Abstract
Non-steroidal anti-inflammatory drugs such as sulindac are promising chemoprevention agents against colon cancer, but their weak potency and side effects limit their use for both chemoprevention and chemotherapy. Here, we evaluated the effect of a new sulindac derivative, phospho-sulindac or OXT-922, on the growth of human cancer cell lines and its mechanism of action. OXT-922 inhibited the growth of human cancer cell lines originating from colon, pancreas and breast ∼11- to 30-fold more potently than sulindac. This effect was mediated by a strong cytokinetic effect. Compared with control, OXT-922 inhibited cell proliferation by up to 67%, induced apoptosis 4.1-fold over control and blocked the G1 to S cell cycle phase transition. OXT-922 suppressed the levels of cell cycle regulating proteins, including cyclins D1 and D3 and Cyclin-dependent kinases (CDK) 4 and 6. The levels of intracellular reactive oxygen species (ROS), especially those of mitochondrial , were markedly elevated (5.5-fold) in response to OXT-922. ROS collapsed the mitochondrial membrane potential and triggered apoptosis, which was largely abrogated by antioxidants. OXT-922 suppressed nuclear factor-kappaB activation and downregulated thioredoxin-1 expression. It also suppressed the production of prostaglandin E2 and decreased cyclooxygenase-1 expression. Similar to sulindac, OXT-922 enhanced spermidine/spermine N1-acetyltransferase activity, reduced the cellular polyamine content and synergized with difluoromethylornithine to inhibit cancer cell proliferation and induce apoptosis. Our results suggest that OXT-922 possesses promising anticancer properties and deserves further evaluation.
Introduction
Substantive epidemiological, in vitro, and animal studies indicate that non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin, sulindac and piroxicam have antitumorigenic activities against colorectal cancer. NSAIDs inhibit cell cycle progression and cell proliferation and induce apoptosis in various cancer cell lines; several underlying molecular mechanisms have been suggested (1). Inhibition of cyclooxygenase (COX) activity by NSAIDs, which leads to reduced prostaglandin synthesis, is considered important for their antitumorigenic activity (2). On the other hand, an extensive array of non-COX targets of NSAIDs relevant to their anticancer properties has been identified. For example, NSAIDs also inhibit the growth of cancer cells expressing no COX-1/COX-2, and this effect might depend on nuclear factor-kappaB (NF-κB) signaling inhibition, oxidative stress or reduced polyamine synthesis (3–5).
Among the nearly 50 approved NSAIDs, aspirin and sulindac are probably the best studied. Interventional clinical trials have demonstrated the cancer preventive effect of both but neither had an effect strong enough to be clinically useful (6–9). As for any agent, besides efficacy, the safety of NSAIDs is another relevant consideration. NSAIDs, even though widely used, do carry significant toxicity, primarily gastrointestinal and renal (10–11). Such toxicity is expected to be more pronounced if they are to be used on a long-term basis for cancer prevention. Several attempts have been made to overcome the limitations of conventional NSAIDs, mainly those concerning their efficacy and safety. Some of these efforts include the chemical modification of the NSAID molecule in the hopes of generating pharmacologically superior derivatives. Two examples are the nitric oxide-donating NSAIDs (12) and a modified sulindac (13). These and other studies suggest that modifying the NSAIDs molecule may lead to useful new compounds.
In the present study, we evaluated the growth inhibitory effect of OXT-922, the novel sulindac derivative shown in Figure 1. We found that the growth inhibitory effect of OXT-922 in cancer cells was largely mediated by (i) elevated levels of reactive oxygen species (ROS), which, in turn, activated relevant cell signaling, leading to apoptosis and (ii) enhanced spermidine/spermine N1-acetyltransferase (SSAT) activity, which reduced polyamine levels and inhibited cell proliferation (cell renewal). Our work elucidates the anticancer mechanism of action of OXT-922 and highlights the potential of this compound as an anticancer drug.
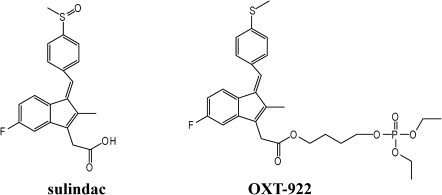
Fig. 1.
The chemical structure of sulindac and OXT-922.
Materials and methods
Reagents and cell culture
OXT-922 was provided by Medicon Inc. (Stony Brook, NY). Annexin V-fluorescein isothiocyanate, dihydroethidium, 2′,7′-dichlorodihydrofluorecein diacetate (DCFDA), 4-amino-5-methylamino-2′,7′-difluorofluorescein and MitoSOX Red were purchased from Invitrogen (Carlsbad, CA). N-acetyl-l-cysteine (NAC) was purchased from EMD4BioSciences (Brookfield, WI). McCoy's 5A medium (modified), modified Eagle's medium (Eagle), RPMI 1640 and antibiotics were from Mediatech (Manassas, VA). Fetal calf serum was from Thermo Scientific (Waltham, MA). Trolox and tempol were from Sigma (St Louis, MO). Melittin was from Enzo Life Sciences (Plymouth Meeting, PA). Human colon (HT-29 and SW480), pancreatic (BxPC-3 and MIA PaCa-2) and breast (MDA-MB-231 and MCF-7) cancer cell lines (American Type Culture Collection, Manassas, VA) were grown in media recommended by American Type Culture Collection.
Cell kinetic assays
Cells were seeded at a density of 5 × 104 cells/cm2 and allowed to attach for 24 h, when various treatments were applied. Cell viability was measured by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay following the protocol of the manufacturer (Roche Diagnostics, Indianapolis, IN, USA). For cell proliferation assay, HT-29 cells, treated with OXT-922, were pulse-labeled with 10 μM bromodeoxyuridine (BrdU) (BD Bioscience, San Jose, CA) 30 min prior to harvesting and analyzed by flow cytometry. To measure cell death, cells were treated with OXT-922 for 24 h, harvested by trypsinization, stained with fluorescein isothiocyanate-conjugated Annexin V and propidium iodide (PI) according to the manufacturer’s protocol and analyzed by flow cytometry. For cell cycle analysis, cells were fixed by cold 70% ethanol and stained with PI following standard protocols prior to flow cytometric analysis.
Determination of ROS
We determined ROS levels with confocal microscopy and flow cytometry. To determine mitochondrial production, cells were seeded in 35 mm glass bottom culture dishes. After each treatment, cells were stained with 5 μM MitoSOX Red for 10 min or with 5 μM dihydrorhodamine for 15 min. Live cells were kept in a 5% CO2 chamber and examined under a Zeiss LSM510 confocal microscope. For flow cytometry, after treatment with the OXT-922 in six-well plates, cells were trypsinized and stained with 10 μM DCFDA for 30 min at 37°C and their fluorescence intensity was analyzed by FACS Caliber (BD Bioscience).
Determination of mitochondrial membrane potential
The mitochondrial membrane potential was determined by flow cytometry using the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide cationic dye (Invitrogen). Briefly, SW480 cells were incubated with OXT-922 at 1 × IC50 (half maximal inhibitory concentration) for 3 h, when cells were trypsinized and washed once with phosphate-buffered saline. The supernatant was discarded and cells were incubated with 5 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide for 30 min at 37°C, protected from light and analyzed by flow cytometry.
Western blotting
After each treatment, cells were lysed on ice with 1% Triton X-100 lysis buffer with 2.5 mM 4-nitrophenylphosphate, 1% sodium dodecyl sulfate and 0.25% sodium deoxycholate for 30 min. For each sample, 30 μg of cell lysate were loaded onto sodium dodecyl sulfate electrophoresis gel and transferred onto a nitrocellulose membrane. The membrane was then immunoblotted with primary antibodies followed by secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence was used to visualize the bands on X-ray film.
Electrophoretic mobility shift assay
After the indicated treatment, cell nuclear fractions were isolated from 3 × 106 cells using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific). The NF-κB activity was detected by using LightShift chemiluminescent EMSA kit (Thermo Scientific) according to the manufacturer’s instructions. Briefly, the nuclear extracts were incubated with biotin-labeled DNA probes at 37°C for 20 min, then loaded onto the polyacrylamide gel and transferred to a nylon membrane. The membrane was exposed to Ultraviolet light for 10 min for cross-linking of the transferred DNA, then incubated with stabilized streptavidin-horseradish peroxide conjugate in blocking buffer for 15 min and covered with substrate working solution, followed by exposure to X-ray film.
Prostaglandin E2 assay
Prostaglandin E2 levels in culture media were determined using the kit from Cayman Chemical (Ann Arbor, MI, USA) according to the manufacturer’s instruction.
SSAT assay
Cells (3 × 106) were seeded in plates overnight and then treated with OXT-922 for 24 h. Cells were washed twice with cold homogenization buffer (10 mM Tris−HCl, pH 7.5; 2.5 mM dithiothreitol; 1 mM ethylenediaminetetraacetic acid), scraped, disrupted by sonification and then centrifuged at 15 000g at 4°C for 10 min. Twenty-five microliters of supernatant were incubated with 3 mM spermidine and 10 μM [14C] acetyl-CoA in a final volume of 50 μl for 10 min at 37°C. The reaction was stopped by the addition of 20 μl 1M NH2OH−HCl and heating in boiling water for 3 min. The resulting samples were centrifuged, 30 μl of supernatant were spotted onto P-81 phosphocellulose discs and scintillation was counted. Results were expressed as a percentage of control.
Results
OXT-922 inhibits the growth of various human cancer cell lines: a strong cytokinetic effect
The growth inhibitory effect of OXT-922 was evaluated by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay in human cancer cell lines. As shown in Table 1, the 24 h IC50 of cell lines originating from the colon, pancreas and breast ranged from 18 μM (MDA-MB-231) to 92 μM (MIA-PaCa-2). The breast cancer cell lines were more sensitive to OXT-922 than others. We also tested the growth inhibitory effect of sulindac. In agreement with previous reports (14), its effect was weak and the IC50 values varied from 489 μM (BxPC-3) to 1173 μM (HT-29). Compared with sulindac, OXT-922 was more potent in all six cancer cell lines tested; the potency enhancement ranged between 11- and 30-fold. However, the normal human colon mucosal epithelial cell line NCM460 is resistant to OXT-922, with an IC50 of 181 μM (2.5-fold higher than the average IC50 of the two colon cancer cell lines); the potency enhancement was only 3-fold compared with sulindac.
Table I.
The growth inhibitory effect of OXT-922 on human cells
| Cell lines | IC50: μM |
Enhancement (fold) | |
| Sulindac | OXT-922 | ||
| Colon | |||
| HT-29 | 1173 | 70 | 17 |
| SW480 | 900 | 73 | 12 |
| NCM 460 | 528 | 181 | 3 |
| Breast | |||
| MCF-7 | 1128 | 38 | 30 |
| MDA-MB-231 | 530 | 18 | 30 |
| Pancreatic | |||
| BxPC-3 | 489 | 32 | 15 |
| MIA-PaCa-2 | 1036 | 92 | 11 |
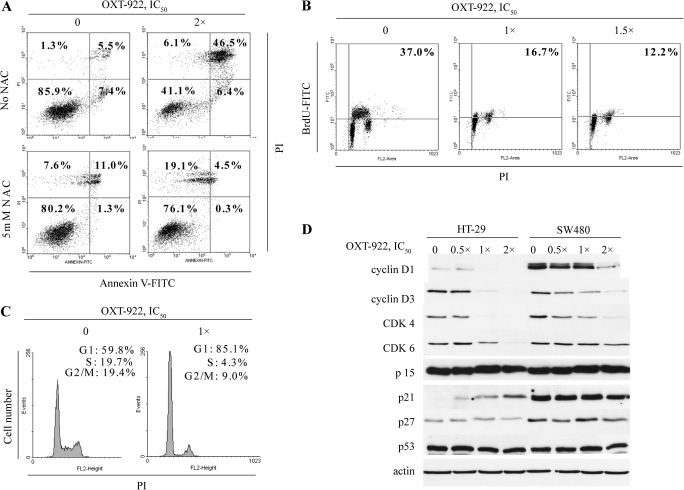
We explored the cytokinetic effect of OXT-922 to assess the mechanism of its growth inhibitory effect. OXT-922 induced apoptosis in HT-29 cells after 24 h treatment with OXT-922 at 2 × IC50 concentration (Figure 2A). The proportion of apoptotic cells [Annexin V(+)/PI(−) and Annexin V(+)/PI(+)] increased ∼4.1-fold, from 12.9 to 52.9%. Late apoptotic cells [Annexin V (+)/PI(+)] increased even more (∼8.5-fold over control). The antioxidant NAC effectively blocked the apoptosis induced by OXT-922. After treatment with OXT-922 2 × IC50 and NAC 2 mM, 76.1% cells survived, whereas only 41.1% cells survived in the absence of NAC. Cell proliferation was evaluated by the BrdU incorporation method. As shown in Figure 2B, OXT-922 reduced BrdU incorporation in HT-29 cells in a concentration-dependent manner. At 1 × IC50 concentration, OXT-922 decreased BrdU-positive cells from 37.0 to 16.7%, and at 1.5 × IC50 concentration, it decreased them further to 12.2%. After 24 h of incubation with 1 × IC50 OXT-922, a significant G1 to S arrest was observed, with the proportion of cells in G1 phase increasing from 59.8 to 85.1% (Figure 2C). In both colon cancer cell lines tested, OXT-922 suppressed the expression of proteins regulating this part of the cell cycle. For example, the levels of cyclin D1, cyclin D3, CDK4 and CDK6 were suppressed in a concentration-dependent manner, more prominently in HT-29 cells. CDK4 and CDK6, each complexes with cyclin D, an interaction that is critical for mitotic cells to overcome the G1 restriction point (15). Thus, our findings suggest that OXT-922 blocks the transition through the G1 restriction point. OXT-922 also upregulated the CDK inhibitor p21 in HT-29 (but not in SW480) cells and left unaffected the protein levels of p15, p27 and p53 (Figure 2D). Taken together, these changes provide at least a partial explanation of the cell cycle effect of OXT-922.
Fig. 2.
The cytokinetic effect of OXT-922. (A) HT-29 cells treated with OXT-922 for 24 h were stained with PI and Annexin V and analyzed by flow cytometry. The numbers inside each box represent the percentage of cells in each category. Lower panel: cells were pretreated with 5 mM NAC for 2 h. (B) BrdU incorporation assay in HT-29 cells following OXT-922 treatment for 24 h. The number in the right upper box indicates the percentage of cells in S phase. (C) Cell cycle profiles generated from fluorescence-activated cell sorting of PI-stained cells 24 h after OXT-922 treatment. (D) Cells were exposed to the indicated concentrations of OXT-922 for 18 h and analyzed by western blotting to determine protein levels. These results are representative of three independent experiments, which generated similar results.
OXT-922 enhances ROS levels in colon cancer cells
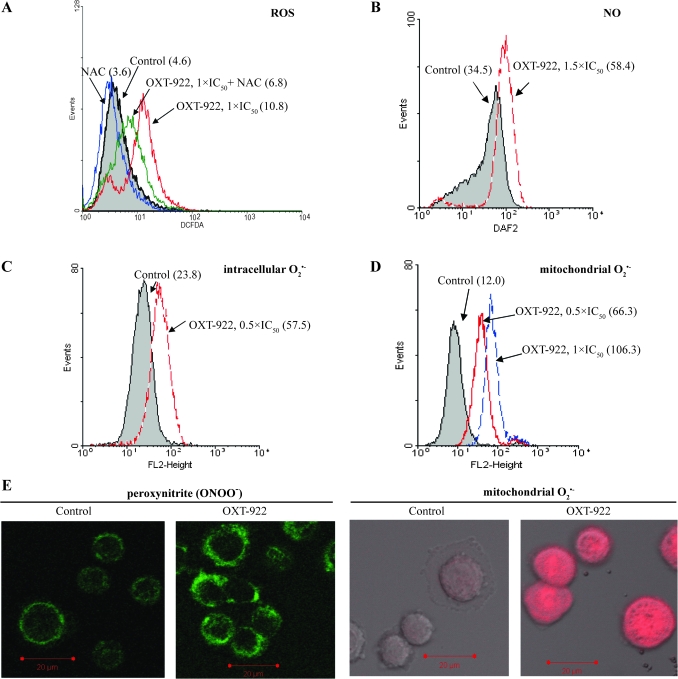
ROS play a critical role in the mechanism of action of some anticancer compounds, including paclitaxel, arsenic trioxide and NO-aspirin (16–19). Therefore, we determined the ROS levels in HT-29 cells after their treatment with OXT-922. As shown in Figure 3A, OXT-922 increased the fluorescence of the general ROS probe, DCFDA, which reacts with nearly 10 individual ROS (20−21). Compared with control, OXT-922 1 × IC50 enhanced ROS levels in SW480 cells by ∼135% in 1 h. Pretreatment of the cells with 2 mM NAC for 2 h suppressed the upregulated ROS levels by 65%; the same result was obtained with HT-29 cells (supplementary Figure S1A is available at Carcinogenesis Online).
Fig. 3.
OXT-922 enhances ROS levels in SW480 cells. (A) Fluorescence intensity histograms of SW480 cells loaded with DCFDA for 30 min and subjected to flow cytometry. Cell were treated with OXT-922 for 1 h and pretreated, as indicated, with 2 mM NAC for 2 h. (B–D) Similar analyses were performed using the molecular probes 4-amino-5-methylamino-2′,7′-difluorofluorescein (for NO), dihydroethidium (for intracellular ) and MitoSOX Red (for mitochondria ). The numbers in parentheses (A–D) are the corresponding geometric means of fluorescence intensity. (E) Peroxyntirte (left) and mitochondrial (right) were detected by confocal microscopy as in Materials and Methods in SW480 cells treated with or without OXT-922 (1 × IC50) for 1 h and then loaded with 5 μM MitoSOX Red or 5 μM dihydrorhodamine for 15 min.
As shown in Figure 3, we determined the levels of individual ROS using specific probes (indicated in parentheses): (i) NO (4-amino-5-methylamino-2′,7′-difluorofluorescein); (ii) intracellular superoxide anion, , (dihydroethidium); (iii) mitochondrial , (MitoSOX Red) and (iv) peroxynitrite, ONOO−, (DHR). Compared with control, OXT-922 treatment increased the intracellular levels of NO by 69% and of by 141%. Of note, mitochondrial levels of were markedly elevated in response to OXT-922, increasing 5.5-fold (the geometric mean of probe fluorescence went from 12.0 to 66.3). The elevated mitochondrial levels were confirmed using fluorescence confocal microscopy (Figure 3E, right panel). The levels of ONOO− were also elevated 128% in SW480 cells (Figure 3E, left panel). Elevation of all these ROS levels was also observed in HT-29 cells in response to OXT-922 (supplementary Figure S1 is available at Carcinogenesis Online).
OXT-922 induces redox-dependent apoptosis
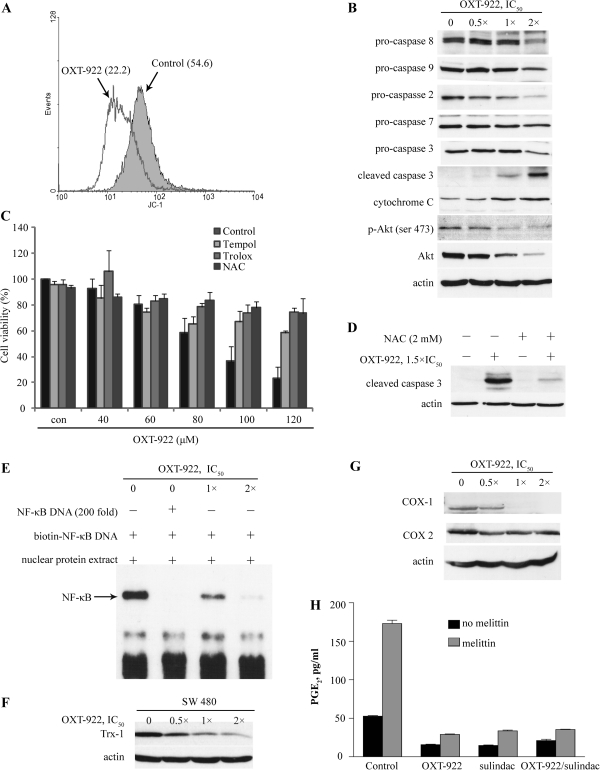
ROS accumulation is critical for the induction of apoptosis by many anticancer agents (16,22). As the levels in mitochondria were markedly enhanced by OXT-922, we first tested for the loss of mitochondrial membrane potential, which is indicative of apoptosis (23−24). To this end, we used the unique fluorescent cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide, which exhibits potential-dependent accumulation in mitochondria and forms red fluorescent J-aggregates (25). As shown in Figure 4A, treatment with OXT-922 1 × IC50 for 3 h decreased red fluorescence signal intensity by 59.4%, indicating the collapse of mitochondrial membrane potential. We then examined the status of apoptosis-related proteins in HT-29 cells treated with OXT-922. The caspase cascade was probed in a concentration-dependent manner after treatment with various concentrations of OXT-922 for 24 h (Figure 4B). Initiator caspases such as caspase-8 and -9 and the effector caspase-2, -3, and -7 (26) were cleaved and activated in HT-29 cells following OXT-922 treatment. OXT-922 also suppressed the expression levels of phosphorylated and total Akt, which is critical for cell proliferation and survival (27).
Fig. 4.
OXT-922 induces redox-dependent apoptosis and modulates NF-κB and COX signaling. (A) HT-29 cells were treated with OXT-922 (1 × IC50) for 3 h and then stained with 2 μM 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide for 30 min and fluorescence intensity was detected by flow cytometry. The numbers in parentheses are the corresponding geometric means of fluorescence intensity. (B) Immunoblots for HT-29 cells treated with the indicated concentrations of OXT-922 for 24 h. Cytochrome C was determined in the cytosolic fraction. (C) HT-29 cells were plated in 96-well plates (1 × 104 cells per well). After pretreatment with or without 2 mM NAC or 200 μM Trolox or 250 μM Tempol for 2 h, cells were treated with OXT-922 for another 36 h, and cell viability was tested by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. (D) HT-29 cells were treated with or without 2 mM NAC for 2 h and then cells were incubated with OXT-922 for an additional 24 h. Protein levels of cleaved caspase-3 were tested by western blot. (E) After a 6 h treatment with OXT-922, NF-κB activation was determined by non-radioactive electrophoretic mobility shift assay using a biotinylated NF-κB DNA probes as in Materials and Methods. (F) Cells were treated with the indicated concentrations of OXT-922 for 3 h and the Trx-1 levels were determined by immunoblot. Loading control: actin. (G) HT-29 cells were treated with the indicated concentrations of OXT-922 for 18 h and COX-1/2 levels were determined by immunoblot. (H) HT-29 cells were treated with the indicated compounds (1 × IC50) for 0.5 h and then treated with or without mellittin (10 μM) for another 2.5 h. Prostaglandin E2 levels were determined in the culture media.
Oxidative stress can modulate cell survival through multiple mechanisms (28). However, cell death can also lead to ROS enrichment (29). To distinguish the causality between OXT-922-induced ROS production and apoptosis, we evaluated the effect of antioxidants on OXT-922-induced apoptosis. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay showed that OXT-922 reduced cell viability in a concentration-dependent manner and that this effect was effectively blocked by the antioxidants NAC, tempol and trolox (30−31) (Figure 4C). NAC pretreatment also greatly blocked OXT-922-induced caspase-3 cleavage (Figure 4D) and protected cells from OXT-922-induced apoptosis (Figure 2A), indicating that oxidative stress is a major player in the induction of apoptosis by OXT-922.
The effect of OXT-922 on mitogen-activated protein kinase, thioredoxin, NF-κB and COX signaling pathways in colon cancer cells
Prior work has demonstrated the extensive effects of NSAIDs on cell signaling pathways, including mitogen-activated protein kinases, NF-κB and thioredoxin (Trx) (32–34). Thus, we explored whether OXT-922 affected these signaling pathways. The levels of phosphorylated p38, c-jun N-terminal kinase and extracellular signal-regulated kinase were not significantly affected by OXT-922 (data not shown). In contrast, the activation of NF-κB was largely inhibited by OXT-922, as revealed by the electrophoretic mobility shift assay shown in Figure 4E. Certain anticancer agents modulate NF-κB signaling through Trx oxidation and activation of apoptosis signal-regulating kinase-1 (17,35). However, OXT-922 did not alter the oxidation level of Trx-1 neither did it reduce the physical association between apoptosis signal-regulating kinase-1 and Trx-1(supplementary Figure S2 is available at Carcinogenesis Online). Instead, it directly downregulated the Trx-1 protein level (Figure 4F and supplementary Figure S3 is available at Carcinogenesis Online). Finally, OXT-922 suppressed the expression of COX-1, notably at its IC50 concentration and above, but had no effect on COX-2 expression over the same range of concentrations (Figure 4G). OXT-922, however, suppressed both the baseline and the stimulated (by melittin) production of prostaglandin E2 by HT-29 cells; the latter effect was pronounced reaching 86% (Figure 4H).
The effect of OXT-922 on polyamines; synergy with difluoromethylornithine
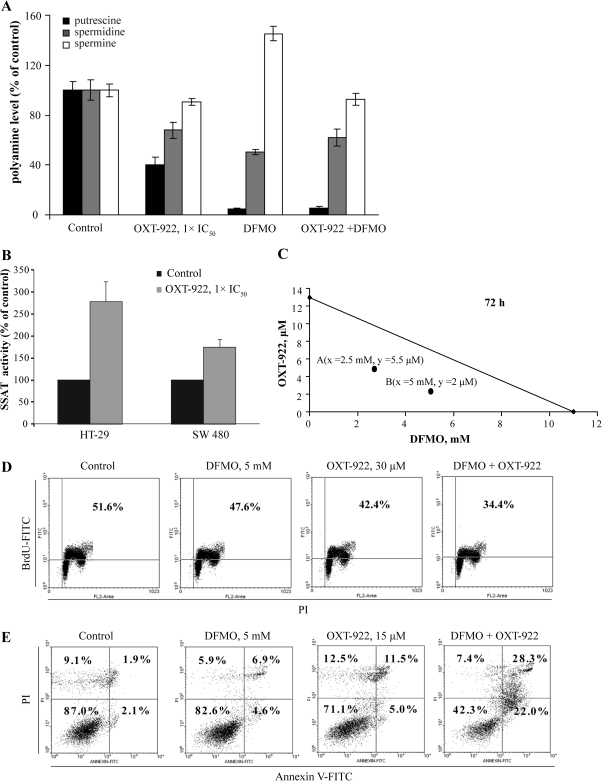
It is reported that the growth inhibitory effect of sulindac on colon cancer cells is due, in part, to its ability to change polyamine metabolism (5). The polyamines (putrescine, spermidine and spermine) are abundant polycations, which are often elevated in neoplastic cells and affect numerous processes in carcinogenesis. Polyamine levels are tightly regulated by the biosynthetic enzyme ornithine decarboxylase (36) and the catabolic enzyme SSAT (37). Sulindac enhances SSAT activity by increasing its transcription leading to suppressed polyamine content of cells (5). We, therefore, evaluated whether OXT-922 affects polyamine levels. As shown in Figure 5, compared with control, OXT-922 significantly reduced the levels of polyamines in SW480 cells, whereas its combination with difluoromethylornithine (DFMO) further decreased putrescine levels. In addition, treatment of HT-29 cells with OXT-922 1 × IC50 for 24 h enhanced SSAT activity by >150%. Similar results were obtained in SW480 cells, explaining in part the effect of OXT-922 on polyamines. However, OXT-922 enhanced ornithine decarboxylase activity in SW480 cells, which may reflect feedback by suppressed polyamine levels; this enhancement was completely inhibited by DFMO (supplementary Figure S4 is available at Carcinogenesis Online).
Fig. 5.
Synergy between difluoromethylornithine and OXT-922. (A) SW480 cells were incubated with 1 × IC50 OXT-922 for 24 h and polyamine levels were determined by high-performance liquid chromatography. Polyamine levels of control group are putrescine, 0.64 nmol/mg protein; spermidine, 4.17 nmol/mg protein and spermine, 4.19 nmol/mg protein. (B) Cells were treated with 1 × IC50 OXT-922 for 24 h and SSAT activity was determined as in Materials and Methods. Values in A and B are mean ± SEM. All differences from the corresponding controls are significant (P values <0.01). SSAT activity of control groups are 9.6 ± 2.4 (SW480) and 7.0 ± 0.4 (HT-29) pmol/mg protein/min. (C) The isobologram, based on cell viability, establishes the synergy between OXT-922 and difluoromethylornithine. The additivity line connects the IC50 value of each compound when used alone. A and B, representing two different concentration pairs of each compound (x = abscissa; y = ordinate), are well below the additivity line. (D) The BrdU incorporation assay was used to detect the de novo DNA synthesis of cells following treatment with OXT-922 and difluoromethylornithine as indicated. The number in the right upper box indicates the percentage of cells in S phase. (E) Annexin V and PI staining, as in Materials and Methods, was used to detect apoptosis in HT-29 cells after 48 h treatment with OXT-922 and difluoromethylornithine. The Annexin V(+)/PI(−) and Annexin V(+)/PI(+) (two right quadrants) are apoptotic cells. The numbers inside each box represent the percentage of cells in each category.
Given the clinically important effect of the combination of sulindac with DFMO, the most widely studied inhibitor of polyamine synthesis, on the prevention of colon cancer (38), we evaluate whether OXT-922 synergizes with DFMO to inhibit colon cancer cell growth. The isobolograms depicted in Figure 5, indeed, establish the synergy between OXT-922 and DFMO, in inhibiting cell growth evaluated both at 72 h. Each of the two concentration pairs of OXT-922 and DFMO are well below the additivity line. This synergistic effect on cell growth is brought about by synergistic effects on cell proliferation (cell renewal) and cell death. Treatment with 5 mM DFMO or 30 μM OXT-922 alone had a very modest antiproliferative effect on SW480 cells but when applied together, they suppressed the proportion of BrdU-positive cells from 51.6 to 34.4%. Similarly, 48 h treatment with 5 mM DFMO or 15 μM OXT-922 alone showed a weak proapoptotic effect, but when these agents were used together, they greatly induced apoptosis (50.3% apoptotic cells as opposed to the expected 28%).
Discussion
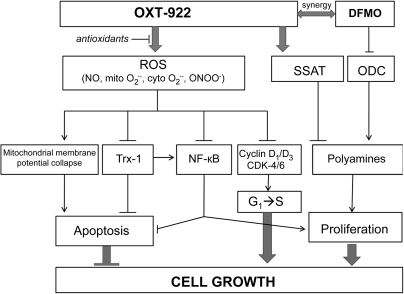
Our findings establish that OXT-922 displays a far greater growth inhibitory effect on human cancer cell lines than conventional sulindac. This effect, studied in detail in colon cancer cell lines, is brought about through two distinct effects, one on ROS-dependent cell signaling and the other on the polyamine pathway. Both of these effects culminate in strong cytokinetic changes, consisting of diminished cell proliferation, enhanced apoptosis and a block in cell cycle. The net effect is marked inhibition of cell growth. These effects are depicted in Figure 6. Of potential clinical importance was the finding that OXT-922 synergized with DFMO, the clinically applicable inhibitor of ornithine decarboxylase.
Fig. 6.
Proposed mechanism of the cancer growth inhibitory effect of OXT-922. OXT-922 induces oxidative stress and enhances SSAT activity. Oxidative stress induces apoptosis by inducing mitochondrial membrane potential collapse, downregulating Trx-1 and suppressing NF-κB activity and blocks G1–S transition through downregulating the expression of cyclin D1/D3 and CDK-4/6. Enhanced SSAT activity decreases the levels of polyamines (synergy with DFMO) and inhibits cell proliferation. Arrows, positive effect; T-shaped arrows, negative effect.
Our results reveal the uniform enhancement of OXT-922 potency compared with conventional sulindac. In the tested six cancer cell lines originating from different human tissues, the average enhancement is 19.2-fold. The six IC50s are within the fairly narrow range of 18−92 μM. Taken together, these findings suggest that OXT-922 may possess a broad spectrum of anticancer activity. In colon cancer cells, which were studied in greater detail, the growth inhibitory effect of OXT-922 is associated with G1 to S cell cycle arrest, inhibition of proliferation and induction of apoptosis. Low concentration of OXT-922 (1 × IC50) significantly reduced the proportion of cells in S phase and blocked DNA synthesis inhibiting cell renewal, whereas high concentration of OXT-922 (2 × IC50) remarkably induced cell death in >50% of the cells (1 × IC50 caused 14.8% more apoptotic cells than control group, data not shown). The relative contribution of these two effects to the overall growth inhibition may vary according to the concentration of OXT-922.
A key finding of our study is that OXT-922 is able to significantly induce ROS production in colon cancer cells and that this effect is primarily responsible for the induction of apoptosis. Prior work has revealed that sulindac induces ROS production in cancer cells (39−41). However, this effect is relatively weak, requiring prolonged exposure to high concentrations of sulindac. In contrast, OXT-922 at low concentration and within 1 h significantly enhanced ROS levels (detected with the ‘general ROS probe’ DCFDA). When we explored the effect of OXT-922 on individual ROS species, it was clear that the most pronounced effect concerned the induction of mitochondrial , which was elevated 5.5-fold compared with control. The remaining three species, NO, ONOO- and whole cell , were modestly induced (about twice of control). Based on these findings, it appears that the mitochondria are the main target of OXT-922 for ROS induction.
ROS have a dual function in living cells: they can serve either as essential signaling molecules, mainly at low concentrations, or they can harm many cell constituents, especially at high concentrations (42). Maintaining ROS homeostasis is crucial for normal cell growth and survival. In HT-29 cells, treatment with OXT-922 (1 × IC50) for 3 h collapsed the mitochondrial membrane potential, an early indication of the initiation of cellular apoptosis. Longer exposure to OXT-922 caused cleavage of caspases and evident apoptosis. The ROS scavenger NAC was able to block caspase-3 cleavage and abrogate the apoptosis induced by OXT-922. Other antioxidants like Trolox and Tempol also abrogated the cytotoxicity of OXT-922. These results confirm the essential role of ROS in OXT-922-induced apoptosis.
Prior work by us has demonstrated that derivatives of NSAIDs modulate signaling pathways, including mitogen-activated protein kinases, COX, the Trx system and NF-κB (17,33,43). Our current results show that OXT-922 exerts no effect on mitogen-activated protein kinase signaling and suppresses NF-κB activity without Trx-1 oxidation. Interestingly, OXT-922 directly downregulates the expression of Trx-1. Considering the importance of Trx-1 in cancer development (44-45), the downregulation of Trx-1 by OXT-922 may contribute to its anticancer activity, a potential mechanism that deserves further study. OXT-922 had a rather unusual effect on the COX isozymes, in that, it suppressed the expression of COX-1 while not affecting that of COX-2. The production of prostaglandin E2, the dominant PG produced by HT-29 cells, was markedly suppressed by OXT-922, especially following stimulation by melittin (3). Of note, the combination of OXT-922 with conventional sulindac had no additive effect, indicating perhaps maximal inhibition by either compound.
OXT-922 has a significant effect on the polyamine pathway, suppressing the levels of all three members (putrescine, spermidine and spermine) that were assayed. This effect is partly due to its ability to stimulate the activity of SSAT, the enzyme that acetylates spermine and spermidine and exports them form the cell. An important finding was the synergy between OXT-922 and DFMO, which was due mainly to a marked enhancement of apoptotic cell death and to a lesser extent to inhibition of proliferation.
Taken together, our findings indicate that OXT-922, a derivative of sulindac belonging to the broader pharmacological category of modified NSAIDs, displays properties that are relevant to the control of cancer. Of particular interest are (i) its cell signaling mechanism involving two pathways that bear heavily on the fate of the cancer cell and (ii) its ability to synergize with DFMO. OXT-922 is a promising novel agent worthy of further probing for its anticancer properties.
Supplementary material
Supplemental Figures S1–S4 can be found at http://carcin.oxfordjournals.org/.
Funding
National Institutes of Health (R01-CA139453).
Supplementary Material
Acknowledgments
Conflict of Interest Statement: The authors have nothing to declare except for Basil Rigas who has an equity position in Medicon Inc.
Glossary
Abbreviations
- BrdU
bromodeoxyuridine
- COX
cyclooxygenase
- DCFDA
2′,7′-dichlorodihydrofluorecein diacetate
- DFMO
difluoromethylornithine
- NAC
N-acetyl-L-cysteine
- NF-κB
nuclear factor-kappaB
- NSAID
Non-steroidal anti-inflammatory drugs
- PI
propidium iodide
- ROS
reactive oxygen species
- SSAT
spermidine/spermine N1-acetyltransferase
- Trx
Thioredoxin
- CDk
cyclin-dependent kinase
- IC50
half maximal inhibitory concentration
References
- 1.Husain SS, et al. NSAID inhibition of GI cancer growth: clinical implications and molecular mechanisms of action. Am. J. Gastroenterol. 2002;97:542–553. doi: 10.1111/j.1572-0241.2002.05528.x. [DOI] [PubMed] [Google Scholar]
- 2.Shiff SJ, et al. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs) J. Exp. Med. 1999;190:445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanif R, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, et al. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc. Natl Acad. Sci. USA. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babbar N, et al. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J. Biol. Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 6.Mahmud SM, et al. Use of non-steroidal anti-inflammatory drugs (NSAIDs) and prostate cancer risk: a meta-analysis. Int. J. Cancer. 2010 [Google Scholar]
- 7.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog. Exp. Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 8.Giardiello FM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N. Engl. J. Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan AN, et al. Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res. 2008;68:2507–2513. doi: 10.1158/0008-5472.CAN-07-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knights KM, et al. Novel mechanisms of nonsteroidal anti-inflammatory drug-induced renal toxicity. Expert Opin. Drug Metab. Toxicol. 2005;1:399–408. doi: 10.1517/17425255.1.3.399. [DOI] [PubMed] [Google Scholar]
- 11.Lazzaroni M, et al. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69:51–69. doi: 10.2165/00003495-200969010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr. Opin. Gastroenterol. 2007;23:55–59. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- 13.Piazza GA, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev. Res. (Phila Pa) 2009;2:572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiff SJ, et al. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J. Clin. Invest. 1995;96:491–503. doi: 10.1172/JCI118060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malumbres M, et al. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Rigas B, et al. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br. J. Cancer. 2008;98:1157–1160. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, et al. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandre J, et al. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 19.Woo SH, et al. Arsenic trioxide sensitizes CD95/Fas-induced apoptosis through ROS-mediated upregulation of CD95/Fas by NF-kappaB activation. Int. J. Cancer. 2004;112:596–606. doi: 10.1002/ijc.20433. [DOI] [PubMed] [Google Scholar]
- 20.Bass DA, et al. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J. Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 21.LeBel CP, et al. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 22.Trachootham D, et al. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 23.Zamzami N, et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vayssiere JL, et al. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl Acad. Sci. USA. 1994;91:11752–11756. doi: 10.1073/pnas.91.24.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smiley ST, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl Acad. Sci. USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedl SJ, et al. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy BT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 28.Fruehauf JP, et al. Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 29.Bubici C, et al. The NF-kappaB-mediated control of ROS and JNK signaling. Histol. Histopathol. 2006;21:69–80. doi: 10.14670/HH-21.69. [DOI] [PubMed] [Google Scholar]
- 30.Perez MJ, et al. Spin trapping agents (Tempol and POBN) protect HepG2 cells overexpressing CYP2E1 against arachidonic acid toxicity. Free Radic. Biol. Med. 2001;30:734–746. doi: 10.1016/s0891-5849(01)00461-0. [DOI] [PubMed] [Google Scholar]
- 31.Giulivi C, et al. Inhibition of protein radical reactions of ferrylmyoglobin by the water-soluble analog of vitamin E, Trolox C. Arch. Biochem. Biophys. 1993;303:152–158. doi: 10.1006/abbi.1993.1266. [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, et al. Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis. 2009;30:512–519. doi: 10.1093/carcin/bgp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JL, et al. NO-donating aspirin inhibits the activation of NF-kappaB in human cancer cell lines and Min mice. Carcinogenesis. 2008;29:390–397. doi: 10.1093/carcin/bgm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JL, et al. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–7618. [PubMed] [Google Scholar]
- 35.Saitoh M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woodcock-Mitchell J, et al. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J. Cell Biol. 1982;95:580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerner EW, et al. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 38.Meyskens FL, Jr., et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minami T, et al. Sulindac enhances the proteasome inhibitor bortezomib-mediated oxidative stress and anticancer activity. Clin. Cancer Res. 2005;11:5248–5256. doi: 10.1158/1078-0432.CCR-05-0085. [DOI] [PubMed] [Google Scholar]
- 40.Seo SK, et al. Combined effects of sulindac and suberoylanilide hydroxamic acid on apoptosis induction in human lung cancer cells. Mol. Pharmacol. 2008;73:1005–1012. doi: 10.1124/mol.107.041293. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti M, et al. Sulindac enhances the killing of cancer cells exposed to oxidative stress. PLoS One. 2009;4:e5804. doi: 10.1371/journal.pone.0005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Autreaux B, et al. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 43.Hundley TR, et al. Nitric oxide-donating aspirin inhibits colon cancer cell growth via mitogen-activated protein kinase activation. J. Pharmacol. Exp. Ther. 2006;316:25–34. doi: 10.1124/jpet.105.091363. [DOI] [PubMed] [Google Scholar]
- 44.Hatfield DL, et al. Selenoproteins that function in cancer prevention and promotion. Biochim. Biophys. Acta. 2009;1790:1541–1545. doi: 10.1016/j.bbagen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee A, et al. The thioredoxin system: a key target in tumour and endothelial cells. Br. J. Radiol. 2008;81 doi: 10.1259/bjr/34180435. Spec No 1, S57–S68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.