T. Stern DM. Hori O. Ogawa S. Expression of the endoplasmic reticulum molecular chaperone (ORP150) rescues hippocampal neurons from glutamate toxicity. J Clin Invest. 2001;108:1439–1450. doi: 10.1172/JCI12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Klucken J. Shin Y. Maslia E. Hyman BT. Mclean PJ. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 236.Kobayashi A. Kang MI. Watai Y. Tong KI. Shibata T. Uchida K. Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kobayashi M. Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 238.Komatsu M. Kurokawa H. Waguri S. Taguchi K. Kobayashi A. Ichimura Y. Sou YS. Ueno I. Sakamoto A. Tong KI. Kim M. Nishito Y. Iemura S. Natsume T. Ueno T. Kominami E. Motohashi H. Tanaka K. Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 239.Kosaka K. Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol Pharm Bull. 2003;26:1620–1622. doi: 10.1248/bpb.26.1620. [DOI] [PubMed] [Google Scholar]
- 240.Kraft AD. Johnson DA. Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kraft AD. Lee JM. Johnson DA. Kan YW. Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- 242.Kruman I. Bruce-Keller AJ. Bredesen D. Waeg G. Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kruman II. Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- 244.Kuroda K. Takagi K. Physiologically active substance in Capsella bursa-pastoris. Nature. 1968;220:707–708. doi: 10.1038/220707a0. [DOI] [PubMed] [Google Scholar]
- 245.Kuroda K. Akao M. Reduction by fumaric acid of side effects of mitomycin C. Biochem Pharmacol. 1980;29:2839–2844. doi: 10.1016/0006-2952(80)90020-9. [DOI] [PubMed] [Google Scholar]
- 246.Lacassagne A. Buu-Hoi NP. Rudali G. Induction of the carcinogenic action produced by a weakly carcinogenic hydrocarbon on a highly active carcinogenic hydrocarbon. Br J Exp Pathol. 1945;26:5–12. [Google Scholar]
- 247.Lagouge M. Argmann C. Gerhart-Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P. Geny B. Laakso M. Puigserver P. Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 248.Lavu S. Boss O. Elliott PJ. Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 249.Lee JM. Shih AY. Murphy TH. Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 250.Lee SH. Blair IA. Oxidative DNA damage and cardiovascular disease. Trends Cardiovasc Med. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 251.Lee M. Schwab C. Yu S. McGeer E. McGeer PL. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 252.Leonarduzzi G. Chiarpotto E. Biasi F. Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- 253.Levonen AL. Landar A. Ramachandran A. Ceaser EK. Dickinson DA. Zanoni G. Morrow JD. Darley-Usmar VM. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lewén A. Matz P. Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 255.Li A. Xi Q. Umstot ES. Bellner L. Schwartzman ML. Jaggar JH. Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle cell KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li L. Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 257.Li X. Zhang D. Hannink M. Beamer LJ. Crystal structure of the Kelch domain of human Keap1. J Biol Chem. 2004;279:54750–54758. doi: 10.1074/jbc.M410073200. [DOI] [PubMed] [Google Scholar]
- 258.Li Y. Xu W. McBurney MW. Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lim GP. Yang F. Chu T, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 261.Liu D. Chan SL. de Souza-Pinto NC. Slevin JR. Wersto RP. Zhan M. Mustafa K. de Cabo R. Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 262.Liu D. Gharavi R. Pitta M. Gleichmann M. Mattson MP. Nicotinamide prevents NAD + depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD + consumption by SIRT1 may endanger energetically compromised neurons. Neuromol Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Liu D. Pitta M. Mattson MP. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann NY Acad Sci. 2008;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Liu H. Dinkova-Kostova AT. Talalay P. Coordinate regulation of enzyme markers for inflammation and for protection against oxidants and electrophiles. Proc Natl Acad Sci USA. 2008;105:15926–15931. doi: 10.1073/pnas.0808346105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lu M. Hu LF. Hu G. Bian JS. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Rad Biol Med. 2008;45:1705–1713. doi: 10.1016/j.freeradbiomed.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 266.Lu M. Kim HE. Li CR. Kim S. Kwak IJ. Lee YJ. Kim SS. Moon JY. Kim CH. Kim DK. Kang HS. Park JS. Two distinct disulfide bonds formed in human heat shock transcription factor 1 act in opposition to regulate its DNA binding activity. Biochemistry. 2008;47:6007–6015. doi: 10.1021/bi702185u. [DOI] [PubMed] [Google Scholar]
- 267.Luckey TD. Radiation Hormesis. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- 268.Ma ZC. Hong Q. Wang YG. Tan HL. Xiao CR. Liang QD. Zhang BL. Gao Y. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol Pharm Bull. 2010;33:29–34. doi: 10.1248/bpb.33.29. [DOI] [PubMed] [Google Scholar]
- 269.Macario AJ. Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- 270.Macario AJ. Conway de Macario E. Molecular chaperones: multiple functions, pathologies, and potential applications. Front Biosci. 2007;12:2588–2600. doi: 10.2741/2257. [DOI] [PubMed] [Google Scholar]
- 271.Macario AJ. Conway de Macario E. Chaperonopathies by defect, excess, or mistake. Ann NY Acad Sci. 2007;1113:178–191. doi: 10.1196/annals.1391.009. [DOI] [PubMed] [Google Scholar]
- 272.Madhavan L. Ourednik V. Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells. 2008;26:254–265. doi: 10.1634/stemcells.2007-0221. [DOI] [PubMed] [Google Scholar]
- 273.Maines MD. Gibbs PEM. 30 some years of heme oxygenase: From a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun. 2005;338:568–577. doi: 10.1016/j.bbrc.2005.08.121. [DOI] [PubMed] [Google Scholar]
- 274.Maines MD. The heme oxygenase system; a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 275.Malhotra D. Thimmulappa R. Navas-Acien A. Sandford A. Elliott M. Singh A. Chen L. Zhuang X. Hogg J. Pare P. Tuder RM. Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 276.Mancuso C. Pani G. Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11:207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 277.Mancuso C. Scapagnini G. Curro D. Giuffrida Stella AM. De Marco C. Butterfield DA. Calabrese V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 278.Mark RJ. Hensley K. Butterfield DA. Mattson MP. Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mark RJ. Pang Z. Geddes JW. Uchida K. Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Masoro EJ. Role of hormesis in life extension by caloric restriction. Dose Response. 2007;5:163–173. doi: 10.2203/dose-response.06-005.Masoro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mattson MP. Goodman Y. Luo H. Fu W. Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 282.Mattson MP. Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci. 1998;21:53–57. doi: 10.1016/s0166-2236(97)01188-0. [DOI] [PubMed] [Google Scholar]
- 283.Mattson MP. LaFerla FM. Chan SL. Leissring MA. Shepel PN. Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 284.Mattson MP. Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromolecular Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- 285.Mattson MP. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev. 2003;2:329–342. doi: 10.1016/s1568-1637(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 286.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mattson MP. Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mattson MP. Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 289.Mattson MP. Son TG. Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5:174–186. doi: 10.2203/dose-response.07-004.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 291.Mattson MP. Calabrese EJ. Best in small doses. The New Scientist. 2008 Aug 6;199:36–39. [Google Scholar]
- 292.Mattson MP. Gleichmann M. Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit Rev Toxicol. 2008;38:633–639. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Maulik N. Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 296.McCord JM. Superoxide dismutase in aging and disease: an overview. Methods Enzymol. 2002;349:331–341. doi: 10.1016/s0076-6879(02)49348-2. [DOI] [PubMed] [Google Scholar]
- 297.McCoubrey WK. Huang TJ. Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- 298.McMahon M. Itoh K. Yamamoto M. Chanas SA. Henderson CJ. McLellan LI. Wolf CR. Cavin C. Hayes JD. The Cap‘n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 299.McMahon M. Thomas N. Itoh K. Yamamoto M. Hayes JD. Dimerization of substrate adaptors can facilitate Cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem. 2006;281:24756–24768. doi: 10.1074/jbc.M601119200. [DOI] [PubMed] [Google Scholar]
- 300.McNally SJ. Harrison EM. Ross JA. Garden OJ. Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 301.Mendes CS. Levet C. Chatelain G. Dourlen P. Fouillet A. Dichtel-Danjoy ML. Gambis A. Ryoo HD. Steller H. Mollereau B. ER stress protects from retinal degeneration. EMBO J. 2009;28:1296–1307. doi: 10.1038/emboj.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Michalak M. Robert Parker JM. Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 303.Miller CA. Campbell SL. Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formaion and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Miller JA. Miller EC. The carcinogenic aminoazo dyes. Adv Cancer Res. 1953;1:339–396. doi: 10.1016/s0065-230x(08)60007-x. [DOI] [PubMed] [Google Scholar]
- 305.Milne JC. Lambert PD. Schenk S. Carney DP. Smith JJ. Gagne DJ. Jin L. Boss O. Perni RB. Vu CB. Bemis JE. Xie R. Disch JS. Ng PY. Nunes JJ. Lynch AV. Yang H. Galonek H. Israelian K. Choy W. Iffland A. Lavu S. Medvedik O. Sinclair DA. Olefsky JM. Jirousek MR. Elliott PJ. Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mitchel REJ. Low doses of radiation are protective in vitro and in vivo: Evolutionary origins. Dose Response. 2006;4:75–90. doi: 10.2203/dose-response.04-002.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mitchel REJ. Low doses of radiation reduce risk in vivo. Dose Response. 2007;5:1–10. doi: 10.2203/dose-response.06-109.Mitchel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Moi P. Chan K. Asunis I. Cao A. Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Morimoto RI. Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 310.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 311.Morimoto RI. Stress, aging, and neurodegenerative disease. New Engl J Med. 2006;355:2254–2255. doi: 10.1056/NEJMcibr065573. [DOI] [PubMed] [Google Scholar]
- 312.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Mothersill C. Seymour CB. Radiation-induced bystander effects and the DNA paradigm: An “out of field” perspective. Mutat Res. 2006;597:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 314.Motohashi H. O'Connor T. Katsuoka F. Engel JD. Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- 315.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 316.Motterlini R. Foresti R. Bassi R. Calabrese V. Clark JE. Green CJ. Endothelial Heme oxygenase-1 induction by hypoxia: modulation by inducible nitric oxide synthase (iNOS) and S-nitrosothiols. J Biol Chem. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- 317.Motterlini R. Foresti R. Bassi R. Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 318.Muller P. Hrstka R. Coomber D. Lane DP. Vojtesek B. Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene. 2008;27:3371–3383. doi: 10.1038/sj.onc.1211010. [DOI] [PubMed] [Google Scholar]
- 319.Munday R. Munday CM. Induction of phase II enzymes by aliphatic sulfides derived from garlic and onions: an overview. Methods Enzymol. 2004;382:449–456. doi: 10.1016/S0076-6879(04)82024-X. [DOI] [PubMed] [Google Scholar]
- 320.Naidoo N. ER and aging—protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 321.Nakamura T. Lipton SA. S-Nitrosylation and uncompetitive/fast off-rate (UFO) drug therapy in neurodegenerative disorders of protein misfolding. Cell Death Differ. 2007;14:1305–1314. doi: 10.1038/sj.cdd.4402138. [DOI] [PubMed] [Google Scholar]
- 322.Napoli C. Ignaro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharm Res. 2009;32:1103–1108. doi: 10.1007/s12272-009-1801-1. [DOI] [PubMed] [Google Scholar]
- 323.Navarro A. Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev. 2008;60:1534–1544. doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 324.Nguyen T. Nioi P. Pickett CB. The nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nguyen T. Sherratt PJ. Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 326.Ni M. Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Nieboer C. de Hoop D. van Loenen AC. Langendijk PN. van Dijk E. Systemic therapy with fumaric acid derivates: new possibilities in the treatment of psoriasis. J Am Acad Dermatol. 1989;20:601–608. doi: 10.1016/s0190-9622(89)70071-2. [DOI] [PubMed] [Google Scholar]
- 328.Nisoli E. Clementi E. Paolucci C. Cozzi V. Tonello C. Sciorati C. Bracale R. Valerio A. Francolini M. Moncada S. Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–893. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 329.Noyan-Ashraf MH. Sadeghinejad Z. Juurlink BH. Dietary approach to decrease aging-related CNS inflammation. Nutr Neurosci. 2005;8:101–110. doi: 10.1080/10284150500069470. [DOI] [PubMed] [Google Scholar]
- 330.Noyan-Ashraf MH. Wu L. Wang R. Juurlink BH. Dietary approaches to positively influence fetal determinants of adult health. FASEB J. 2006;20:371–373. doi: 10.1096/fj.05-4889fje. [DOI] [PubMed] [Google Scholar]
- 331.Nystul TG. Roth MB. Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101:9133–9136. doi: 10.1073/pnas.0403312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Okada K. Shoda J. Taguchi K. Maher JM. Ishizaki K. Inoue Y. Ohtsuki M. Goto N. Takeda K. Utsunomiya H. Oda K. Warabi E. Ishii T. Osaka K. Hyodo I. Yamamoto M. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 333.Okawa H. Motohashi H. Kobayashi A. Aburatani H. Kensler TW. Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 334.Okun E. Griffioen KJ. Lathia JD. Tang SC. Mattson MP. Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ono K. Ikemoto M. Kawarabayashi T. Ikeda M. Nishinakagawa T. Hosokawa M. Shoji M. Takahashi M. Nakashima M. A chemical chaperone, sodium 4-phenylbutyric acid, attenuates the pathogenic potency in human alpha-synuclein A30P + A53T transgenic mice. Parkinsonism Relat Disord. 2009;15:649–654. doi: 10.1016/j.parkreldis.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 336.Osburn WO. Yates MS. Dolan PD. Chen S. Liby KT. Sporn MB. Taguchi K. Yamamoto M. Kensler TW. Genetic or pharmacologic amplification of nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;104:218–227. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002;4:309–319. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 338.Otterbein LE. Soares MP. Yamashita K. Bach F. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–466. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 339.Otterbein LE. Zuckerbraun BS. Haga M. Liu F. Song R. Usheva A. Stachulak C. Bodyak N. Smith RN. Csizmadia E. Tyagi S. Akamatsu Y. Flavell RJ. Billiar TR. Tzeng E. Bach FH. Choi AM. Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 340.Outeiro TF. Kontopoulos E. Altmann SM. Kufareva I. Strathearn KE. Amore AM. Volk CB. Maxwell MM. Rochet JC. McLean PJ. Young AB. Abagyan R. Feany MB. Hyman BT. Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 341.Outeiro TF. Marques O. Kazantsev A. Therapeutic role of sirtuins in neurodegenerative disease. Biochim Biophys Acta. 2008;1782:363–369. doi: 10.1016/j.bbadis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 342.Pacchioni AM. Vallone J. Melendez RI. Shih A. Murphy TH. Kalivas PW. Nrf2 gene deletion fails to alter psychostimulant-induced behavior or neurotoxicity. Brain Res. 2007;1127:26–35. doi: 10.1016/j.brainres.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Padmanabhan B. Scharlock M. Tong KI. Nakamura Y. Kang MI. Kobayashi A. Matsumoto T. Tanaka A. Yamamoto M. Yokoyama S. Purification, crystallization and preliminary X-ray diffraction analysis of the Kelch-like motif region of mouse Keap1. Acta Crystallogr Sect F Struct Biol Crystalliz Commun. 2005;61:153–155. doi: 10.1107/S1744309104032506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Padmanabhan B. Tong KI. Kobayashi A. Yamamoto M. Yokoyama S. Structural insights into the similar modes of Nrf2 transcription factor recognition by the cytoplasmic repressor Keap1. J Synchrotron Radiat. 2008;15:273–276. doi: 10.1107/S090904950705114X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Padmanabhan B. Tong KI. Ohta T. Nakamura Y. Scharlock M. Ohtsuji M. Kang MI. Kobayashi A. Yokoyama S. Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 346.Panahian N. Yoshiura M. Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 347.Park MK. Choi YM. Kang YK. Petersen OH. The endoplasmic reticulum as an integrator of multiple dendritic events. Neuroscientist. 2008;14:68–77. doi: 10.1177/1073858407305691. [DOI] [PubMed] [Google Scholar]
- 348.Parker JA. Arango M. Abderrahmane S. Lambert E. Tourette C. Catoire H. Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 349.Parola M. Bellomo G. Robino G. Barrera G. Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 350.Paterniti I. Genovese T. Mazzon E. Crisafulli C. Di Paola R. Galuppo M. Bramanti P. Cuzzocrea S. Liver X receptor agonist treatment regulates inflammatory response after spinal cord trauma. J Neurochem. 2010;112:611–624. doi: 10.1111/j.1471-4159.2009.06471.x. [DOI] [PubMed] [Google Scholar]
- 351.Perluigi M. Joshi G. Sultana R. Calabrese V. De Marco C. Coccia R. Cini C. Butterfield DA. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. J Neurosci Res. 2006;84:418–426. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- 352.Petrache I. Natarajan V. Zhen L. Medler TR. Richter AT. Cho C. Hubbard WC. Berdyshev EV. Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Pfister JA. Ma C. Morrison BE. D'Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One. 2008;3:e4090. doi: 10.1371/journal.pone.0004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Piantadosi CA. Zhang J. Levin ED. Folz RJ. Schmechel DE. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol. 1997;147:103–114. doi: 10.1006/exnr.1997.6584. [DOI] [PubMed] [Google Scholar]
- 355.Piantadosi CA. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic Biol Med. 2008;45:562–569. doi: 10.1016/j.freeradbiomed.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Prestera T. Zhang Y. Spencer SR. Wilczak CA. Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- 357.Prochaska HJ. Santamaria AB. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 358.Prochaska HJ. Bregman HS. De Long MJ. Talalay P. Specificity of induction of cancer protective enzymes by analogues of tert-butyl-4-hydroxyanisole (BHA) Biochem Pharmacol. 1985;34:3909–3914. doi: 10.1016/0006-2952(85)90443-5. [DOI] [PubMed] [Google Scholar]
- 359.Prochaska HJ. De Long MJ. Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci USA. 1985;82:8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Prochaska HJ. Santamaria AB. Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci USA. 1992;89:2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Prozorovski T. Schulze-Topphoff U. Glumm R. Baumgart J. Schröter F. Ninnemann O. Siegert E. Bendix I. Brüstle O. Nitsch R. Zipp F. Aktas O. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 362.Przemyslaw W. Vaarmann A. Choubey V. Safiulina D. Liiv J. Kuum M. Kaasik A. PGC-1[alpha] AND PGC-1[beta] regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Qin W. Yang T. Ho L. Zhao Z. Wang J. Chen L. Zhao W. Thiyagarajan M. MacGrogan D. Rodgers JT. Puigserver P. Sadoshima J. Deng H. Pedrini S. Gandy S. Sauve AA. Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 364.Qu K. Lee SW. Bian JS. Low CM. Wong PT. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int. 2008;52:155–165. doi: 10.1016/j.neuint.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 365.Ramesh Babu J. Lamar Seibenhener M. Peng J. Strom AL. Kemppainen R. Cox N. Zhu H. Wooten MC. Diaz-Meco MT. Moscat J. Wooten MW. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 366.Randall WA. Price CW. Welch H. Demonstration of hormesis (increase in fatality rate) by penicillin. Am J Pub Health. 1947;37:421–425. [PubMed] [Google Scholar]
- 367.Redpath JL. Suppression of neoplastic transformation in vitro by low doses of low LET radiation. Dose Response. 2006;4:302–308. doi: 10.2203/dose-response.06-114.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Redpath JL. Elmore E. Radiation-induced neoplastic transformation in vitro, hormesis and risk assessment. Dose Response. 2007;5:123–130. doi: 10.2203/dose-response.06-010.Redpath. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Reisman SA. Yeager RL. Yamamoto M. Klaassen CD. Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species. Toxicol Sci. 2009;108:35–47. doi: 10.1093/toxsci/kfn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Robertson CH. Griffin AC. Richardson HL. The inhibitory action of p-hydroxypropiophenone on hepatic carcinoma induced by azo dye. J Natl Cancer Inst. 1954;15:519–527. [PubMed] [Google Scholar]
- 371.Riegel B. Wartman WB. Hill WT. Reeb BB. Shubik P. Stanger DW. Delay of methylcholanthrene skin carcinogenesis in mice by 1,2,5,6-dibenzofluorene. Cancer Res. 1951;11:301–303. [PubMed] [Google Scholar]
- 372.Ristow M. Zarse K. Oberbach A. Klöting N. Birringer M. Kiehntopf M. Stumvoll M. Kahn CR. Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Rossi A. Trotta E. Brandi R. Arisi I. Coccia Santoro MG. AIRAP: a new human Heat shock gene regulated by Heat shock factor 1. J Biol Chem. 2010;285:13607–13615. doi: 10.1074/jbc.M109.082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Roth MB. Nystul T. Buying time in suspended animation. Sci Am. 2005;292:48–55. doi: 10.1038/scientificamerican0605-48. [DOI] [PubMed] [Google Scholar]
- 375.Rushmore TH. Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 376.Rushmore TH. Morton MR. Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–11639. [PubMed] [Google Scholar]
- 377.Sachdev S. Davies KJ. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 378.Sadruddin S. Arora R. Resveratrol: biologic and therapeutic implications. J Cardiometab Syndr. 2009;4:102–106. doi: 10.1111/j.1559-4572.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- 379.Sagan LA. On radiation, paradigms, and hormesis. Science. 1989;245:574–621. doi: 10.1126/science.2669125. [DOI] [PubMed] [Google Scholar]
- 380.Saibil HR. Chaperone machines in action. Curr Opin Struct Biol. 2008;18:35–42. doi: 10.1016/j.sbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 381.Sakai K. Nomura T. Ina Y. Enhancement of bio-protective functions by low dose/dose-rate radiation. Dose Response. 2006;4:327–332. doi: 10.2203/dose-response.06-115.Sakai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Salminen A. Kauppinen A. Suuronen T. Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? Bioessays. 2008;30:939–942. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- 383.Salminen A. Kaarniranta K. SIRT1 regulates the ribosomal DNA locus: epigenetic candles twinkle longevity in the Christmas tree. Biochem Biophys Res Commun. 2009;378:6–9. doi: 10.1016/j.bbrc.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 384.Sandur SK. Deorukhkar A. Pandey MK. Pabón AM. Shentu S. Guha S. Aggarwal BB. Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Satoh T. Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 386.Satoh T. Kosaka K. Itoh K. Kobayashi A. Yamamoto M. Shimojo Y. Kitajima C. Cui J. Kamins J. Okamoto S. Izumi M. Shirasawa T. Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Satoh T. Okamoto SI. Cui J. Watanabe Y. Furuta K. Suzuki M. Tohyama K. Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Satoh T. Saitoh S. Hosaka M. Kosaka K. Simple ortho- and para-hydroquinones as compounds neuroprotective against oxidative stress in a manner associated with specific transcriptional activation. Biochem Biophys Res Commun. 2009;379:537–541. doi: 10.1016/j.bbrc.2008.12.106. [DOI] [PubMed] [Google Scholar]
- 389.Scapagnini G. Butterfield DA. Colombrita C. Sultana R. Pascale A. Calabrese V. Ethyl ferulate, a lipophilic polyphenol, induces HO-1 and protects rat neurons against oxidative stress. Antioxid Redox Signal. 2004;6:811–818. doi: 10.1089/ars.2004.6.811. [DOI] [PubMed] [Google Scholar]
- 390.Scapagnini G. Colombrita C. Amadio M. D'Agata V. Arcelli E. Sapienza M. Quattrone A. Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 391.Schilling S. Goelz S. Linker R. Luehder F. Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Schimrigk S. Brune N. Hellwig K. Lukas C. Bellenberg B. Rieks M. Hoffmann V. Pöhlau D. Przuntek H. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol. 2006;13:604–610. doi: 10.1111/j.1468-1331.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 393.Schipper HM. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–830. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 394.Schipper HM. Song W. Zukor H. Hascalovici JR. Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem. 2009;110:469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- 395.Schmidt U. Holsboer F. Rein T. Role of the hsp70 cochaperone BAG1 in glucocorticoid receptor function and stress-related diseases. Proc Natl Acad Sci USA. 2008;105:E101. doi: 10.1073/pnas.0809354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Schulz H. Uber Hefegifte. Pfluger's Archiv Gesemmte Physiol. 1888;42:517–541. [Google Scholar]
- 397.Scott BR. DiPalma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose Response. 2006;5:230–255. doi: 10.2203/dose-response.06-002.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Scott BR. It's time for a new low-dose-radiation risk assessment paradigm—one that acknowledges hormesis. Dose Response. 2008;6:333–351. doi: 10.2203/dose-response.07-005.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sergent O. Griffon B. Morel I. Chevanne M. Dubos MP. Cillard P. Cillard J. Effect of nitric oxide on iron-mediated oxidative stress in primary rat hepatocyte culture. Hepatology. 1977;25:122–127. doi: 10.1002/hep.510250123. [DOI] [PubMed] [Google Scholar]
- 400.Sethi G. Ahn KS. Pandey MK. Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-κB activation. Blood. 2007;109:2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 401.Sethi JM. Otterbein LE. Choi AM. Differential modulation of exogenous carbon monoxide of TNF-alpha stimulated mitogen-activated protein kinases n rat pulmonary artery endothelial cells. Antioxid Redox Signal. 2002;4:241–248. doi: 10.1089/152308602753666299. [DOI] [PubMed] [Google Scholar]
- 402.Shan X. Chi L. Ke Y. Luo C. Qian S. Gozal D. Liu R. Manganese superoxide dismutase protects mouse cortical neurons from chronic intermittent hypoxia-mediated oxidative damage. Neurobiol Dis. 2007;28:206–215. doi: 10.1016/j.nbd.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sharma SK. Christen P. Goloubinoff P. Disaggregating chaperones: an unfolding story. Curr Protein Pept Sci. 2009;10:432–446. doi: 10.2174/138920309789351930. [DOI] [PubMed] [Google Scholar]
- 404.Shibuya N. Mikami Y. Kimura Y. Nagahara N. Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 405.Shih AY. Erb H. Murphy TH. Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem. 2007;101:109–119. doi: 10.1111/j.1471-4159.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- 406.Shih AY. Imbeault S. Barakauskas V. Erb H. Jiang L. Li P. Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 407.Shih AY. Johnson DA. Wong G. Kraft AD. Jiang L. Erb H. Johnson JA. Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–33940. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Shih AY. Li P. Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Shimazu T. Horinouchi S. Yoshida M. Multiple histone deacetylases and the CREB-binding protein regulate pre-mRNA 3′-end processing. J Biol Chem. 2007;282:4470–4478. doi: 10.1074/jbc.M609745200. [DOI] [PubMed] [Google Scholar]
- 410.Siebert A. Desai V. Chandrasekaran K. Fiskum G. Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;87:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 411.Siems W. Carluccio F. Radenkovic S. Grune T. Hampl H. Oxidative stress in renal anemia of hemodialysis patients is mitigated by epoetin treatment. Kidney Blood Press Res. 2005;28:295–301. doi: 10.1159/000090184. [DOI] [PubMed] [Google Scholar]
- 412.Siow RC. Ishii T. Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 413.Sirabella R. Secondo A. Pannaccione A. Scorziello A. Valsecchi V. Adornetto A. Bilo L. Di Renzo G. Annunziato L. Anoxia-induced NF-kappaB-dependent upregulation of NCX1 contributes to Ca2+ refilling into endoplasmic reticulum in cortical neurons. Stroke. 2009;40:922–929. doi: 10.1161/STROKEAHA.108.531962. [DOI] [PubMed] [Google Scholar]
- 414.Sjorstrand T. Endogenous formation of carbon monoxide in man under normal and pathological conditions. Scand J Clin Lab Invest. 1949;1:201–214. [Google Scholar]
- 415.Smith EL. Schuchman EH. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 2008;22:3419–3431. doi: 10.1096/fj.08-108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Söti C. Csermely P. Aging cellular networks: chaperones as major participants. Exp Gerontol. 2007;42:113–119. doi: 10.1016/j.exger.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 417.Söti C. Csermely P. Protein stress and stress proteins: implications in aging and disease. J Biosci. 2007;32:511–515. doi: 10.1007/s12038-007-0050-z. [DOI] [PubMed] [Google Scholar]
- 418.Sõti C. Nagy E. Giricz Z. Vígh L. Csermely P. Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Sparnins VL. Barany G. Wattenberg LW. Effects of organosulfur compounds from garlic and onions on benzo[a]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis. 1988;9:131–134. doi: 10.1093/carcin/9.1.131. [DOI] [PubMed] [Google Scholar]
- 420.Spencer SR. Wilczak CA. Talalay P. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990;50:7871–7875. [PubMed] [Google Scholar]
- 421.Spencer SR. Xue LA. Klenz EM. Talalay P. The potency of inducers of NAD(P)H:(quinone-acceptor) oxidoreductase parallels their efficiency as substrates for glutathione transferases. Structural and electronic correlations. Biochem J. 1991;1:711–717. doi: 10.1042/bj2730711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Stanford SJ. Walters MJ. Mitchell JA. Carbon monoxide inhibits endothelin-1 release by human pulmonary artery smooth muscle cells. Eur J Pharmacol. 2004;486:349–352. doi: 10.1016/j.ejphar.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 423.Stebbing ARD. Effects of low metal levels on a clonal hydroid. J Marine Biol Assoc UK. 1976;56:977–994. [Google Scholar]
- 424.Stebbing ARD. Hormesis—the stimulation of growth by low-levels of inhibitors. Sci Total Environ. 1982;22:213–234. doi: 10.1016/0048-9697(82)90066-3. [DOI] [PubMed] [Google Scholar]
- 425.Stocker R. Yamamoto Y. McDonagh AF. Glazer AN. Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 426.Stoof TJ. Flier J. Sampat S. Nieboer C. Tensen CP. Boorsma DM. The antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cells. Br J Dermatol. 2001;144:1114–1120. doi: 10.1046/j.1365-2133.2001.04220.x. [DOI] [PubMed] [Google Scholar]
- 427.Suliman HB. Carraway MS. Tatro LG. Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 428.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 429.Sun MM. Bu H. Li B. Yu JX. Guo YS. Li CY. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol Res. 2009;31:23–27. doi: 10.1179/174313208X332959. [DOI] [PubMed] [Google Scholar]
- 430.Suzuki K. Koike T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem Biophys Res Commun. 2007;359:665–671. doi: 10.1016/j.bbrc.2007.05.164. [DOI] [PubMed] [Google Scholar]
- 431.Sykes PJ. Morley AA. Hooker AM. The pKZ1 recombination mutation assay: a sensitive assay for low dose studies. Dose Response. 2006;4:91–105. doi: 10.2203/dose-response.05-035.Sykes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Sykes PJ. Day TK. Requirements for identification of low dose and non-linear mutagenic responses to ionizing radiation. Dose Response. 2007;5:308–314. doi: 10.2203/dose-response.07-018.Sykes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Szabadi E. Model of 2 functionally antagonistic receptor populations activated by same agonist. J Theor Biol. 1977;69:101–112. doi: 10.1016/0022-5193(77)90390-3. [DOI] [PubMed] [Google Scholar]
- 434.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 435.Talalay P. Batzinger RP. Benson AM. Bueding E. Cha YN. Biochemical studies on the mechanisms by which dietary antioxidants suppress mutagenic activity. Adv Enzyme Regul. 1978;17:23–36. doi: 10.1016/0065-2571(79)90006-2. [DOI] [PubMed] [Google Scholar]
- 436.Talalay P. Benson AM. Elevation of quinone reductase activity by anticarcinogenic antioxidants. Adv Enzyme Regul. 1982;20:287–300. doi: 10.1016/0065-2571(82)90021-8. [DOI] [PubMed] [Google Scholar]
- 437.Talalay P. De Long MJ. Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors. 2000;12:5–11. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- 439.Talalay P. Dinkova-Kostova AT. Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 440.Talalay P. Dinkova-Kostova AT. Role of nicotinamide quinone oxidoreductase 1 (NQO1) in protection against toxicity of electrophiles and reactive oxygen intermediates. Methods Enzymol. 2004;382:355–364. doi: 10.1016/S0076-6879(04)82019-6. [DOI] [PubMed] [Google Scholar]
- 441.Talalay P. Fahey JW. Healy ZR. Wehage SL. Benedict AL. Min C. Dinkova-Kostova AT. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci USA. 2007;104:17500–17505. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Tan BH. Wong PT. Bian JS. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 443.Tangerman A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J Chromatogr B. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 444.Teiten MH. Eifes S. Reuter S. Duvoix A. Dicato M. Diederich M. Gene expression profiling related to anti-inflammatory properties of curcumin in K562 leukemia cells. Ann NY Acad Sci. 2009;1171:391–398. doi: 10.1111/j.1749-6632.2009.04890.x. [DOI] [PubMed] [Google Scholar]
- 445.Tenhunen R. Marver HS. Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 446.Terada LS. Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol. 2006;174:615–623. doi: 10.1083/jcb.200605036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Thom ER. Fisher D. Xu YA. Notarfrancesco K. Ischiropoulos H. Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc Natl Acad Sci USA. 2000;97:1305–1310. doi: 10.1073/pnas.97.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Thong H-Y. Maibach HI. Hormesis [biological effects of low level exposure (BELLE)] and dermatology. Dose Response. 2008;6:1–15. doi: 10.2203/dose-response.07-029.Thong. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Tong KI. Kobayashi A. Katsuoka F. Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 450.Tong KI. Katoh Y. Kusunoki H. Itoh K. Tanaka T. Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Tong KI. Padmanabhan B. Kobayashi A. Shang C. Hirotsu Y. Yokoyama S. Yamamoto M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27:7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Trott A. West JD. Klaić L. Westerheide SD. Silverman RB. Morimoto RI. Morano KA. Activation of heat shock and antioxidant responses by the natural product celastrol: transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19:1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Turcanu V. Dhouib M. Poindron P. Nitric oxide synthase inhibition by haem oxygenase decreases macrophagenitric-oxide-dependent cytotoxicity: a negative feedback mechanism for the regulation of nitric oxide production. Res Immunol. 1998;149:741–744. doi: 10.1016/s0923-2494(99)80050-9. [DOI] [PubMed] [Google Scholar]
- 454.Um HS. Kang EB. Leem YH. Cho IH. Yang CH. Chae KR. Hwang DY. Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer's disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529–539. [PubMed] [Google Scholar]
- 455.Umemura K. Kimura H. Hydrogen sulfide enhances reducing activity in neurons: neurotrophic role of H2S in the brain? Antioxid Redox Signal. 2007;9:2035–2041. doi: 10.1089/ars.2007.1802. [DOI] [PubMed] [Google Scholar]
- 456.Vargas MR. Johnson DA. Sirkis DW. Messing A. Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Verkhratsky A. The endoplasmic reticulum and neuronal calcium signalling. Cell Calcium. 2002;32:393–404. doi: 10.1016/s0143416002001896. [DOI] [PubMed] [Google Scholar]
- 458.Verma A. Hirsch DJ. Glatt CE. Ronnett GV. Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 459.Violi F. Marino R. Milite MT. Loffredo L. Nitric oxide and its role in lipid peroxidation. Diabetes Metab Res Rev. 1999;15:283–288. doi: 10.1002/(sici)1520-7560(199907/08)15:4<283::aid-dmrr42>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 460.Wagner F. Asfar P. Calzia E. Radermacher P. Szabó C. Bench-to-bedside review: Hydrogen sulfide—the third gaseous transmitter: applications for critical care. Crit Care. 2009;13:213. doi: 10.1186/cc7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Wakabayashi N. Dinkova-Kostova AT. Holtzclaw WD. Kang MI. Kobayashi A. Yamamoto M. Kensler TW. Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Wang W. Fang H. Groom L. Cheng A. Zhang W. Liu J. Wang X. Li K. Han P. Zheng M. Yin J. Wang W. Mattson MP. Kao JP. Lakatta EG. Sheu SS. Ouyang K. Chen J. Dirksen RT. Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wattenberg LW. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by phenolic antioxidants and ethoxyquin. J Natl Cancer Inst. 1972;48:1425–1430. [PubMed] [Google Scholar]
- 464.Wattenberg LW. Coccia JB. Lam LK. Inhibitory effects of phenolic compounds on benzo(a)pyrene-induced neoplasia. Cancer Res. 1980;40:2820–2823. [PubMed] [Google Scholar]
- 465.Welch H. Price CW. Randall WA. Increase in fatality rate of E. Typhosa for white mice by streptomycin. J Am Pharm. 1946;35:155–158. doi: 10.1002/jps.3030350505. [DOI] [PubMed] [Google Scholar]
- 466.Westerheide SD. Anckar J. Stevens SM. Sistonen L., Jr. Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 323. 2009:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Westerheide SD. Bosman JD. Mbadugha BN. Kawahara TL. Matsumoto G. Kim S. Gu W. Devlin JP. Silverman RB. Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 468.Wierinckx A. Brevé J. Mercier D. Schultzberg M. Drukarch B. Van Dam AM. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol. 2005;166:132–143. doi: 10.1016/j.jneuroim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 469.Yang F. Lim GP. Begum AN. Ubeda OJ. Simmons MR. Ambegaokar SS. Chen PP. Kayed R. Glabe CG. Frautschy SA. Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 470.Yao CJ. Lin CW. Lin-Shiau SY. Roles of thapsigargin-sensitive Ca2+ stores in the survival of developing cultured neurons. J Neurochem. 1999;73:457–465. doi: 10.1046/j.1471-4159.1999.0730457.x. [DOI] [PubMed] [Google Scholar]
- 471.Yates MS. Tran QT. Dolan PM. Osburn WO. Shin S. McCulloch CC. Silkworth JB. Taguchi K. Yamamoto M. Williams CR. Liby KT. Sporn MB. Sutter TR. Kensler TW. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Yoshida T. Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 473.Young LJ. Caughey WS. Oxygenation of carbon monoxide by bovine heart cytochrome c oxidase. Biochemistry. 1986;25:152–161. doi: 10.1021/bi00349a022. [DOI] [PubMed] [Google Scholar]
- 474.Yu Z. Luo H. Fu W. Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 475.Yu ZF. Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 476.Yu ZF. Nikolova-Karakashian M. Zhou D. Cheng G. Schuchman EH. Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 477.Zhang DD. Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Zhang K. Kaufman RJ. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb Exp Pharmacol. 2006;172:69–91. doi: 10.1007/3-540-29717-0_3. [DOI] [PubMed] [Google Scholar]
- 479.Zhang K. Zhao T. Huang X. Liu ZH. Xiong L. Li MM. Wu LY. Zhao YQ. Zhu LL. Fan M. Preinduction of HSP70 promotes hypoxic tolerance and facilitates acclimatization to acute hypobaric hypoxia in mouse brain. Cell Stress Chaperones. 2009;14:407–415. doi: 10.1007/s12192-008-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Zhang Y. Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 481.Zhang Y. Talalay P. Cho CG. Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Zhao J. Kobori N. Aronowski J. Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 483.Zhao J. Moore AN. Clifton GL. Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- 484.Zhao J. Moore AN. Redell JB. Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Zhao X. Sun G. Zhang J. Strong R. Dash PK. Kan YW. Grotta JC. Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 486.Zhou P. Qian L. Iadecola C. Nitric oxide inhibits caspase activation and apoptotic morphology but does not rescue neuronal death. J Cereb Blood Flow Metab. 2005;25:348–357. doi: 10.1038/sj.jcbfm.9600036. [DOI] [PubMed] [Google Scholar]
- 487.Zlotkin S. A new approach to control of anemia in “at risk” infants and children around the world. 2004 Ryley-Jeffs memorial lecture. Can J Diet Pract Res. 2004;65:136–138. doi: 10.3148/65.3.2004.136. [DOI] [PubMed] [Google Scholar]