Summary
Dendritic cells (DCs) comprise distinct functional subsets including CD8− and CD8+ classical DCs (cDCs) and interferon-secreting plasmacytoid DCs (pDCs). The cytokine Flt3 ligand (Flt3L) controls the development of DCs, and is particularly important for the pDC, CD8+ cDC and their CD103+ tissue counterparts. We report that mammalian target of rapamycin (mTOR) inhibitor rapamycin impaired Flt3L-driven DC development in vitro, with the pDCs and CD8+-like cDCs most profoundly affected. Conversely, deletion of the phosphoinositide 3-kinase (PI3K)-mTOR negative regulator Pten facilitated Flt3L-driven DC development in culture. DC-specific Pten targeting in vivo caused the expansion of CD8+ and CD103+ cDC numbers, which was reversible by rapamycin. The increased CD8+ cDC numbers caused by Pten deletion correlated with increased susceptibility to the intracellular pathogen Listeria. Thus, PI3K-mTOR signaling downstream of Flt3L controls DC development, and its restriction by Pten ensures optimal DC pool size and subset composition.
Introduction
Dendritic cells (DCs) provide the key link between innate and adaptive immunity by efficiently recognizing pathogens through pattern recognition receptors such as Toll-like receptors, and priming pathogen-specific immune responses. The DC compartment comprises several distinct subsets, with their total and relative numbers maintained constant throughout adult life (Merad and Manz, 2009; Pulendran et al., 2008). In the lymphoid organs of mice, the CD8− classical, or conventional DCs (cDCs) efficiently present major histocompatibility complex (MHC) class II (MHC II)-restricted exogenous antigens to CD4+ T cells. In contrast, the CD8+ cDCs can prime cytotoxic CD8+ T cells due to their ability to process dead or dying cells and cross-present antigens on MHC class I molecules (den Haan et al., 2000). Consistent with these properties, CD8+ DCs are critical for the capture, transport and presentation of intracellular pathogens such as Listeria monocytogenes (LM) (Neuenhahn et al., 2006). The same dichotomy was documented in tissues, in which the CD103+ cDC subset serves as a functional and genetic counterpart of CD8+ cDCs (Bedoui et al., 2009b; Bogunovic et al., 2009; Ginhoux et al., 2009; Varol et al., 2009). In addition to cDCs, plasmacytoid dendritic cells (pDCs) efficiently recognize viral nucleic acids and secrete large amounts of type I interferon (IFN) and other cytokines.
Both cDCs and pDCs develop through a distinct cellular pathway involving a common DC progenitor (CDP, or pro-DC) in the bone marrow (BM) (Naik et al., 2007; Onai et al., 2007). The CDP gives rise to pDCs directly in the BM, and produces a common cDC precursor (pre-DC) that differentiates into cDCs in the lymphoid organs through subset-specific intermediates (Bedoui et al., 2009a; Liu et al., 2009; Naik et al., 2006). This common pathway of DC development critically depends on cytokine Flt3 ligand (Flt3L) which signals through its receptor Flt3 expressed on CDP, pre-DC and their progeny. Both cDC and pDC numbers are reduced in the lymphoid organs of Flt3L- or Flt3-deficient animals (McKenna et al., 2000; Tussiwand et al., 2005; Waskow et al., 2008), suggesting a key role of Flt3 in DC development from CDP. In the tissues, the CD8+-like CD103+ cDCs preferentially require Flt3 for their development (Bogunovic et al., 2009; Ginhoux et al., 2009; Varol et al., 2009). Conversely, administration of Flt3L causes a substantial expansion in numbers of the DC compartment (Maraskovsky et al., 1996), particularly of the CD8+ cDCs (Bedoui et al., 2009a; O’Keeffe et al., 2002; Vollstedt et al., 2004) and the corresponding CD103+ tissue DCs (Varol et al., 2009). Thus, Flt3 provides an essential signal for the development and homeostasis of DCs, particularly of the CD8+ and CD103+ cDCs. However, the molecular basis of Flt3 signaling in the DC lineage is poorly understood, and the signal transduction pathways downstream of Flt3 remain to be elucidated.
The phosphoinositide 3-kinase (PI3K) pathway is activated by multiple growth factors and cytokines to control metabolism and promote survival, proliferation and/or differentiation (Engelman et al., 2006). Receptor-mediated activation of PI3K family kinases generates inositol phospholipids that activate the protein kinase Akt, which phosphorylates multiple substrates including Foxo transcription factors, glycogen synthase kinase (GSK3β), and the mammalian target of rapamycin (mTOR). mTOR is a serine and threonine kinase that serves as a nutrient and energy sensor regulating protein metabolism. The PI3K-Akt-mediated activation of mTOR leads to the phosphorylation of ribosomal protein S6, a key regulator of ribosome biogenesis, protein translation and cell size. The activity of PI3K-Akt pathway is tightly controlled by multiple negative regulators and feedback loops. A key cell-intrinsic inhibitor of Akt signaling is phosphatase and tensin homolog (Pten), a lipid phosphatase that prevents Akt activation by dephosphorylating PI3K-generated inositol phospholipids. The deletion of Pten leads to the constitutive activation of PI3K-Akt signaling, and is a common event in malignant transformation. For instance, constitutive or inducible Pten deletion in the BM causes major hematopoietic abnormalities, myeloid leukemia and/or T cell lymphoma (Yilmaz et al., 2006; Zhang et al., 2006).
PI3K-mTOR has emerged as an important signaling pathway regulating both innate and adaptive immunity (Thomson et al., 2009; Weichhart and Saemann, 2009). In the DC lineage, the role of mTOR signaling has been studied largely in the context of Toll-like receptor (TLR)-induced cytokine secretion. In myeloid cells and cDCs, PI3K-mTOR was shown to promote maturation (Hackstein et al., 2003) and facilitate anti-inflammatory responses such as secretion of interleukin-10 (IL-10) (Ohtani et al., 2008; Weichhart et al., 2008), whereas in pDCs it is required for TLR-induced type I interferon production (Cao et al., 2008; Guiducci et al., 2008). In this work, we explored the role of PI3K-mTOR signaling in DC development and homeostasis. We now report that mTOR signaling is induced by Flt3L and is required in vitro for Flt3L-driven development of DCs, particularly of pDCs and CD8+ cDC equivalents. Conversely, the activation of PI3K-mTOR signaling by deletion of Pten accelerated Flt3L-driven DC development in vitro and caused the expansion of CD8+ and CD103+ cDC numbers in vivo. Our results suggest that PI3K-mTOR pathway is essential for Flt3-mediated DC development, whereas DC-intrinsic expression of Pten restricts PI3K-mTOR signaling to maintain optimal DC subset composition.
Results
Rapamycin blocks Flt3L-induced DC development in culture
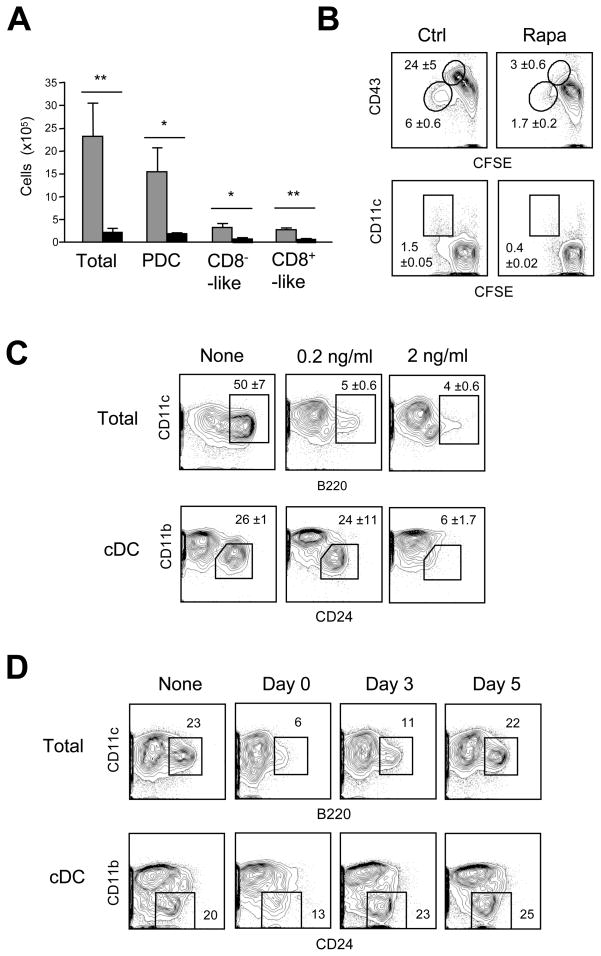
DC development can be faithfully recapitulated in Flt3L-supplemented BM cultures, which give rise to pDCs and the two cDC subsets (Naik et al., 2005). The addition of mTOR inhibitor rapamycin to these cultures severely reduced the numbers of all DC subsets at the endpoint (Fig. 1A). The analysis of CFSE-labeled BM cultures on day 3 (Fig. 1B) showed that rapamycin blocked the proliferation of DC and pDC progenitors including CD43hi pro-DCs and CD43+ CD11c+ pre-DCs (Naik et al., 2007). In contrast, rapamycin did not prevent DC development in GM-CSF-supplemented BM cultures (Fig. S1), although it affected the maturation status of the resulting CD11b+ DCs as described previously (Hackstein et al., 2003).
Figure 1. Rapamycin inhibits Flt3L-driven DC development in vitro.
(A) The effect of rapamycin on DC development in vitro. Wild-type BM was cultured in the presence of Flt3L with or without 10 ng/ml rapamycin, and analyzed by flow cytometry on day 8 for the resulting pDCs (CD11c+ B220+ CD11b−), CD8−-like cDCs (CD11c+ B220− CD11bhi) and CD8+-like cDCs (CD11c+ B220− CD11blo). Shown are absolute cell numbers per 2x106 input BM cells (mean ± S.D. of three independent cultures); *P<0.05; **P<0.01.
(B) The effect of rapamycin on DC progenitor expansion. CFSE-labeled BM cells were cultured with Flt3L with or without rapamycin, and analyzed on day 3. Highlighted are CFSE-diluting pro-DCs with high or low CD43 expression (upper panels) and CFSElo CD11c+ pre-DCs (lower panels), with average percentages ± range of two independent cultures.
(C) The effect of rapamycin at low doses. BM cells were cultured with Flt3L and the indicated doses of rapamycin. Shown are staining profiles on day 8, highlighting CD11c+ B220+ pDCs (top) and CD24+ CD11blo CD8+-like cDCs among the gated CD11c+ MHC II+ cDCs (bottom). Average percentages ±S.D. of three independent cultures are indicated.
(D) Time-dependent rapamycin activity in Flt3L-supplemented BM cultures. Rapamycin (10 ng/ml) was added at the indicated days, and the cultures were analyzed on day 9. Representative of 4 independent cultures.
The pDC population was particularly affected by rapamycin, even when the drug was added at lower concentration (Fig. 1C) or at a later time point (day 3, Fig. 1D). The proliferation of DC and pDC progenitors was not affected in the latter case, suggesting an additional rapamycin effect on pDC maturation (not shown). Among the cDC subsets, the CD11blo CD24+ equivalents of CD8+ cDCs were more affected by lower rapamycin concentrations than the CD11bhi equivalents of CD8− cDCs. Thus, mTOR activity is essential for Flt3L-dependent development of all DC in vitro, with pDCs and CD8+-like cDCs being most sensitive to mTOR inhibition.
Pten deletion in the bone marrow facilitates Flt3L-induced DC development
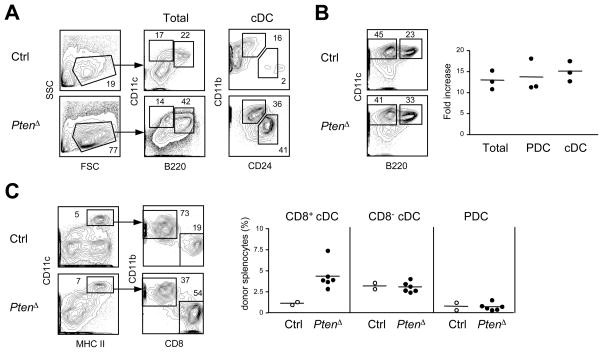
To activate PI3K-mTOR signaling during DC development, we deleted PI3K inhibitor Pten in the adult BM using tamoxifen-inducible Gt(ROSA)26Sor-CreER deleter strain. Tamoxifen was administered to Ptenfl/fl Gt(ROSA)26Sor-CreER+ mice to induce global Pten deletion (Pten&Δ), and 5 days later the BM was cultured with Flt3L. The PtenΔ BM cultures showed greatly accelerated DC development, yielding phenotypically mature pDCs and cDCs as early as day 5 (Fig. 2A). At the endpoint (days 8–9), the absolute numbers of resulting DCs were increased more than 10-fold in PtenΔ BM cultures (day 8, Fig. 2B). Importantly, Pten deletion partially restored DC development in the presence of rapamycin (Fig. S2), confirming the functional hyperactivation of mTOR signaling after Pten loss. No acceleration or increased DC yield were observed in GM-CSF-supplemented BM cultures (Fig. S2). Thus, Pten deletion in hematopoietic progenitors facilitates Flt3L-driven development of all DC subsets in culture.
Figure 2. Pten deletion in the BM facilitates DC development.
Global Pten deletion was induced by tamoxifen administration to Ptenfl/fl Gt(ROSA)26Sor-CreER+ animals (PtenΔ) or littermate controls (Ctrl) 5 days prior to BM isolation.
(A) DC development in Flt3L-supplemented BM cultures on day 5. Shown are staining profiles representative of two independent experiments, with total live cells (forward versus side scatter gate), CD11c+ B220+ pDCs, CD11c+ B220− cDCs and their subsets highlighted.
(B) DC development in Flt3L-supplemented BM cultures at the endpoint. Cultures described in panel (A) were analyzed on days 8–9. Shown are representative staining profiles of total live cells, and fold increase of the absolute DC numbers in PtenΔ over control cultures (three independent experiments).
(C) DC development in vivo in the BM chimeras. BM cells from each tamoxifen-treated animal (one control and three PtenΔ) were transferred into two irradiated recipients, and splenic DCs were analyzed 6 wk thereafter. Shown are representative staining profiles of donor-derived (CD45.2+) DCs, and the fractions of cDCs (CD11chi MHC II+ CD8+ or CD8−) and pDCs (CD11clo B220+ Bst2+) among the total donor-derived splenocytes.
To test the effect of Pten deletion on DC development in vivo, irradiated recipients were reconstituted with the BM from tamoxifen-induced mice. The chimeras reconstituted with PtenΔ BM showed the expected hematopoietic abnormalities such as myeloid shift and impaired B cell development (Zhang et al., 2006), confounding the analysis of absolute cell numbers. Notably, the proportion of CD8+ cDCs was increased >3-fold among PtenΔ donor-derived splenocytes, whereas the fractions of pDCs and CD8− cDCs were unchanged (Fig. 2C). A similarly increased fraction of CD8+ cDCs was observed in the spleens of donor PtenΔ mice 5 days after tamoxifen administration (data not shown). Thus, Pten deletion in the hematopoietic compartment in vivo favors the development of CD8+ cDCs, the subset preferentially expanded by Flt3L administration.
Flt3 signaling activates mTOR in DCs
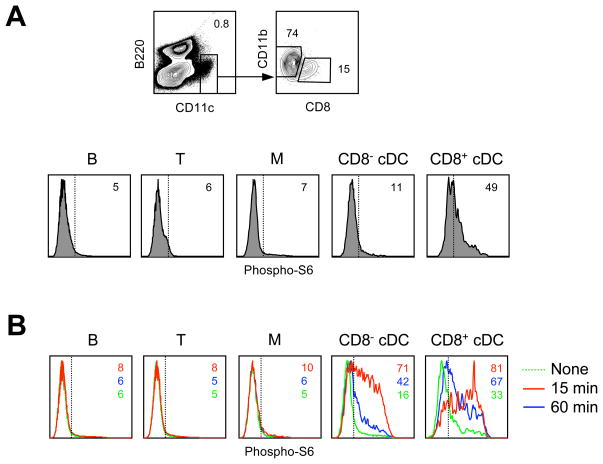
The observed correlation between mTOR signaling and Flt3L-driven DC development prompted us to analyze mTOR activity in DCs in vivo. As a readout, we measured phosphorylated S6 (p-S6) in DCs after instant fixation of freshly isolated splenocytes (Fig. 3A). In naïve animals, CD8+ cDCs showed higher basal amounts of pS6 (Fig. 3A). The injection of recombinant Flt3L induced a robust and transient pS6 signal in the CD11c+ DCs but not in other cell types, reflecting DC-specific Flt3 expression (Fig. 3B). In contrast, TLR ligand administration induced a prolonged pS6 signal in multiple cell types (data not shown). Notably, Flt3L-induced p-S6 signal was higher and more sustained in CD8+ compared to CD8− cDCs, consistent with the preferential expansion of this subset by Flt3L. These data show that Flt3L induces mTOR signaling in DCs in vivo, with the highest activity in CD8+ cDCs.
Figure 3. mTOR signaling in DCs in vivo.
(A) The expression of phosphorylated S6 protein (p-S6) in the splenocytes of naïve wild-type mice. Shown is the definition of cDC subsets in ex vivo splenocytes after instant fixation, and p-S6 expression in T cells (CD90+), B cells (B220+ CD11c−), monocytes or macrophages (M, CD11blo CD11c−) and cDCs. Positive staining threshold is indicated by the dotted line.
(B) The induction of p-S6 by Flt3L in vivo. Shown are histograms of intracellular p-S6 fluorescence in the indicated cell types 15 or 60 minutes after Flt3L administration.
The expansion of CD8+ cDC numbers after DC-specific Pten deletion
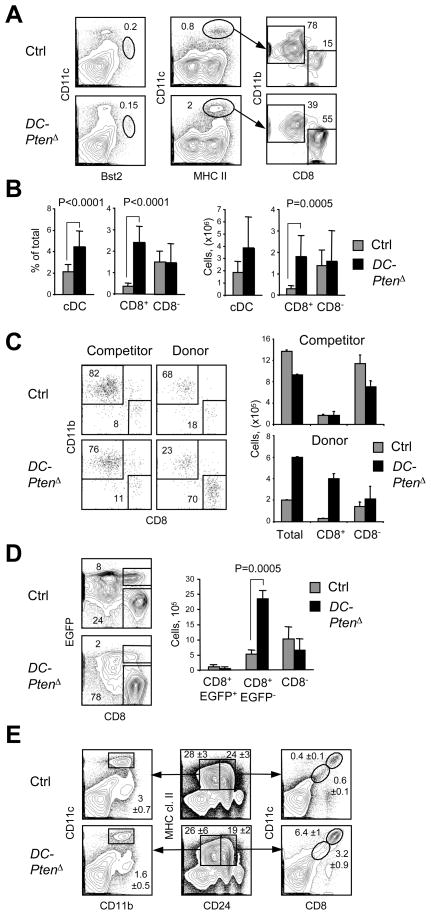
To activate PI3K-mTOR signaling specifically in the DC lineage, we targeted Pten with the DC-specific Itgax-Cre deleter strain (Fig. S3). The resulting Ptenfl/fl Itgax-Cre+ animals should lack Pten in the CD11c (Itgax)-expressing DC (DC-PtenΔ). Indeed, DC-PtenΔ mice carrying a fluorescent Cre reporter allele showed Cre recombination in nearly all cDCs but only in 4–12% of T and B lymphocytes. In older DC-PtenΔ animals, the “leaky” Pten loss in T lymphocytes eventually caused T cell lymphomas and death, therefore only young adult mice (4–10 wk) were used for analysis. The expression of Pten was nearly abolished in cDCs and markedly reduced in pDCs but normal in myeloid non-DCs, supporting a DC-specific Pten deletion. In vitro, DC-PtenΔ BM-derived cDCs showed the expected hyperactivation of the PI3K-Akt pathway, including hyperphosphorylated Akt and GSK3β (Fig. S3).
The analysis of splenic DC subsets in naïve DC-PtenΔ mice revealed a 5–6-fold increase in the fraction and absolute numbers of CD8+ cDCs, whereas CD8− cDC and pDC populations remained normal (Fig. 4A, B). To confirm the cell-intrinsic nature of CD8+ cDC expansion, we established competitive BM chimeras from control or DC-PtenΔ (CD45.2) donor mice and CD45.1 congenic competitor mice. Donor chimerism reached 30–40% in lymphocytes and 15–20% in myeloid cells and DCs (Fig. S3). Even at this relatively low chimerism, a prominent expansion of CD8+ cDCs was observed in DC-PtenΔ-derived splenocytes but not in control-derived or competitor-derived splenocytes (Fig. 4C). Thus, the DC-specific loss of Pten leads to the cell-intrinsic expansion of CD8+ cDC numbers, recapitulating the effect of global Pten deletion. Recently, Baron et al. used Cx3cr1-EGFP reporter strain to sub-divide CD8+ cDCs into two populations, and showed that only the Cx3cr1-EGFP− population represents Flt3L-responsive, cross-presenting CD8+ cDCs (Bar-On et al., 2010). The analysis of Cx3cr1-EGFP+ DC-PtenΔ mice showed the increase of EGFP− but not of EGFP+ CD8+ cDCs (Fig. 4D), confirming that the increase in numbers is specific to the Flt3L-responsive CD8+ cDC subset.
Figure 4. DC-specific Pten deletion causes the expansion of CD8+ cDCs.
(A) Staining profiles of splenic DC populations in Ptenfl/fl Itgax-Cre+ animals with DC-specific Pten deletion (DC-PtenΔ) and in littermate controls (Ctrl), highlighting CD11clo Bst2+ pDCs, CD11chi MHC II+ cDCs and their subsets.
(B) The fraction and absolute number of cDC subsets in DC-PtenΔ and control spleens (n=10–11). The P values of statistically significant differences are indicated.
(C) The analysis of DCs in hematopoietic chimeras reconstituted with a mixture of the control or DC-PtenΔ BM (CD45.2+) and wild-type CD45.1+ competitor BM. Shown are representative staining profiles and the absolute numbers of donor- and competitor-derived splenic cDC subsets (mean ± S.D. of 3 recipient animals).
(D) Splenic cDC subsets in Cx3cr1-EGFP+ DC-PtenΔ and littermate control mice. Shown are staining profiles of CD11chi MHC II+ cDCs with EGFP+ and EGFP− CD8+ cDC subsets highlighted, and absolute numbers of cDC subsets (n=4–5).
(E) Immature splenic cDC populations in DC-PtenΔ and control mice. Shown are staining profiles of gated MHC II+ CD24+ or CD24− populations containing mature CD11chi cDC subsets as well as CD11clo CD8lo immature cDCs (mean ± S.D. of three animals).
Next, we sought to determine the developmental origin of the observed CD8+ cDC proliferation. BrdU pulse-chase experiments revealed normal incorporation and dilution rates in the CD8+ cDCs (not shown), suggesting that Pten-deficient mature cDCs have normal cell division and turnover. The CD11clo MHC II− Cx3cr1-EGFP+ pre-cDCs (Liu et al., 2009) were not increased in DC-PtenΔ spleens (Fig. S3), consistent with inefficient Cre recombination in early cDC progenitors (Caton et al., 2007). In contrast, we observed the expansion of MHCII+ CD24hi CD11clo CD8lo Cx3cr1-EGFP− population likely representing immature CD8+ cDCs (Fig. 4E). A comparable CD11clo immature population was not obvious among the MHCII+ CD24lo cells encompassing CD8− cDCs in control or DC-PtenΔ mice. Thus, Pten loss causes the expansion of CD8+ cDC numbers that originates at the immature CD8lo differentiation stage.
The expansion of CD103+ cDC numbers in DC-PtenΔ mice
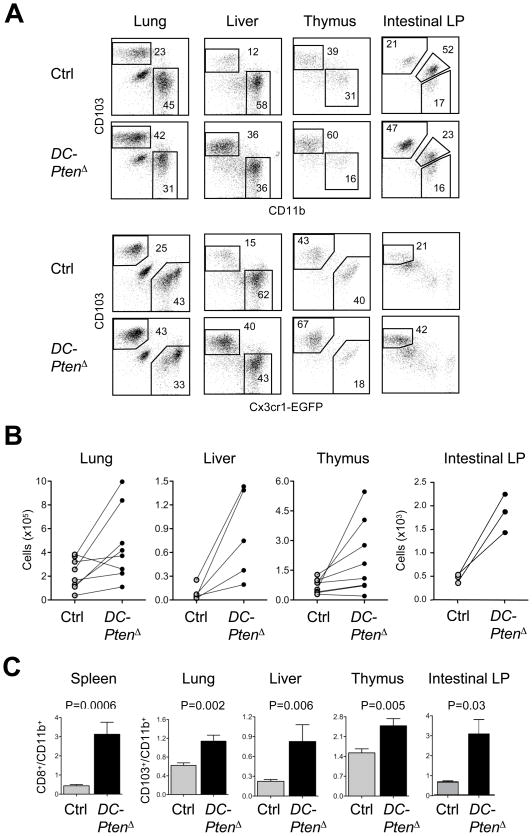
The CD103+ cDC subset in tissues represents the functional and genetic equivalent of CD8+ cDCs, and is strongly dependent on Flt3 signaling. As shown in Fig. 5, the fraction and absolute numbers of CD103+ CD11b− cDCs were increased in the thymus, liver, lung and intestinal lamina propria of DC-PtenΔ mice. This observation was confirmed by the analysis of Cx3cr1-EGFP+ DC-PtenΔ mice, in which the corresponding Cx3cr1-EGFP− CD103+ cDC population was similarly expanded. This resulted in the significantly increased ratio of CD103+ to CD103− cDCs in these organs (Fig. 5C) as well as in the Peyer’s patches and mesenteric lymph nodes (not shown). Thus, Pten deletion results in the expansion of CD8+-like cDCs both in lymphoid organs and in tissues.
Figure 5. The expansion of CD103+ cDCs following Pten deletion.
(A) The CD103+ versus CD11b+ cDC subset distribution in the tissues of DC-specific DC-PtenΔ mice. Shown are representative staining profiles of DAPI− CD45+ CD11chi MHC II+ cDCs from the indicated tissues of Cx3cr1-EGFP+ DC-PtenΔ and control mice. The populations of CD103+ and CD11b+ or Cx3cr1-EGFP+ DCs are highlighted; the intestinal lamina propria (LP) contains an additional CD103+ CD11b+ population.
(B) Absolute numbers of CD103+ cDCs in individual DC-PtenΔ mice shown by pairwise comparison to the corresponding littermate control.
(C) The ratio of CD103+ CD11b− to CD103− CD11b+ fractions among cDCs from control and DC-PtenΔ mice (mean ± S.E.M. of 8–9 animals for all tissues except intestinal LP, for which n=3).
The expansion of Pten-deficient CD8+ cDC numbers is mTOR-dependent
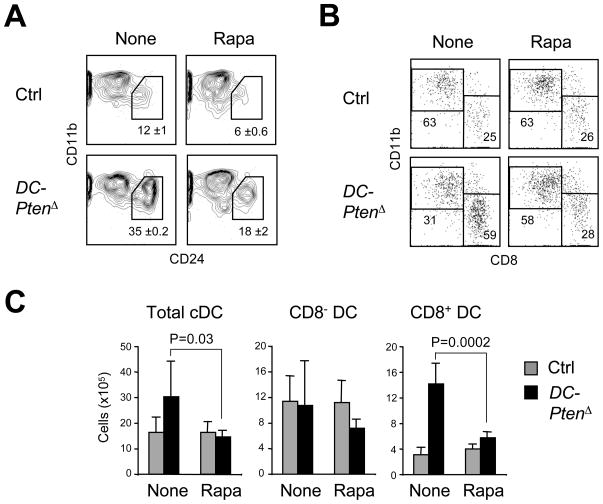
We tested whether the mTOR activity was mediating the CD8+ cDC expansion caused by Pten loss. Consistent with the in vivo phenotype, the CD11b−CD24+ population of CD8+-like cDCs was increased in Flt3L-supplemented cultures of the DC-PtenΔ BM; this expansion was reduced by rapamycin (Fig. 6A). As with the global Pten deletion, DC-PtenΔ BM cultures showed partial rescue of CD24+ DC development in the presence of rapamycin, confirming mTOR activation. Furthermore, daily administration of rapamycin reduced the CD8+ cDC compartment of DC-PtenΔ mice almost to control numbers (Fig. 6B). Thus, the expansion of CD8+ cDC numbers following DC-specific Pten deletion requires the activation of mTOR. To test whether mTOR activation alone would affect DC development, we performed DC-specific targeting of Tsc1, a key negative regulator of mTOR. The Tsc1fl/fl Itgax-Cre+ mice harbored a normal DC compartment (Fig. S4), suggesting that mTOR activity is necessary but not sufficient for CD8+ cDC expansion.
Figure 6. mTOR blockade reverses the expansion of Pten-deficient DCs.
(A) Rapamycin treatment of Flt3L-supplemented BM cultures from DC-PtenΔ and control mice. Rapamycin was added on day 3 of culture. Shown are staining profiles of gated CD11c+ B220− cDCs highlighting the CD11blo CD24hi CD8+ cDC-like subset (mean ± range of two independent cultures).
(B) Rapamycin treatment of DC-PtenΔ and control animals. Shown are representative staining profiles of splenic CD11chi MHC II+ cDCs from mice treated for 7 days with rapamycin or vehicle only.
(C) Absolute numbers of splenic cDC subsets from DC-PtenΔ and control animals treated with rapamycin (5–6 animals per group).
DC-specific Pten deletion impairs control of Listeria infection
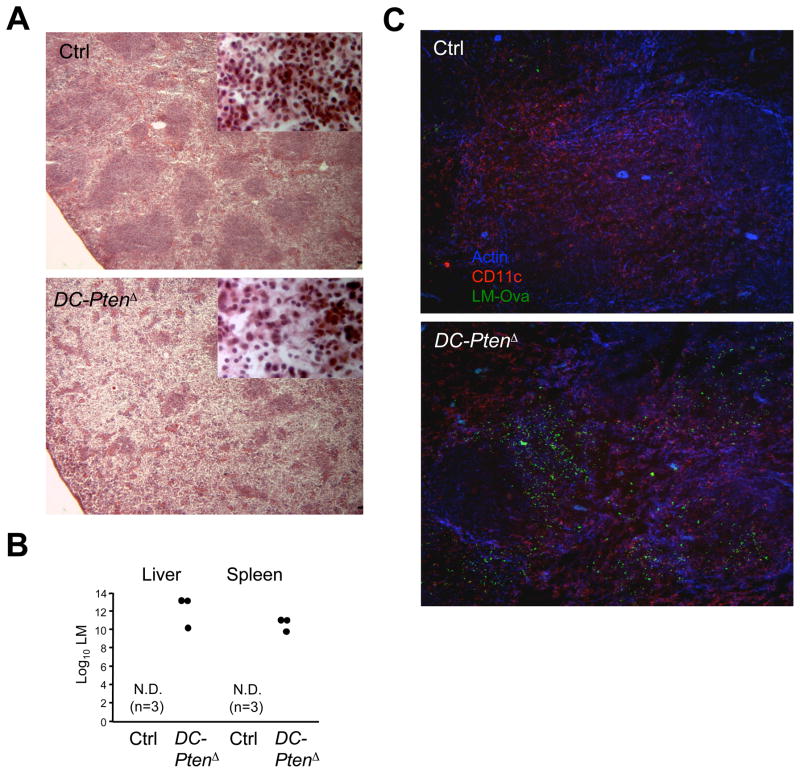
The expansion of DC compartment by Flt3L and other cytokines paradoxically impairs immunity to intracellular bacteria such as Listeria (LM) and pneumococci (Alaniz et al., 2004; Winter et al., 2007). We therefore tested the ability of DC-PtenΔ mice to control LM infection, using the attenuated ovalbumin-expressing LM (LM-OVA). At low LM-OVA doses DC-PtenΔ mice survived until day 6 but showed a severe disorganization of the spleen architecture with necrotic foci, hypocellularity and dissolution of lymphoid follicles (Fig. 7A). At higher LM-OVA doses, control mice survived and cleared the infection by day 6, whereas all DC-PtenΔ mice became moribund and harbored high bacterial titers in the spleens and livers (Fig. 7B). By immunochemistry, DC-PtenΔ spleens showed a greatly increased bacterial load as early as 24 hr after infection (Fig. 7C). Similarly, at 36 hr high LM titers were detected in the livers of all DC-PtenΔ mice, while most control livers remained sterile (data not shown). On the other hand, both the initial clustering of OVA-specific CD8+ T cells and their subsequent expansion were unaffected, suggesting that Pten-deficient cDCs can efficiently prime T cells (Fig. S5). These data demonstrate that DC-specific deletion of Pten causes CD8+ cDC expansion but increases susceptibility to LM, similar to cytokine-induced DC expansion. Thus, Pten expression in DCs controls optimal DC subset homeostasis to ensure protection against bacterial infections.
Figure 7. DC-specific Pten deletion increases sensitivity to Listeria infection.
(A) Spleen histology of control and DC-PtenΔ mice on day 6 after infection with 2x104 LM-OVA bacteria (H&E staining, 25x magnification; inset, 400x magnification). Uninfected control or DC-PtenΔ mice showed no difference by histology (not shown).
(B) Bacterial titers on day 6 after infection with 2x105 LM-OVA. All DC-PtenΔ mice were moribund and showed prominent inflammation of the spleen and liver. Symbols represent individual animals; N.D., not detected.
(C) Immunohistochemical analysis of the spleen 24 hr after infection with 105 LM-OVA. Frozen spleen sections were stained for polymerized actin, CD11c and ovalbumin to detect LM-OVA.
Discussion
DCs represent the only immune cell type expressing Flt3 receptor throughout all developmental stages as well as in mature cells (Karsunky et al., 2003). Accordingly, Flt3L is a key regulator of steady-state DC development, facilitating the proliferation of earliest DC progenitors in the BM (D’Amico and Wu, 2003; Karsunky et al., 2003) and the differentiation of pre-DCs into mature DCs in the periphery (Waskow et al., 2008). However, the pathways transducing Flt3 signals in DCs have been poorly characterized. Although transcription factor Stat3 is important for Flt3-driven pDC and cDC development (Laouar et al., 2003; Onai et al., 2006), more proximal signaling pathways downstream of Flt3 remain unknown. The PI3K-mTOR signaling plays an important role downstream of the constitutive Flt3 receptor activation in myeloid leukemias (Mohi et al., 2004), and activates Stat3 in TLR-stimulated cells (Weichhart et al., 2008). We therefore tested its role in the physiological Flt3 signaling during DC development. We found that mTOR signaling is activated by Flt3 in DCs, and is essential for Flt3L-mediated but not GM-CSF-mediated DC development in vitro. Conversely, genetic activation of PI3K-mTOR by the loss of Pten facilitated Flt3L-driven DC development in vitro, and caused in vivo expansion of CD8+ cDCs in a manner resembling Flt3L administration. This genetic evidence collectively suggests that the PI3K-mTOR pathway represents a key transducer of Flt3L-Flt3 signals in DCs.
In cultures of total BM cells, Flt3L promotes proliferation of common DC and pDC progenitors (Naik et al., 2007) and drives the development of all DC subsets (Naik et al., 2005). We found that mTOR inhibitor rapamycin severely impaired DC development in BM cultures by blocking Flt3L-driven proliferation of early progenitors. This effect was due to on-target rapamycin activity, because it was observed at concentrations commonly used for mTOR inhibition (Hackstein et al., 2003; Cao et al., 2008; Ohtani et al., 2008) and was partially rescued by Pten deletion. The requirement for mTOR in Flt3L signaling may be particularly evident in BM cultures, because DC development in this system is rapid, inducible and driven solely by Flt3L. Furthermore, these in vitro results are in line with the reported ~50% reduction of total DC numbers in vivo after prolonged rapamycin administration to mice (Hackstein et al., 2003). Athough all DC subsets were affected by rapamycin, the pDCs were particularly sensitive, consistent with the more severe decrease of pDCs in Flt3-deficient or mutant animals (Eidenschenk et al., 2010; Waskow et al., 2008). Among cDCs, the CD24hi CD8+-like DCs were more susceptible to mTOR blockade, in agreement with the selective role of Flt3 in the development of CD8+ and CD103+ cDCs (Bogunovic et al., 2009; Ginhoux et al., 2009; Varol et al., 2009). Conversely, PI3K-mTOR hyperactivation caused by deletion of Pten in the BM ameliorated the effect of rapamycin and greatly increased Flt3L-driven DC development in BM cultures. Both rapamycin and Pten deletion acted specifically in Flt3L cultures and did not affect DC development driven by GM-CSF. Overall, the efficiency of Flt3-driven DC development in BM cultures directly correlated with the blockade or hyperactivation of mTOR activity, suggesting that mTOR mediates Flt3 signaling in this system.
To analyze the consequences of PI3K-mTOR hyperactivation in vivo, we deleted Pten in hematopoietic progenitors (PtenΔ) or in committed DCs (DC-PtenΔ). In both systems Pten deletion caused a dramatic expansion of CD8+ cDC subset in the spleen and a less pronounced increase of the corresponding CD103+ cDC subset in tissues. Although Pten deletion activates multiple pathways downstream of PI3K, the phenotype was fully reversed by mTOR blockade. Notably, preferential expansion of CD8+ cDCs is a hallmark of Flt3L administration in vivo, as documented in several studies (Bedoui et al., 2009a; O’Keeffe et al., 2002; Vollstedt et al., 2004). This phenotype is unique to Flt3L, as other cytokines such as GM-CSF preferentially expand CD8− cDCs (Mach et al., 2000; O’Keeffe et al., 2002). Furthermore, only a distinct Cx3cr1-EGFP− subset of CD8+ cDCs is responsive to Flt3L (Bar-On et al., 2010), the same subset that is specifically expanded in DC-PtenΔ mice. Thus, genetic activation of PI3K-mTOR signaling in DCs recapitulates the subset preference (albeit not the magnitude) of Flt3L-induced DC expansion, supporting the role of mTOR in Flt3 signaling in vivo.
An important question is why Pten deletion does not increase the number of CD8− cDCs or pDCs in vivo, as it does in BM cultures. One possibility is that these two subsets or their progenitor “saturate” their respective anatomic niches, such as the splenic marginal zone for CD8− cDCs. Another likely explanation is that their development does not involve extensive proliferation between lineage commitment and terminal differentiation. Indeed, CD8− cDCs and pDCs are relatively poorly responsive to Flt3L administration in vivo, yielding ~2–3-fold expansion at best compared to >30-fold expansion attainable with CD8+ cDCs (O’Keeffe et al., 2002; Vollstedt et al., 2004). It is noteworthy that the CD8− cDC expansion caused by B16-Flt3L tumor cell injection (Mach et al., 2000) may reflect an antitumor immune reaction, as it is not recapitulated by recombinant Flt3L (O’Keeffe et al., 2002). Furthermore, transgenic expression of the transforming SV40 T antigens in all CD11c+ cDCs caused selective expansion of CD8+ but not of CD8− cDCs (Steiner et al., 2008). Finally, DC expansion due to Pten loss likely requires endogenous Flt3L, whose concentration is tightly regulated and may be limiting. Thus, Pten deletion recapitulates the specificity of Flt3L administration, but does not reach the higher threshold required for the expansion of CD8− cDCs and pDCs in vivo.
The CD8+ and CD103+ cDC subsets appears particularly responsive to Flt3 signaling and to Pten deletion that facilitates it. This correlates with increased mTOR signaling after Flt3L administration and in the steady state, the latter possibly reflecting endogenous Flt3L activity. While the molecular basis for such increased mTOR signaling remains to be elucidated, it likely contributes to the Flt3L responsiveness of CD8+ cDCs. Another important factor is the presence of a Flt3-sensitive differentiation stage specifically in this subset. Indeed, Pten deletion caused marked expansion of MHC II+CD24+CD11cloCD8lo population, the likely immature intermediate between pre-DCs and CD8+ CD11chi cDCs. This population at least partially overlaps with the CD8int cDCs and with the recently identified CD24hi CD8− precursors of CD8+ cDCs, both of which are specifically expanded by Flt3L treatment (Bedoui et al., 2009a; O’Keeffe et al., 2002). Furthermore, the cell-intrinsic restriction of CD8lo precursor expansion by Pten appears necessary to maintain the characteristic low numbers of CD8+ cDCs. A similar role of Pten has been reported in the B cell lineage, where Pten deletion enhances the generation of only certain B cell subsets such as marginal zone B cells (Anzelon et al., 2003; Suzuki et al., 2003). Thus, in both DCs and B cells Pten acts as a critical regulator of differentiation that maintains proper ratios of peripheral cell subsets.
This newly established function of Pten in the negative regulation of CD8+ cDC numbers is critical for the optimal immune response, as demonstrated by impaired control of Listeria infection after DC-specific Pten loss. Again, this result resembles the consequences of Flt3L treatment, which increases susceptibility to several pathogenic bacteria including S.pneumoniae (Winter et al., 2007), Listeria and M.tuberculosis (Alaniz et al., 2004). Neither Pten deletion (this study) nor Flt3L treatment (Alaniz et al., 2004) impaired T cell responses to Listeria, suggesting that the T cell priming capacity of DCs remains intact. Indeed, increased Listeria replication in Pten-targeted mice was observed as early as 24 hours post infection, suggesting an early defect that precedes T cell priming. Although functional DC defects such as impaired IL-12 production are possible (Ohtani et al., 2008), IL-12 production was relatively normal both in Flt3L-expanded DCs (O’Keeffe et al., 2002) and in Pten-deficient DCs (data not shown). A more likely possibility is the sheer increase in DC numbers, which creates a larger reservoir for early bacterial replication. Indeed, splenic cDCs provide the key cellular niche of Listeria shortly after infection (Aoshi et al., 2009; Aoshi et al., 2008), and CD8+ cDCs in particular are responsible for Listeria spread and transport into the white pulp (Neuenhahn et al., 2006). At later time points cDCs are prominently infected but, unlike macrophages, neither kill bacteria nor become targets of cytotoxic T cells (Alaniz et al., 2004). Therefore, the expansion of DCs in general and of CD8+ cDCs in particular appears detrimental for the control of bacterial infections, and must be restricted by specific regulatory mechanisms. Our results identify the DC-intrinsic negative control of PI3K-mTOR signaling by Pten as one such mechanism that ensures proper balance of peripheral DC subsets, thereby facilitating optimal immune responses.
In conclusion, our study identifies a novel role of PI3K-mTOR signaling as a key mediator of DC development downstream of Flt3, and underscores the importance of its precise regulation by Pten for DC homeostasis and immunity to pathogens. These data have implications for the therapeutic use of mTOR inhibitor drug rapamycin, which in the long term may affect DC development in human patients. In addition, these results suggest that the commonly used Flt3L-induced DC expansion may not adequately represent steady-state DC development, due to skewed subset composition and excessive mTOR signaling in DCs. These caveats of cytokine-induced DC expansion should be considered in the design of DC-based therapeutic strategies.
Experimental Procedures
Animals
The Ptenfl (Trotman et al., 2003), Gt(ROSA)26Sor-CreER (Cisse et al., 2008), Itgax-Cre (originally designated CD11c-Cre, Caton et al., 2007) and Cx3cr1-EGFP (Jung et al., 2000) strains and crosses thereof were on C57BL/6 background (N10+). Because Itgax-Cre+ or Gt(ROSA)26Sor-CreER+ animals show no phenotypic or functional differences from wild-type (Caton et al., 2007) and data not shown), Cre-negative littermates were used as controls. Wild-type C57BL/6 and CD45.1 congenic B6.SJL mice were from Taconic.
Cre recombination in Gt(ROSA)26Sor-CreER+ animals and control littermates was induced by oral tamoxifen administration as described (Cisse et al., 2008). Five days later, BM was removed, confirmed for Pten deletion by genomic PCR and cultured in vitro or injected i.v. into lethally irradiated B6.SJL mice. The recipients were analyzed after 6 weeks and showed >90% donor chimerism. For competitive reconstitution from Itgax-Cre+ mice, control or DC-PtenΔ BM cells were mixed 1:1 with B6.SJL BM cells and injected into irradiated B6.SJL recipients. The resulting chimeras were analyzed 8–10 wk later. For in vivo rapamycin treatment, mice were injected i.p. with 30 μg rapamycin (LC Laboratories) in PBS with 5% DMSO and 10% ethanol or with vehicle only for 7 consecutive days. For in vivo Flt3 stimulation, mice were injected i.v. with 1 μg of recombinant murine Flt3L (R&D Systems) in PBS, and the spleen were harvested at the indicated times. All animal studies were performed according to the investigators’ protocols approved by the respective Institutional Animal Care and Use Committees.
Cell analysis
Cells were isolated ex vivo from lymphoid organs and stained for cell surface markers as described (Caton et al., 2007; Cisse et al., 2008). The isolation and analysis of DCs from the thymus, lymph nodes and tissues was done as in (Bogunovic et al., 2009; Ginhoux et al., 2009). For the analysis of S6 phosphorylation in vivo, splenocytes were isolated directly into PBS with paraformaldehyde and permeabilized with ice-cold methanol as described (O’Gorman et al., 2009). Fixed cells were simultaneously stained for cell surface markers and phospho-S6 Ser235–236 (clone 2F9, Cell Signaling Technology) using pre-determined antibody per cell number ratios. The samples were acquired on LSR II flow cytometer or sorted on FACSAria flow sorter (BD Immunocytometry Systems), and analyzed using FlowJo software (Treestar Inc.).
DC culture
For DC development in vitro, total BM cells (2 × 106/ml) were cultured for 8–10 days with 50–100 ng/ml recombinant murine Flt3L (for all experiments involving rapamycin treatment), or with 20% supernatant of Flt3L-expressing B16 cell line (Mach et al., 2000). CFSE labeling was done as described (Naik et al., 2007). Rapamycin was added at 10 ng/ml unless indicated otherwise.
LM-OVA infection
Freshly grown LM-OVA (Dudani et al., 2002) was diluted in PBS and injected i.v. at the indicated number. For LM titration, one half of the spleen and liver was lysed in PBS with 0.1% Tween 20 and plated on BHI (BD Biosciences) agar plates. For histological analysis, spleens were fixed, paraffin-embedded and stained with hematoxylin-eosin. The detection of LM-OVA in the spleens by immunochemistry was performed as described (Aoshi et al., 2009).
Statistical analysis
Statistical significance was estimated using unpaired, two-tailed Student’s t-test.
Highlights.
Flt3L induces mammalian target of rapamycin (mTOR) signaling in dendritic cells (DCs);
Flt3L-driven DC development is impaired by rapamycin and accelerated by Pten deletion;
DC-specific deletion of Pten causes mTOR-dependent expansion of CD8+ DC numbers;
DC-specific deletion of Pten impairs immunity to Listeria infection
Supplementary Material
Acknowledgments
We thank A. Ferrando, T. Diacovo and T. Ludwig for animal strains, N. Serbina for LM-OVA, A. Fay and J. Dworkin for help with bacterial culture, E. Simonds for technical assistance and the Reizis lab members for help and discussions. Supported by the SPAR-American Asthma Foundation Award (B.R.), NIH grants AI067804 and AI072571 (B.R.), AI057229 and HHSN272200700038C (G.P.N.).
Footnotes
Competing Interests Statement
Technologies associated with phospho-flow are licensed in part to BD Biosciences, and G.P.N. is a consultant for BD Biosciences, a supplier of the reagents used in this report. Other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaniz RC, Sandall S, Thomas EK, Wilson CB. Increased dendritic cell numbers impair protective immunity to intracellular bacteria despite augmenting antigen-specific CD8+ T lymphocyte responses. J Immunol. 2004;172:3725–3735. doi: 10.4049/jimmunol.172.6.3725. [DOI] [PubMed] [Google Scholar]
- Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- Aoshi T, Carrero JA, Konjufca V, Koide Y, Unanue ER, Miller MJ. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur J Immunol. 2009;39:417–425. doi: 10.1002/eji.200838718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshi T, Zinselmeyer BH, Konjufca V, Lynch JN, Zhang X, Koide Y, Miller MJ. Bacterial entry to the splenic white pulp initiates antigen presentation to CD8+ T cells. Immunity. 2008;29:476–486. doi: 10.1016/j.immuni.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Bar-On L, Birnberg T, Lewis KL, Edelson BT, Bruder D, Hildner K, Buer J, Murphy KM, Reizis B, Jung S. CX3CR1+ CD8alpha+ dendritic cells are a steady state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:14745–50. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoui S, Prato S, Mintern J, Gebhardt T, Zhan Y, Lew AM, Heath WR, Villadangos JA, Segura E. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009a;182:4200–4207. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross- presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009b;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudani R, Chapdelaine Y, Faassen Hv H, Smith DK, Shen H, Krishnan L, Sad S. Multiple mechanisms compensate to enhance tumor-protective CD8(+) T cell response in the long-term despite poor CD8(+) T cell priming initially: comparison between an acute versus a chronic intracellular bacterium expressing a model antigen. J Immunol. 2002;168:5737–5745. doi: 10.4049/jimmunol.168.11.5737. [DOI] [PubMed] [Google Scholar]
- Eidenschenk C, Crozat K, Krebs P, Arens R, Popkin D, Arnold CN, Blasius AL, Benedict CA, Moresco EM, Xia Y, Beutler B. Flt3 permits survival during infection by rendering dendritic cells competent to activate NK cells. Proc Natl Acad Sci U S A. 2010;107:9759–9764. doi: 10.1073/pnas.1005186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4--induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In Vivo Analysis of Dendritic Cell Development and Homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohi MG, Boulton C, Gu TL, Sternberg DW, Neuberg D, Griffin JD, Gilliland DG, Neel BG. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci U S A. 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O’Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- O’Gorman WE, Dooms H, Thorne SH, Kuswanto WF, Simonds EF, Krutzik PO, Nolan GP, Abbas AK. The initial phase of an immune response functions to activate regulatory T cells. J Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- Onai N, Obata-Onai A, Tussiwand R, Lanzavecchia A, Manz MG. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J Exp Med. 2006;203:227–238. doi: 10.1084/jem.20051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Denning TL. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr Opin Immunol. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner QG, Otten LA, Hicks MJ, Kaya G, Grosjean F, Saeuberli E, Lavanchy C, Beermann F, McClain KL, Acha-Orbea H. In vivo transformation of mouse conventional CD8alpha+ dendritic cells leads to progressive multisystem histiocytosis. Blood. 2008;111:2073–2082. doi: 10.1182/blood-2007-06-097576. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tussiwand R, Onai N, Mazzucchelli L, Manz MG. Inhibition of natural type I IFN-producing and dendritic cell development by a small molecule receptor tyrosine kinase inhibitor with Flt3 affinity. J Immunol. 2005;175:3674–3680. doi: 10.4049/jimmunol.175.6.3674. [DOI] [PubMed] [Google Scholar]
- Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Vollstedt S, O’Keeffe M, Odermatt B, Beat R, Glanzmann B, Riesen M, Shortman K, Suter M. Treatment of neonatal mice with Flt3 ligand leads to changes in dendritic cell subpopulations associated with enhanced IL-12 and IFN-alpha production. Eur J Immunol. 2004;34:1849–1860. doi: 10.1002/eji.200324443. [DOI] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Winter C, Taut K, Langer F, Mack M, Briles DE, Paton JC, Maus R, Srivastava M, Welte T, Maus UA. FMS-like tyrosine kinase 3 ligand aggravates the lung inflammatory response to Streptococcus pneumoniae infection in mice: role of dendritic cells. J Immunol. 2007;179:3099–3108. doi: 10.4049/jimmunol.179.5.3099. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.