Abstract
BACKGROUND
The active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25D) reduces the growth of several prostate cancer cell lines, most commonly by inducing a cell cycle arrest in G1. This is mediated, in part, through down-regulation of c-Myc, a positive regulator of the transcription factor, E2F. There is evidence that prostate cancer cells lacking functional retinoblastoma protein (Rb), a negative regulator of E2F activity, are poorly responsive to 1,25D treatment. Since up to 60% of prostate cancers demonstrate a loss of heterozygosity for Rb, we sought to determine whether Rb is required for the growth inhibitory effects of 1,25D.
METHODS
Using siRNA, Rb was reduced in C4-2 prostate cancer cells, and the response of cells to 1,25D treatment or depletion of c-myc measured by [3H]-thymidine incorporation and flow cytometry. The effects of 1,25D treatment on E2F levels and activity, and E2F target gene expression were also measured.
RESULTS
1,25D treatment and c-Myc depletion both cause a G1 arrest inhibiting C4-2 cell proliferation independently of Rb. 1,25D reduces c-Myc expression and causes a decrease in E2F and E2F target genes. Bcl-2, an E2F target and positive regulator of C4-2 cell growth, also is down-regulated by 1,25D independently of Rb.
CONCLUSIONS
Redundant growth inhibitory pathways compensate for the loss of Rb, and tumors lacking functional Rb may be responsive to 1,25D.
Keywords: Vitamin D, Rb, c-Myc, prostate cancer, E2F
INTRODUCTION
Androgen deprivation is the standard therapy for treatment of metastatic prostate cancer. It is initially effective, but androgen-independent tumors usually recur 2–3 years after initiation of treatment. Therefore, other strategies are needed to treat this disease. One potential therapeutic being studied pre-clinically is the use of vitamin D receptor (VDR) agonists. The biologically active metabolite of vitamin D, 1α,25-dihydroxyvitamin D3 (1,25D), has been reported to reduce the growth of many prostate cancer cell lines by several mechanisms including altering the secretion and signaling of growth factors [1–3], induction of apoptosis [4], and/or induction of a cell cycle arrest, most commonly in G1 [5, 6]. Thus, there may be multiple, possibly redundant pathways by which 1,25D inhibits growth.
The G1 to S phase transition is dependent upon the activity of the transcription factor E2F, which up-regulates genes necessary for continuation of the cell cycle [7]. During G1, E2F activity is suppressed by the retinoblastoma protein (Rb). Active Rb binds to and sequesters E2F [8], until it is inactivated by phosphorylations that allow release of E2F and transition into S phase [9].
In LNCaP prostate cancer cells, the 1,25D induced G1 arrest [5, 6] is accompanied by a decrease in E2F transcriptional activity [6]. Our lab has shown that 1,25D reduces expression of the transcription factor c-Myc, and that reducing c-Myc alone can cause growth inhibition via cell cycle arrest in G1 to a similar extent as 1,25D treatment in C4-2 cells, the androgen-sensitive, but androgen deprivation-resistant derivatives of LNCaPs [10]. c-Myc induces E2F1 expression [11]. c-Myc also positively regulates Rb phosphorylation and inactivation. Rb is phosphorylated by the cyclin D/cyclin dependent kinase 4/6 (cdk4/6) and cyclin E/cdk2 complexes [12, 13]. c-Myc increases cyclin D-cdk4/6 activity [14], cyclin E expression [15], and expression of Cdc25a [16], a phosphatase that removes an inhibitory phosphate from cdk2. c-Myc also represses expression of cdk inhibitors p21 [17] and p27 [14]. Therefore, the down-regulation of c-Myc by 1,25D should decrease Rb phosphorylation, resulting in reduced E2F activity. Also, it has been reported that p21 is an essential factor for the growth inhibitory effects of 1,25D in some prostate cancer cell lines [18, 19].
There is some data suggesting that prostate cancer cells lacking functional Rb are poorly responsive to 1,25D. The DU145 cell line, which lacks functional Rb and expresses mutant p53 [20] is weakly growth inhibited by 1,25D treatment [21]. SV40 large T antigen inactivates Rb and p53, and SV40 transformed lines also are not responsive to 1,25D treatment [20]. In previous studies, we've shown that p53 has only a modest effect on responsiveness to 1,25D [22]. Based on these observations, we sought to determine whether the growth inhibitory effects of 1,25D treatment require the expression of Rb in C4-2 cells. This is of importance because up to 60% of prostate cancers demonstrate a loss of heterozygosity of Rb [23–26]. Our results show that 1,25D can reduce the growth of prostate cancer cells independently of Rb expression, and that reducing c-Myc levels either by using siRNA or by treatment with 1,25D is sufficient to reduce E2F expression and activity independently of Rb. Consistent with this, 1,25D-mediated down-regulation of the anti-apoptotic protein Bcl-2, whose expression is regulated by E2F, also is Rb-independent.
MATERIALS AND METHODS
Cell Culture and Reagents
The C4-2 prostate cancer cell line was obtained from UroCor Inc. (Oklahoma City, OK). The cells were grown in T medium (Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated FBS (56°C for 30 min) in an incubator maintained at 37°C in humidified 5% CO2. 1,25(OH)2D3 (1,25D) was obtained from Solvay Pharmaceuticals (Weesp, The Netherlands). Stock solutions prepared in ethanol were stored in the dark at −80°C.
Small Interfering RNA (siRNA) Transfection
C4-2 cells were plated at a density of 30,000 cells/well in 6 well plates. They were transfected with siRNA in serum free T medium using Lipofectamine (Invitrogen) diluted in OPTI-MEM medium (Invitrogen) for six hours according to the manufacturer's recommendations. Silencer Negative Control #1 siRNA (catalog no. 4635) was purchased from Ambion (Austin, TX). siGENOME SMARTpool siRNA against c-Myc (catalog no. M-003282-07) was purchased from Dharmacon (Lafayette, CO). Custom siRNA targeting Rb was purchased from Dharmacon with sense sequence (GAAACAGAAGAACCUGAUU)dTdT and antisense sequence (AAUCAGGUUCUUCUGUUUC)dTdT. SMARTpool Bcl-2 siRNA (catalog no. M-003307) and ONTARGETplus Duplex Human Bcl-2 siRNA (catalog no. J-003307-19) were purchased from Dharmacon. After transfection, medium was replaced with fresh T medium supplemented with 5% heat inactivated FBS.
Luciferase and β-Galactosidase Plasmid Transfection
Luciferase reporter plasmids pTA-luc and pE2F-luc (Clontech, Mountain View, CA) and a mammalian pCR3.1 β-gal expression plasmid (Invitrogen) [27] were transfected using Lipofectin (Invitrogen) according to the manufacturer's recommendations. Luciferase activity was measured using luciferase assay reagent from Promega (Madison, WI) and β-galactosidase activity was measured to normalize luciferase activity as described previously [28].
[3H]-Thymidine Incorporation and Cell Counting
Cells were pulsed for 2–3 hours with 2 μCi/ml [3H]-thymidine (MP Biomedicals, Solon, OH) and incorporation measured as described previously [29]. To measure cell number, cells were washed with Hank's buffered salt solution and harvested with 0.25% trypsin. Cells were counted using a Coulter particle counter Z1 (Coulter, Hialeah, FL).
Western Blot Analysis
Cells were harvested in Dulbecco's Phosphate Buffered Saline (PBS) (Invitrogen) and protein extracts were prepared by three freeze/thaw cycles in TESH lysis buffer [0.01 M Tris, 1 mM EDTA, 12 mM monothioglycerol, pH7.7, protease inhibitor cocktail]. The Bradford assay was used to determine protein concentrations; the lysates were run on SDS-PAGE and transferred to nitrocellulose membranes. Membranes for Rb, c-Myc, and actin were blocked for one hour at room temperature with 1% milk-Tris-buffered saline with Tween 20 [TBST: 10 mM Tris (pH 7.5), 0.15 M NaCl, 0.1% Tween 20]. Membranes were then incubated with primary antibodies at 4°C overnight. All subsequent incubations and washes were done at room temperature. Primary mouse monoclonal Rb antibody clone LM95.1 (Calbiochem, San Diego, CA) was diluted 1:200 in 0.5% milk-TBST. Primary mouse monoclonal c-Myc antibody clone 9E-10 (11 667 149 001, Roche Applied Science, Indianapolis, IN) was diluted 1:80 in 1% milk-TBST,and rabbit monoclonal c-Myc antibody Y69 (Abcam, Cambridge, MA) (used in Fig. 5) was diluted 1:5,000 in 5%milk-TBST. Primary mouse monoclonal actin antibody (Millipore, Temecula, CA) was diluted 1:10,000 in 1% milk-TBST. After primary antibody incubation, blots were washed three times with 1% milk-TBST. Actin blots were then incubated with horseradish peroxidase-conjugated anti-mouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ) diluted 1:30,000 in TBST for one hour. c-Myc Y69 blots were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Amersham Pharmacia Biotech) diluted 1:30,000 for one hour in TBST. Rb and c-Myc 9E-10 blots were incubated with rabbit anti-mouse IgG (Zymed Laboratories, South San Francisco, CA) secondary antibody diluted 1:5,000 in 1% milk-TBST for one hour. After secondary antibody incubation, Rb and c-Myc 9E-10 blots were washed three times in 1% milk-TBST, and then incubated for one hour with horseradish peroxidase-conjugated donkey anti-rabbit IgG diluted 1:30,000 in TBST. After horseradish peroxidase incubation, all blots were washed three times with TBST. Membranes for Bcl-2 were first blocked for one hour at room temperature with 1% BSA-phosphate buffered saline with Tween 20 [PBST: 2.67 mM KCl, 1.47 mM KH2PO4, 137.93 mM NaCl, 8.06 mM Na2HPO4-7H2O]. Membranes were then incubated with primary mouse monoclonal Bcl-2 antibody clone 124 (DakoCytomation, Carpinteria, CA) diluted 1:2,000 in 1% BSA-PBST at 4°C overnight. All subsequent incubations and washes were done at room temperature. After primary antibody incubation, blots were washed three times with 1% BSA-PBST, and incubated with rabbit anti-mouse IgG (Zymed Laboratories) secondary antibody diluted 1:5000 in 1% BSA-PBST for one hour. After secondary incubation, blots were washed three times in 1% BSA-PBST, and incubated for one hour with horseradish peroxidase-conjugated donkey anti-rabbit IgG diluted 1:30,000 in PBST. After horseradish peroxidase incubation, blots were washed three times with PBST. All proteins were detected with ECL+ (GE Healthcare, Buckinghamshire, England). The NIH Image Processing and Analysis in Java (ImageJ) software was used to quantify band intensities.
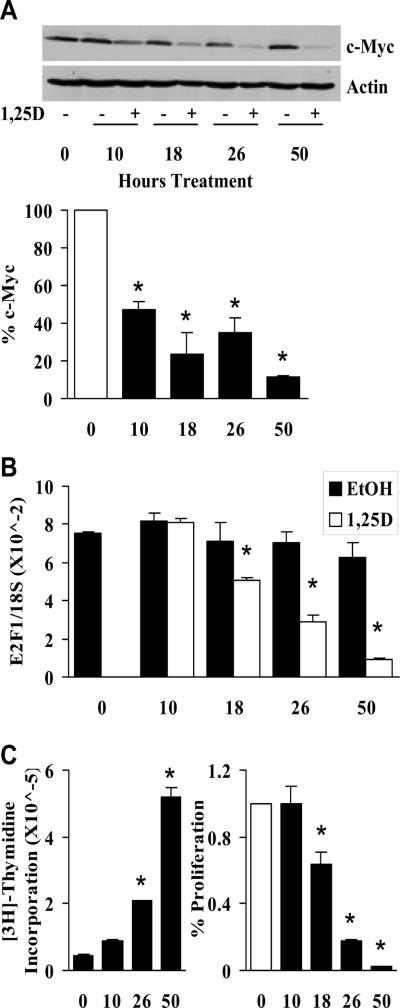
Fig. 5.
c-Myc down-regulation precedes E2F down-regulation and growth inhibition during 1,25D treatment. A, Cells were treated with EtOH or 100 nM 1,25D. At the indicated timepoints, c-Myc and actin levels were measured by Western analysis. Signal intensities from three independent Western blots were measured with a densitometer and quantified. Densitometric values are represented as the percent of c-Myc present in 1,25D treated samples relative to the EtOH controls at each timepoint (white bar – 0 hours of treatment) (*, P < 0.003 significant reduction of c-Myc in 1,25D treated samples versus EtOH treated samples at each timepoint). B, Cells were treated as in (A) and E2F levels were measured by qPCR (*, P < 0.015 significant effect of 1,25D treatment versus EtOH). C, In parallel samples from (B) cell proliferation was measured by [3H]-thymidine incorporation. [3H]-thymidine was added 2 hours prior to the harvest times indicated on the X axis. Left panel shows increase in cell proliferation over time (*, P < 10 × 10−5 significant increase in proliferation relative to T=0). Right panel shows percent of growth in 1,25D treated samples relative to the EtOH controls at each timepoint (white bar – 0 hours of treatment) (*, P < 0.01 significant effect of reduction of growth in 1,25D treated samples versus EtOH treated samples at each timepoint). Note that there is no 18 hour timepoint on the left because the effect of 18 hours of treatment (right) was determined by adding 1,25D in the afternoon and harvesting 18 hours later at the same time as the 26 hour timepoint, so the proliferation at 26 hours served as control for both.
Quantitative RT-PCR (qPCR)
Cells were harvested in PBS and RNA was extracted using Trizol reagent (Invitrogen). cDNA was prepared from 300 ng RNA with Superscript III Reverse Transcriptase (Invitrogen), and primer sets for E2F1, E2F2, cyclin E, cyclin A2, and MCM7 were used for detection of the transcripts with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). For detection of c-Myc, Rb, and Bcl-2, 100 ng of RNA template was analyzed with the Taqman One Step PCR Master Mix (Applied Biosystems). All target genes were normalized to 18S RNA with Applied Biosystems primer and probe set (4319413E-0402011) using Taqman One Step PCR Master Mix (for RNA templates) or Taqman Universal Master Mix (for cDNA templates). See Table 1 for primer and probe sequences.
Table 1. Quantitative Real-Time Primers and Probes.
Sets are listed as forward primer, reverse primer, and FAM/TAMRA probe.
| mRNA | Sequence 5' → 3' | NCBI Access Number |
|---|---|---|
| Bcl-2 | CATGTGTGTGGAGAGCGTCAA GCCGGTTCAGGTACTCAGTCA CCTGGTGGACAACATCGGCCCTGT |
NM_000633.2 (Variant α), NM_000657.2 (Variant β) |
| c-Myc | AGCTGCTTAGACGCTGGATTTT GTTCCTGTTGGTGAAGCTAACGT AGCCTCCCGCGACGATGCC |
NM_002467.3 |
| Cyclin A2 | CGCTCCAAGAGGACCAGGA GGTCCGCGGTTGTTGGAC |
NM_001237.3 |
| Cyclin E | AAAGAAGATGATGACCGGGTTTAC GAGCCTCTGGATGGTGCAAT |
NM_001238.1 (Variant 1), NM_057182.1 (Variant 2) |
| E2F1 | TCCAAGAACCACATCCAGTG CTGGGTCAACCCCTCAAG |
NM_005225.2 |
| E2F2 | TGAAGGAGCTGATGAACACG TTAAAGTTGCCAACAGCACG |
NM_004091.2 |
| MCM7 | GCTCTTTGCTGATGCCGTACA TCTTTATTTACCACTTCCCTCTCCTT |
NM_005916.3 (Variant 1), NM_182776.1 (Variant 2) |
| Rb | ACCTCAAACAAGGAAGAGA ACATCTGTGAGAGACAATG ATCTCAGGACCTTGGTGG |
NM_000321.2 |
Flow Cytometry
Cells were washed and harvested in PBS, fixed in 90% ethanol, and stained with propidium iodide for fluorescence activated cell sorting analysis as described previously [30]. Cell cycle distribution was analyzed using Becton-Dickinson LSRII and CantoII instruments, and quantified using FlowJo version 8.8.6 (Tree Star Inc, Ashland, OR).
Statistics
PASW Statistics 17.0 software by SPSS Inc. (Chicago, IL) was used to determine statistical significance (P < 0.05). The unpaired t test was used to compare the means of two groups. One-way ANOVA was used to compare multiple means to a control group. Two-way ANOVA was used to compare means with two independent variables to a control group. Results are shown as means with error bars indicating SEM. Unless otherwise indicated, all experiments were performed at least three times in triplicate, with one representative experiment shown.
RESULTS
1,25D inhibits the growth of Rb-depleted C4-2 prostate cancer cells
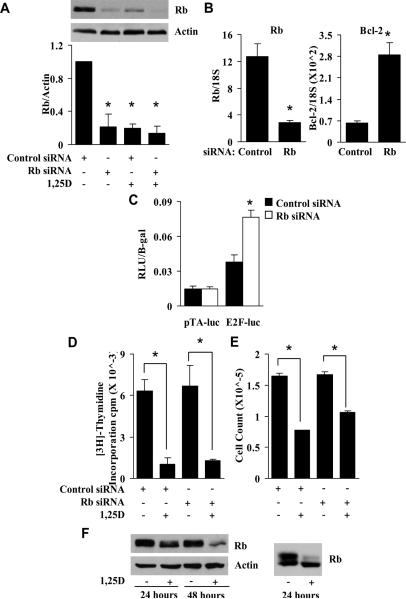
To determine whether Rb is required for the growth inhibitory effects of 1,25D, C4-2 prostate cancer cells were transfected with control siRNA or siRNA specifically targeting Rb, and subsequently treated with 1,25D. Rb siRNA transfection resulted in a strong reduction in Rb protein (Fig. 1A). Densitometric quantification of Western blots from three independent experiments shows a consistent decrease in Rb levels by Rb siRNA (Fig. 1A). Because Bcl-2 expression is induced by active E2F [31], Rb depletion should increase Bcl-2 levels, as this increases the amount of active E2F. Rb siRNA caused a substantial (4-fold) increase in Bcl-2 mRNA (Fig. 1B), confirming that Rb was reduced to sufficient levels to decrease overall Rb activity. Additionally, an E2F-responsive luciferase reporter assay confirmed an increase in E2F activity with reduced Rb expression (Fig. 1C). The specific E2F-dependent activity (E2F luciferase minus the activity of the TA-luciferase lacking the E2F binding site) in Rb siRNA transfected cells was 3-fold higher compared to control siRNA transfected cells, similar to the change in Bcl-2. Despite this, reducing Rb levels alone did not alter proliferation measured by [3H]-thymidine incorporation (Fig. 1D) or cell number measured using a Coulter counter (Fig. 1E), nor did it prevent 1,25D-mediated growth inhibition, indicating that Rb is not required for 1,25D-dependent growth inhibition (Fig. 1D and 1E). During these analyses we observed that Rb expression is consistently reduced by 1,25D treatment (Fig 1A). To confirm that this loss was independent of the conditions required for siRNA transfection, C4-2 cells were treated with 1,25D for 24 and 48 hours in the absence of siRNA transfection, and Western analysis was performed. 1,25D decreased Rb protein levels as a function of time of treatment (Fig. 1F) although the remaining Rb is the hypophosphorylated/activated form as judged by the preferential loss of the slower mobility species (see Fig. 1F right for an example of cells treated for 24 hours followed by separation on an SDS gel until the 40K standard was electrophoresed off the gel to give better resolution of the phosphorylated forms of Rb). Interestingly, there was little or no change in Rb mRNA (data not shown), so the regulation must be post-transcriptional.
Fig. 1.
1,25D-mediated growth inhibition in C4-2 cells is Rb-independent. A, Cells were transiently transfected with 60 pM control or Rb siRNA and grown for 72 hours. 24 hours after transfection, cells were treated with EtOH (vehicle control) or 100 nM 1,25D. Cells were harvested at the end of the 72 hour growth period and Rb and actin protein levels were measured by Western analysis. Signal intensities from three independent Western blots were measured with a densitometer and quantified. Densitometric values represent Rb/Actin with the value in EtOH treated cells set at 1 (*, P < 3.5 × 10−4 significant effect of 1,25D treatment and/or Rb siRNA transfection versus EtOH treatment and control siRNA transfection). B, Cells were transfected with control or Rb siRNA. 72 hours after transfection, RNA was isolated, and Rb and Bcl-2 RNA levels were measured by quantitative RT-PCR (qPCR) (*, P < 0.01 significant effect of Rb siRNA versus control siRNA). C, Cells were treated with EtOH or 100 nM 1,25D for 24 hours. Cells were then transfected with 1.0 ug of pTA-luc (parental control) or pE2F-luc plus 100 ng of pCR3.1 β-galactosidase. After transfection, cells were treated again with EtOH or 1,25D. 24 hours after treatment the activities of luciferase and β-galactosidase were measured. Relative luciferase units (RLU) were normalized for β-galactosidase activity (*, P = 0.001 significant effect of Rb siRNA versus control siRNA within E2F-luc transfected cells). D, Proliferation was measured by [3H]-thymidine incorporation in parallel samples from (A) (*, P < 0.025 significant effect of 1,25D versus EtOH within the siRNA transfection group). E, Cells were treated as in (A) and counted with a Coulter counter (*, P < 2.5 × 10−4). F, Cells were treated with EtOH or 100 nM 1,25D and Rb and actin protein levels were measured by Western analysis at the indicated times. Samples from an independent experiment were run longer on a SDS-PAGE gel to achieve better separation of the hyper-phosphorylated and hypo-phosphorylated species of Rb (right panel).
1,25D-mediated cell cycle accumulation in G0/G1 is Rb-independent
Because 1,25D regulates genes to induce a G2 arrest in some cell lines, we asked whether the 1,25D-dependent growth inhibition in the Rb-depleted cells altered cell cycle distribution relative to control cells treated with 1,25D. Reducing Rb alone did not significantly affect cell cycle distribution of vehicle treated cells, nor did it prevent 1,25D-mediated cell cycle accumulation in G0/G1 (Table 2). Thus, 1,25D-mediated G1 arrest is Rb-independent.
Table 2. 1,25D induces an Rb-independent G0/G1 cell cycle accumulation in C4-2 cells.
Cells were transiently transfected with 60 pM control or Rb siRNA. 24 hours after transfection, cells were treated with EtOH or 100 nM 1,25D. The cell cycle profile was determined using flow cytometry (*, P < 0.02 significant effect of 1,25D versus EtOH within the siRNA transfection group).
| G0/G1 | S | G2/M | |
|---|---|---|---|
| Control siRNA + EtOH | 57%±5.6 | 28%±4.4 | 14%±1 |
| Control siRNA + 1,25D | 82%±0.8* | 4%±0.5* | 14%±0.8 |
| Rb siRNA + EtOH | 51%±4.5 | 31%±3.5 | 15%±.1 |
| Rb siRNA + 1,25D | 79%±1.6* | 6%±0.9* | 16%±0.3 |
Growth inhibition caused by c-Myc depletion also is Rb-independent
One of the critical upstream effectors of Rb activity is c-Myc [14]. We have shown previously that 1,25D treatment reduces c-Myc and that reducing c-Myc to comparable levels using two independent siRNAs mimics the growth inhibition and G1 accumulation caused by 1,25D treatment [10]. To determine whether inhibition of cell growth by c-Myc depletion requires Rb, both c-Myc and Rb were reduced with siRNA and cell proliferation was examined. The reduction in c-Myc and Rb protein levels with their respective siRNAs was confirmed by Western analysis (Fig. 2A). c-Myc siRNA reduced C4-2 cell proliferation as previously demonstrated [10], and Rb siRNA did not prevent the growth inhibition caused by the reduction in c-Myc (Fig. 2B). Rb siRNA also did not reduce the G0/G1 accumulation previously shown to result from reduced c-Myc expression [10] (data not shown). We observed that the combination of c-Myc and Rb siRNAs caused an enhanced reduction of c-Myc protein although Rb siRNA alone did not reduce c-Myc expression. This may contribute to the apparently greater, but not statistically significant, reduction in proliferation seen in the c-Myc and Rb siRNA transfected cells versus the cells that were transfected with c-Myc siRNA alone. As expected, reducing Rb did not prevent the reduction in c-Myc protein caused by 1,25D treatment (Fig. 2C). Thus, the down-regulation of c-Myc by 1,25D treatment causes an Rb-independent G1 arrest.
Fig. 2.
c-Myc is required for cell growth in Rb-depleted cells. A and B, Cells were transiently transfected with 160 pM total of the indicated combinations of either 60 pM Rb siRNA, 100 pM c-Myc siRNA, or the additional amount of control siRNA. A, 72 hours after transfection, Rb, c-Myc, and actin levels were measured by Western analysis. c-Myc is shown as the lower mobility species (arrow), whereas the higher mobility species is non-specific. B, Proliferation was measured by [3H]-Thymidine incorporation in parallel samples from (A) (*, P < 2 × 10−4 significant effect of c-Myc siRNA with or without Rb siRNA versus control siRNA). C, Cells were transiently transfected with 60 pM control or Rb siRNA. 24 hours after transfection, cells were treated with EtOH or 100 nM 1,25D. 72 hours after transfection, Rb, c-Myc, and actin protein levels were determined by Western analysis.
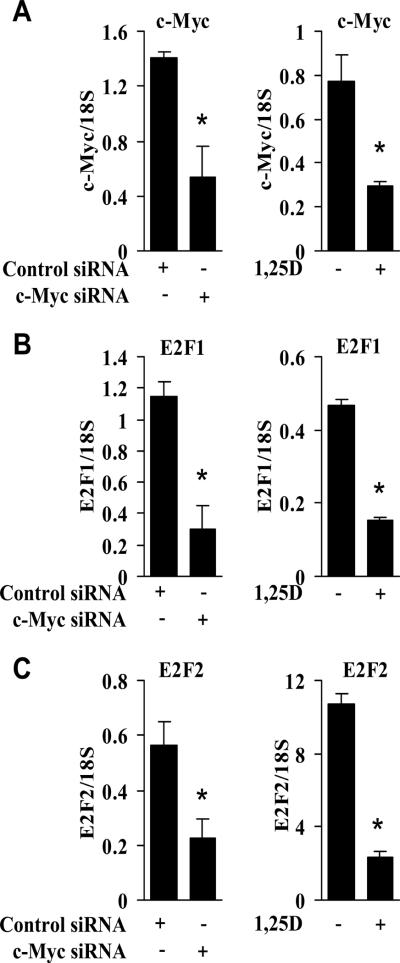
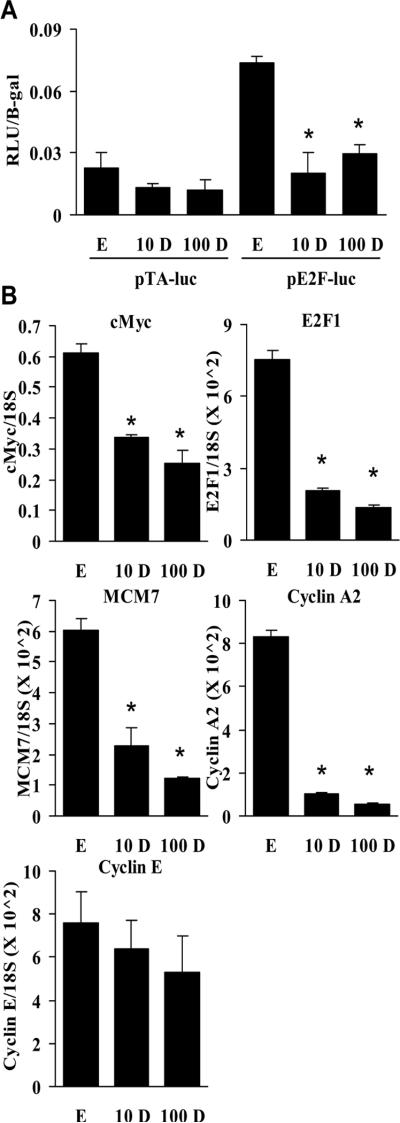
Both 1,25D treatment and c-Myc siRNA reduce E2F activity
In addition to influencing E2F activity through regulation of genes that contribute to inactivation of Rb [14], c-Myc also directly regulates transcription of E2F [11]. We have shown previously that E2F1 expression is reduced as a consequence of reducing c-Myc with siRNA [10], suggesting that 1,25D-mediated down-regulation of c-Myc may influence E2F levels [10]. E2F1 and c-Myc cooperate to induce other E2F genes [11], such as E2F2. Therefore, the levels of E2F2 reflect the activity of E2F1. The reduction in c-Myc by c-Myc siRNA or 1,25D treatment (A) was paralleled by decreased expression of both E2F1 (Fig. 3B) and E2F2 (Fig. 3C). To directly test the effects of 1,25D treatment on E2F activity we used an E2F-responsive luciferase reporter assay. As expected, 1,25D at either 10 or 100 nM reduced E2F activity (Fig. 4A). To further examine the effects of 1,25D treatment on E2F activity, we measured a broader range of E2F target genes including MCM7 [32], cyclin A2 [33], and cyclin E [34]. Both 10 nM and 100 nM 1,25D decreased expression of c-Myc and E2F1. It also decreased expression of MCM7 and cyclin A2, but not cyclin E (Fig. 4B). Thus, 1,25D likely causes a G1 arrest in the absence of Rb through its independent ability to decrease E2F expression and activity.
Fig. 3.
1,25D treatment or depletion of c-Myc reduces E2F levels in C4-2 cells. A, Cells were either transiently transfected with 100 pM of control or c-Myc siRNA, or treated with EtOH or 100 nM 1,25D. 72 hours after transfection or treatment, c-Myc levels were measured by qPCR (*, P < 0.02 significant effect of c-Myc siRNA or 1,25D versus control siRNA or EtOH). B, In parallel samples from (A), E2F1 levels were measured by qPCR (*, P < 0.01 significant effect of c-Myc siRNA or 1,25D versus control siRNA or EtOH). C, In parallel samples from (A), E2F2 levels were measured by qPCR (*, P < 0.035 significant effect of c-Myc siRNA or 1,25D versus control siRNA or EtOH).
Fig. 4.
1,25D treatment reduces E2F activity and expression of c-Myc, E2F1, and E2F target genes in C4-2 cells. A, Cells were transiently transfected with 60 pM control or Rb siRNA. Immediately after transfection, cells were treated with EtOH or 100 nM 1,25D. 48 hours after treatment, cells were transfected with 1.5 ug of pTA-luc (parental control) or pE2F-luc and 100 ng of pCR3.1 β-galactosidase. After transfection, cells were treated again with EtOH or 100 nM 1,25D for 24 hours (*, P < 0.01 significant effect of 1,25D treatment versus EtOH). B, Cells were treated with EtOH, 10 nM 1,25D (10 D), or 100 nM 1,25D (100 D) for 48 hours. c-Myc, E2F1, MCM7, cyclin A2, and cyclin E levels were measured by qPCR (*, P < 0.001 significant effect of 1,25D treatment versus EtOH).
1,25D-mediated c-Myc reduction precedes E2F down-regulation and growth inhibition
The studies above suggest that 1,25D causes down-regulation of c-myc resulting in reduced E2F and reduced proliferation. Thus, we asked whether we could detect a reduction in c-Myc prior to a decrease in E2F levels and growth inhibition with a timecourse of 1,25 treatment. The half lives of both c-Myc mRNA and protein are relatively short, so relatively rapid reductions in c-Myc can be measured. A reduction in c-Myc protein was consistently detected within 10 hours of treatment (Fig. 5A), but was not obvious at 4 hours (data not shown). In contrast, E2F expression (Fig. 5B) and proliferation (Fig. 5C) were unaffected at 10 hours, while both exhibit some reduction by 18 hours with greater reductions at later time points. This is indicates that 1,25D causing down-regulation of c-Myc leading to a reduction in E2F and proliferation rather than c-Myc down-regulation being secondary to other downstream changes that occur as a consequence of cell cycle arrest.
1,25D-mediated down-regulation of Bcl-2 is independent of Rb
One of the previously reported actions of 1,25D is down-regulation of Bcl-2 [4] (Fig. 6A). As mentioned earlier, Rb limits Bcl-2 expression, due to its interaction with and inhibition of E2F, a transcriptional activator of Bcl-2 [31]. Since 1,25D reduces E2F independently of Rb, and Bcl-2 is an E2F target gene, we asked whether 1,25D also decreases Bcl-2 expression independently of Rb. To test this, we compared Bcl-2 expression in control cells and cells depleted of Rb in the absence and presence of 1,25D. 1,25D diminished basal levels of Bcl-2 and also greatly reduced the increased expression of Bcl-2 caused by elimination of Rb (Fig. 6B). This indicates that the down-regulation of E2F by 1,25D is sufficient to reduce Bcl-2 expression although other E2F-independent effects of 1,25D on Bcl-2 expression cannot be excluded.
Fig. 6.
Rb is not required for 1,25D-mediated Bcl-2 down-regulation, and Bcl-2 is required for optimal growth of C4-2 cells. A, Cells were treated for 72 hours with EtOH or 100 nM 1,25D. Bcl-2 and actin levels were measured by Western analysis. Signal intensity was measured with a densitometer. Densitometric values represent Bcl-2/Actin normalized to EtOH. B, Cells were transiently transfected with 60 pM control or Rb siRNA. 24 hours after transfection, cells were treated with EtOH or 100 nM 1,25D. 72 hours after transfection, Bcl-2 RNA levels were measured by qPCR. (*, P < 0.05 significant effect of 1,25D treatment versus EtOH within each siRNA transfection group). C, Cells were transiently transfected with 100 pM control or one of two independent Bcl-2 siRNAs (Bcl-2 A and Bcl-2 B). 72 hours after transfection, cell proliferation was measured with [3H]-Thymidine incorporation, and Bcl-2 and actin levels were measured by Western analysis in parallel samples (*, P < 0.01 significant effect of Bcl-2 siRNA versus control siRNA). The experiment was performed in triplicate two times with each independent Bcl-2 siRNA.
Bcl-2 expression is required for optimal growth of C4-2 Cells
We have shown previously that artificial over-expression of Bcl-2 in LNCaP cells reduces the growth inhibitory effects of 1,25D [4]; however the consequences of 1,25D-mediated down-regulation of Bcl-2 have not been determined. To examine whether Bcl-2 plays a role in cell growth/survival in the absence of an exogenous inducer of apoptosis, Bcl-2 levels were reduced in C4-2 cells using two independent siRNAs, and Bcl-2 expression and cell proliferation were measured. Both Bcl-2 siRNAs significantly reduced cell proliferation (Fig. 6C). Although the down-regulation of Bcl-2 caused by siRNA is greater than that induced by 1,25D, it is clear that Bcl-2 can play a role in cell proliferation/survival under these conditions, but its down-regulation may not be required for the overall response of cells to 1,25D treatment.
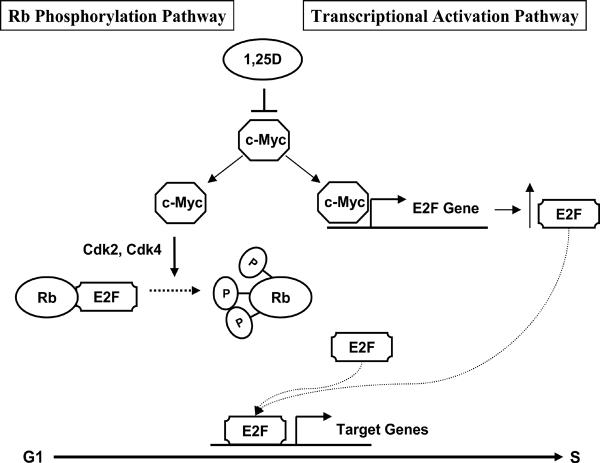
Model: 1,25D inhibits cell cycle progression primarily through Rb-independent pathways in C4-2 cells
Our studies and others have shown that c-Myc plays a dual role in increasing overall E2F activity, through inactivation of Rb, thereby releasing E2F, and through direct up-regulation of E2F (Fig. 7). While 1,25D-mediated down-regulation of c-Myc can influence both pathways, the Rb pathway is not required for reducing overall E2F activity and causing a G1 arrest in C4-2 cells.
Fig. 7.
Model of 1,25D target gene regulation through c-Myc. Functional E2F is required for progression into S phase. Active Rb (hypophosphorylated) binds E2F preventing its actions as a transcription factor. c-Myc induces activation of cdk2 and cdk4, inhibition of Rb activity by phosphorylation, and release and activation of E2F. In addition, c-Myc directly induces transcription of E2F. 1,25D reduces c-Myc expression leading to a net decrease in E2F expression and activity preventing progression from G1 to S phase.
DISCUSSION
While it is well established that 1,25D reduces the growth of prostate cancer cells, frequently by inducing a cell cycle arrest in G1, the mechanisms by which this occurs are not fully understood. Several cell cycle regulators have been suggested as targets of 1,25D action to cause cell cycle arrest. Rb is of particular interest because loss of Rb occurs frequently in prostate cancer and, in previous studies, cells that lacked functional Rb were less responsive to 1,25D treatment compared to cell lines that do express active Rb [20, 21]. However, artificial re-expression of Rb does not increase the weak growth inhibitory response of DU145 cells to 1,25D [20]. Because other factors might be limiting response to 1,25D in DU145 cells, we wanted to determine whether Rb is required for the growth inhibitory effects of 1,25D in cells that are responsive to 1,25D. Using the C4-2 cell line, we found that reducing Rb with siRNA does not prevent growth inhibition caused by 1,25D treatment. Reducing Rb alone had no effect on cell proliferation suggesting that Rb does not have intrinsic growth inhibitory properties under these conditions. One possible explanation for the lack of response to Rb knockdown is that there is functional compensation by redundant Rb family members, p107 and p130. However, we only detected a minor, but inconsistent increase in p107 and no change in p130 when Rb was reduced with siRNA (data not shown). The net increase in E2F activity (Fig. 1B) suggests that, at most, there is a minor compensation for Rb loss by other Rb family members. We also noted that DU-145 cells express much higher levels of p130 than C4-2 cells (not shown), so the basal levels of these proteins do not appear to be unusually high in the C4-2 cells. We also found that 1,25D reduces Rb protein levels in the absence of siRNA transfection, contributing to our conclusion that Rb is not required for the effects of 1,25D in this cell line. Overall Rb expression also is reduced by 1,25D treatment of LNCaP and ALVA31 prostate cancer cells [6], as well as HL-60 myeloid leukemia cells [35], so this response is not unique to C4-2 cells.
It has been reported that 1,25D also can induce a G2/M arrest in some cell lines, via induction of GADD45 [36], a gene that plays a role in cell cycle and DNA repair [37, 38]. Induction of GADD45 is growth inhibitory in prostate cancer cells [39]. In addition, depletion of the 1,25D induced gene Irx5 causes a G2/M arrest in LNCaP cells [40]. Thus, it was possible that alternative actions of 1,25D caused growth inhibition in the absence of Rb. However, our results show that 1,25D treatment causes a G0/G1 accumulation despite the loss of Rb.
Since c-Myc is an indirect upstream regulator of Rb function, via activation of the cyclin dependent kinases that phosphorylate Rb, we asked whether Rb is required for the growth inhibition caused by c-Myc depletion. Our studies show that c-Myc is required for proliferation regardless of the Rb status of the cells. c-Myc directly stimulates E2F expression and activity; it induces E2F1 expression, and the combination of c-Myc and E2F1 up-regulates E2F2 [11]. Our finding that treating cells with 1,25D or depleting c-Myc with siRNA substantially reduces expression of both E2F1 and E2F2, suggests that the Rb-independent cell cycle arrest induced by 1,25D is due to the reduction in E2F. We found that 1,25D treatment also reduced E2F activity, as well as the expression of the E2F target genes cyclin A2 and MCM7, but not cyclin E. However, the extent of dependence of cyclin E expression on E2F in these cells has not been determined experimentally. The time course shown in Fig. 5 demonstrates that 1,25D reduces c-Myc expression prior to changes in proliferation and is consistent with our model suggesting that the reduction in c-Myc, which reduces E2F expression, causes a reduction in proliferation. Interestingly, the failure of SV-40 transformed prostate cell lines to be growth inhibited by 1,25D might be due to one of the lesser known activities of SV-40 large T antigen. SV-40 large T antigen binds to ubiquitin ligase Fbw7 [41], an enzyme that promotes the turnover of its substrates, one of which is c-Myc [42]. In C4-2 cells, 1,25D reduces c-Myc, in part, through increasing its turnover rate [10]. In SV-40 transformed cells, inhibition of Fbw7 may reduce proteasome-mediated degradation of c-Myc, limiting the growth inhibitory effects of 1,25D.
The Cdk2 inhibitors, p21 and p27 also have been implicated in 1,25D-mediated growth inhibition [18, 19, 43]. In a subline of ALVA-31 prostate cancer cells, p21 is required for 1,25D-mediated growth inhibition [19] and p27 −/− MEFs are resistant to 1,25D-mediated growth inhibition [43]. c-Myc represses expression of both p21 and p27 [14, 17]. 1,25D decreases c-Myc expression by a variety of mechanisms [10, 44–47]. In C4-2 cells, 1,25D reduces c-Myc expression to about 10% of basal levels. In lines where down-regulation of c-Myc is less substantial, Rb and the pathway preventing inactivation of Rb may be needed for 1,25D-mediated growth inhibition. The reduction in c-Myc is sufficient to inhibit growth of C4-2 cells, but this does not exclude redundant growth inhibitory pathways.
We have shown previously that 1,25D reduces levels of the anti-apoptotic protein Bcl-2 in prostate cancer cells [4]. Bcl-2 has E2F binding sites in its promoter, and reducing Rb increases Bcl-2 expression [31]. Because 1,25D reduces c-Myc and presumably E2F independently of Rb, we asked whether 1,25D-mediated Bcl-2 down-regulation is also Rb-independent. We found that in Rb siRNA transfected cells, 1,25D treatment does not reduce Bcl-2 to the levels it does in control siRNA transfected cells, but there is still a very significant reduction of Bcl-2. Therefore, Rb may play some role in the down-regulation of Bcl-2, but its presence is not required for 1,25D-mediated regulation.
Since Bcl-2 can serve as a marker for E2F activity, we wanted to determine if Bcl-2, itself, plays a role in proliferation. Previous results in our lab showed that when Bcl-2 is over-expressed in the LNCaP cell line, it reduces the sensitivity of the cells to 1,25D treatment. Cell growth was not reduced to the extent it was in control cells and the cells gained the ability to re-grow when hormone was removed from the cell culture medium [4] In the current study, we show that reducing Bcl-2 with siRNA reduces [3H]-thymidine incorporation, which is a measure of the number of cells going through S phase. Thus, the down-regulation of Bcl-2 by 1,25D may contribute to the overall growth inhibition; some of the effect likely is due to the induction of apoptosis in Bcl-2-depleted cells. Lin, et.al. reported that Bcl-2 is up-regulated during and required for the progression of prostate cancer cells to androgen-independence [48]. Reducing Bcl-2 in androgen-independent but not androgen-dependent prostate cancer cells, sensitized them to UV-induced apoptosis, making Bcl-2 a potential therapeutic target in androgen-independent prostate cancer [48]. It is therefore possible that the down-regulation of Bcl-2 by 1,25D in our androgen-independent C4-2 cells increases the apoptotic effects of 1,25D, and that the strong growth inhibition we see with even greater Bcl-2 knockdown by siRNA may also be due to an increase in apoptosis. Further experiments are needed to verify this hypothesis.
In summary, we show that Rb is not required for the growth inhibitory effects of 1,25D in C4-2 cells and that reducing c-Myc leading to reduced E2F is sufficient to cause a G1 arrest in the absence of Rb. Therefore, tumors with a loss of heterozygosity of Rb or Rb inactivation should still be responsive to vitamin D receptor agonists.
ACKNOWLEDGEMENTS
We thank William E. Bingman III for technical expertise and the Molecular and Cellular Biology Tissue Culture Core for maintaining and plating the cell lines. Flow cytometry analysis was supported by the BCM Cytometry and Cell Sorting Core with funding from the NIH (NCI P30CA125123).
This work was supported by the National Institutes of Health Grant 5R01CA107691, the NIH Initiative for Minority Student Development R25GM56929, and the Training Program in Molecular Endocrinology DK07696.
Grant Information National Institutes of Health Grant 5R01CA107691
NIH Initiative for Minority Student Development R25GM56929
Training Program in Molecular Endocrinology DK07696
NIH NCI P30CA125123
Footnotes
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.Huynh H, Pollak M, Zhang JC. Regulation of insulin-like growth factor (IGF) II and IGF binding protein 3 autocrine loop in human PC-3 prostate cancer cells by vitamin D metabolite 1,25(OH)2D3 and its analog EB1089. Int J Oncol. 1998;13(1):137–43. doi: 10.3892/ijo.13.1.137. [DOI] [PubMed] [Google Scholar]
- 2.Boyle BJ, et al. Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol. 2001;165(4):1319–24. [PubMed] [Google Scholar]
- 3.Tovar Sepulveda VA, Falzon M. Regulation of PTH-related protein gene expression by vitamin D in PC-3 prostate cancer cells. Mol Cell Endocrinol. 2002;190(1–2):115–24. doi: 10.1016/s0303-7207(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 4.Blutt SE, et al. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141(1):10–7. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 5.Blutt SE, et al. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology. 1997;138(4):1491–7. doi: 10.1210/endo.138.4.5063. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang SH, Burnstein KL. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology. 1998;139(3):1197–207. doi: 10.1210/endo.139.3.5770. [DOI] [PubMed] [Google Scholar]
- 7.Sala A, et al. Correlation between E2F-1 requirement in the S phase and E2F-1 transactivation of cell cycle-related genes in human cells. Cancer Res. 1994;54(6):1402–6. [PubMed] [Google Scholar]
- 8.Chellappan SP, et al. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65(6):1053–61. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub SJ, Prater CA, Dean DC. Retinoblastoma protein switches the E2F site from positive to negative element. Nature. 1992;358(6383):259–61. doi: 10.1038/358259a0. [DOI] [PubMed] [Google Scholar]
- 10.Rohan JN, Weigel NL. 1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4-2 prostate cancer cells. Endocrinology. 2009;150(5):2046–54. doi: 10.1210/en.2008-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung JY, et al. A role for Myc in facilitating transcription activation by E2F1. Oncogene. 2008;27(30):4172–9. doi: 10.1038/onc.2008.55. [DOI] [PubMed] [Google Scholar]
- 12.Kato J, et al. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7(3):331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18(2):753–61. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mateyak MK, Obaya AJ, Sedivy JM. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19(7):4672–83. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen-Durr P, et al. Differential modulation of cyclin gene expression by MYC. Proc Natl Acad Sci U S A. 1993;90(8):3685–9. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382(6591):511–7. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 17.Claassen GF, Hann SR. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta -induced cell-cycle arrest. Proc Natl Acad Sci U S A. 2000;97(17):9498–503. doi: 10.1073/pnas.150006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao A, et al. Vitamin D receptor and p21/WAF1 are targets of genistein and 1,25-dihydroxyvitamin D3 in human prostate cancer cells. Cancer Res. 2004;64(6):2143–7. doi: 10.1158/0008-5472.can-03-3480. [DOI] [PubMed] [Google Scholar]
- 19.Moffatt KA, et al. Growth inhibitory effects of 1alpha, 25-dihydroxyvitamin D(3) are mediated by increased levels of p21 in the prostatic carcinoma cell line ALVA-31. Cancer Res. 2001;61(19):7122–9. [PubMed] [Google Scholar]
- 20.Gross C, S.R., Plymate SR, Rhim JS, Peehl DM, Feldman D. Simian virus 40-, but not human papillomavirus0, transformation of prostatic epithelial cells results in loss of growth-inhibition by 1,25-dihydroxyvitamin D3. International Journal of Oncology. 1996;(8):41–47. doi: 10.3892/ijo.8.1.41. [DOI] [PubMed] [Google Scholar]
- 21.Skowronski RJ, Peehl DM, Feldman D. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology. 1993;132(5):1952–60. doi: 10.1210/endo.132.5.7682937. [DOI] [PubMed] [Google Scholar]
- 22.Polek TC, et al. p53 Is required for 1,25-dihydroxyvitamin D3-induced G0 arrest but is not required for G1 accumulation or apoptosis of LNCaP prostate cancer cells. Endocrinology. 2003;144(1):50–60. doi: 10.1210/en.2001-210109. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SM, et al. Loss of the retinoblastoma susceptibility gene (RB1) is a frequent and early event in prostatic tumorigenesis. Br J Cancer. 1994;70(6):1252–7. doi: 10.1038/bjc.1994.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ittmann MM, Wieczorek R. Alterations of the retinoblastoma gene in clinically localized, stage B prostate adenocarcinomas. Hum Pathol. 1996;27(1):28–34. doi: 10.1016/s0046-8177(96)90134-3. [DOI] [PubMed] [Google Scholar]
- 25.Tricoli JV, et al. Alterations of the retinoblastoma gene in human prostate adenocarcinoma. Genes Chromosomes Cancer. 1996;15(2):108–14. doi: 10.1002/(SICI)1098-2264(199602)15:2<108::AID-GCC5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Brooks JD, Bova GS, Isaacs WB. Allelic loss of the retinoblastoma gene in primary human prostatic adenocarcinomas. Prostate. 1995;26(1):35–9. doi: 10.1002/pros.2990260108. [DOI] [PubMed] [Google Scholar]
- 27.Agoulnik IU, et al. Repressors of androgen and progesterone receptor action. J Biol Chem. 2003;278(33):31136–48. doi: 10.1074/jbc.M305153200. [DOI] [PubMed] [Google Scholar]
- 28.Bai W, Weigel NL. Phosphorylation of Ser211 in the chicken progesterone receptor modulates its transcriptional activity. J Biol Chem. 1996;271(22):12801–6. doi: 10.1074/jbc.271.22.12801. [DOI] [PubMed] [Google Scholar]
- 29.Stewart LV, Weigel NL. Role of insulin-like growth factor binding proteins in 1alpha,25-dihydroxyvitamin D(3)-induced growth inhibition of human prostate cancer cells. Prostate. 2005;64(1):9–19. doi: 10.1002/pros.20212. [DOI] [PubMed] [Google Scholar]
- 30.Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005;25(8):2885–98. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, et al. Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene. 2004;23(12):2161–76. doi: 10.1038/sj.onc.1207326. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, et al. Cloning and characterization of human MCM7 promoter. Gene. 1998;216(1):85–91. doi: 10.1016/s0378-1119(98)00323-0. [DOI] [PubMed] [Google Scholar]
- 33.Schulze A, et al. Cell cycle regulation of the cyclin A gene promoter is mediated by a variant E2F site. Proc Natl Acad Sci U S A. 1995;92(24):11264–8. doi: 10.1073/pnas.92.24.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtani K, DeGregori J, Nevins JR. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995;92(26):12146–50. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen A, et al. Coupled down-regulation of the RB retinoblastoma and c-myc genes antecedes cell differentiation: possible role of RB as a “status quo” gene. Eur J Cell Biol. 1992;57(2):210–21. [PubMed] [Google Scholar]
- 36.Jiang F, et al. G2/M arrest by 1,25-dihydroxyvitamin D3 in ovarian cancer cells mediated through the induction of GADD45 via an exonic enhancer. J Biol Chem. 2003;278(48):48030–40. doi: 10.1074/jbc.M308430200. [DOI] [PubMed] [Google Scholar]
- 37.Smith ML, et al. Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to u.v.-irradiation or cisplatin. Oncogene. 1996;13(10):2255–63. [PubMed] [Google Scholar]
- 38.Wang XW, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A. 1999;96(7):3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang F, Wang Z. Gadd45gamma is androgen-responsive and growth-inhibitory in prostate cancer cells. Mol Cell Endocrinol. 2004;213(2):121–9. doi: 10.1016/j.mce.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 40.Myrthue A, et al. The iroquois homeobox gene 5 is regulated by 1,25-dihydroxyvitamin D3 in human prostate cancer and regulates apoptosis and the cell cycle in LNCaP prostate cancer cells. Clin Cancer Res. 2008;14(11):3562–70. doi: 10.1158/1078-0432.CCR-07-4649. [DOI] [PubMed] [Google Scholar]
- 41.Welcker M, Clurman BE. The SV40 large T antigen contains a decoy phosphodegron that mediates its interactions with Fbw7/hCdc4. J Biol Chem. 2005;280(9):7654–8. doi: 10.1074/jbc.M413377200. [DOI] [PubMed] [Google Scholar]
- 42.Yada M, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23(10):2116–25. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wade WN, et al. p27Kip1 is essential for the antiproliferative action of 1,25-dihydroxyvitamin D3 in primary, but not immortalized, mouse embryonic fibroblasts. J Biol Chem. 2002;277(40):37301–6. doi: 10.1074/jbc.M204162200. [DOI] [PubMed] [Google Scholar]
- 44.Pan Q, et al. 1,25-Dihydroxyvitamin D3-regulated binding of nuclear proteins to a c-myc intron element. Endocrinology. 1996;137(10):4154–60. doi: 10.1210/endo.137.10.8828471. [DOI] [PubMed] [Google Scholar]
- 45.Pan Q, Simpson RU. c-myc intron element-binding proteins are required for 1, 25-dihydroxyvitamin D3 regulation of c-myc during HL-60 cell differentiation and the involvement of HOXB4. J Biol Chem. 1999;274(13):8437–44. doi: 10.1074/jbc.274.13.8437. [DOI] [PubMed] [Google Scholar]
- 46.Mangasarian K, Mellon WS. 1,25-Dihydroxyvitamin D-3 destabilizes c-myc mRNA in HL-60 leukemic cells. Biochim Biophys Acta. 1993;1172(1–2):55–63. doi: 10.1016/0167-4781(93)90269-j. [DOI] [PubMed] [Google Scholar]
- 47.Simpson RU, et al. Transcriptional regulation of the c-myc protooncogene by 1,25-dihydroxyvitamin D3 in HL-60 promyelocytic leukemia cells. J Biol Chem. 1987;262(9):4104–8. [PubMed] [Google Scholar]
- 48.Lin Y, et al. Up-regulation of Bcl-2 is required for the progression of prostate cancer cells from an androgen-dependent to an androgen-independent growth stage. Cell Res. 2007;17(6):531–6. doi: 10.1038/cr.2007.12. [DOI] [PubMed] [Google Scholar]