Abstract
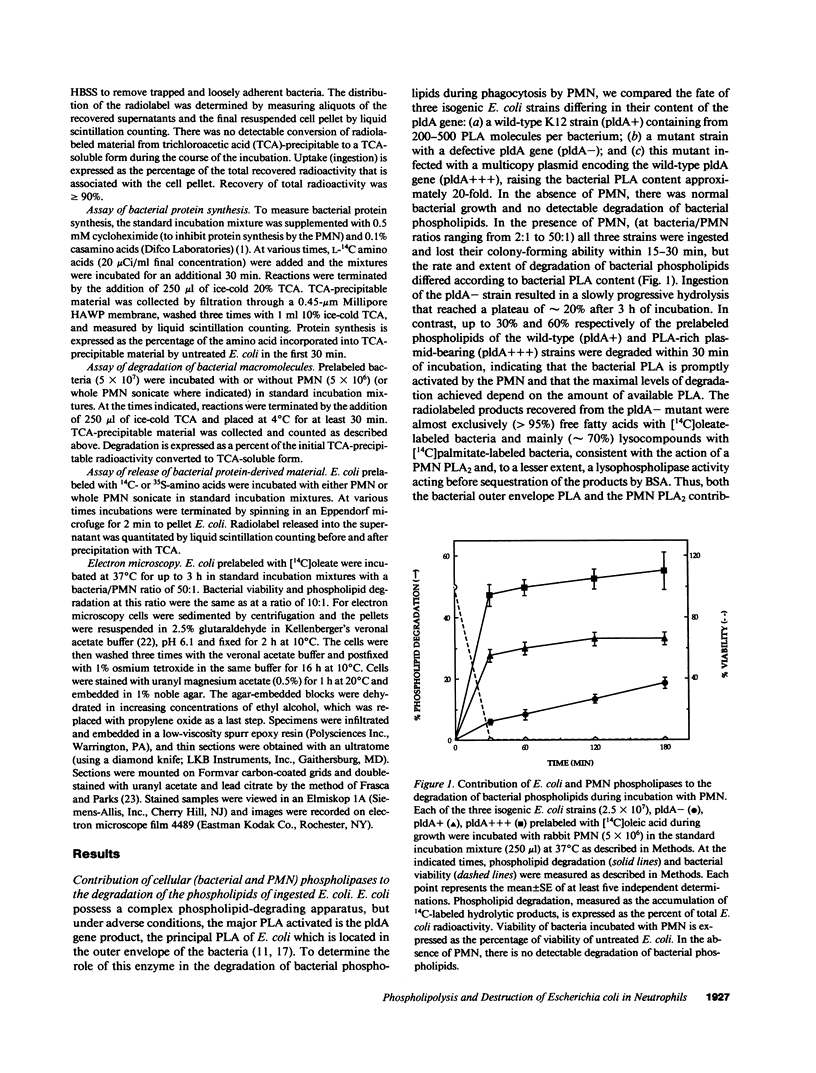
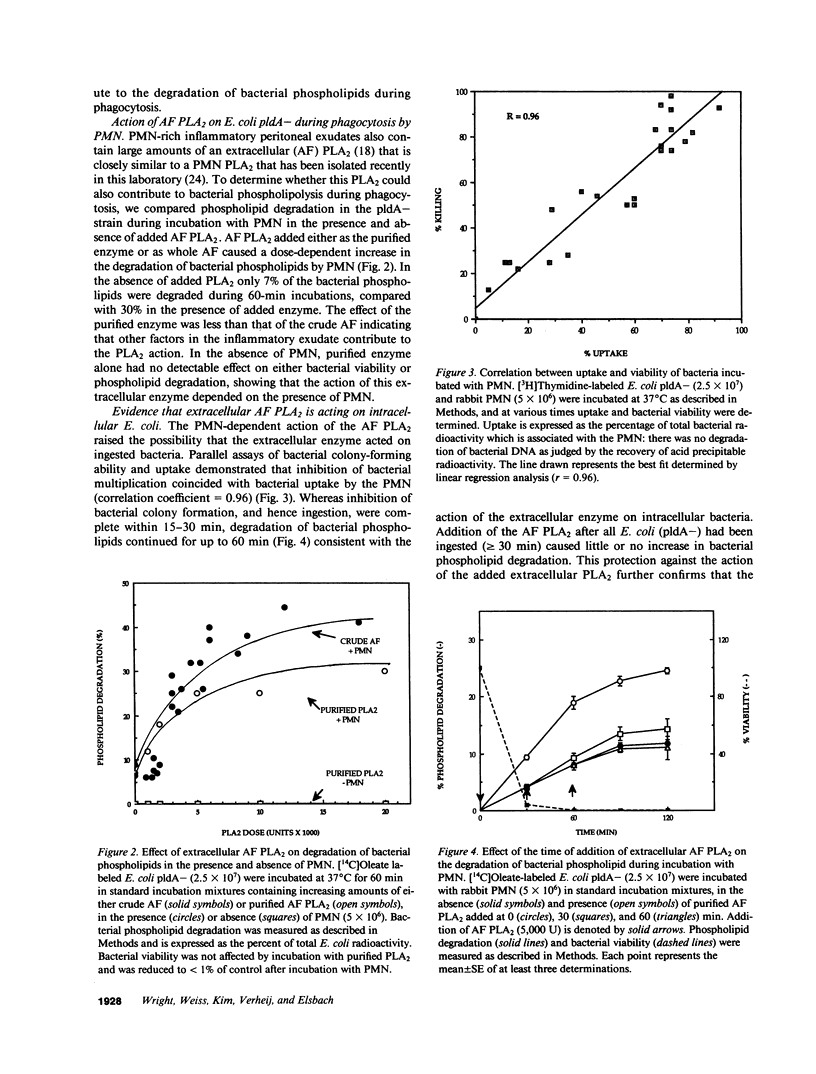
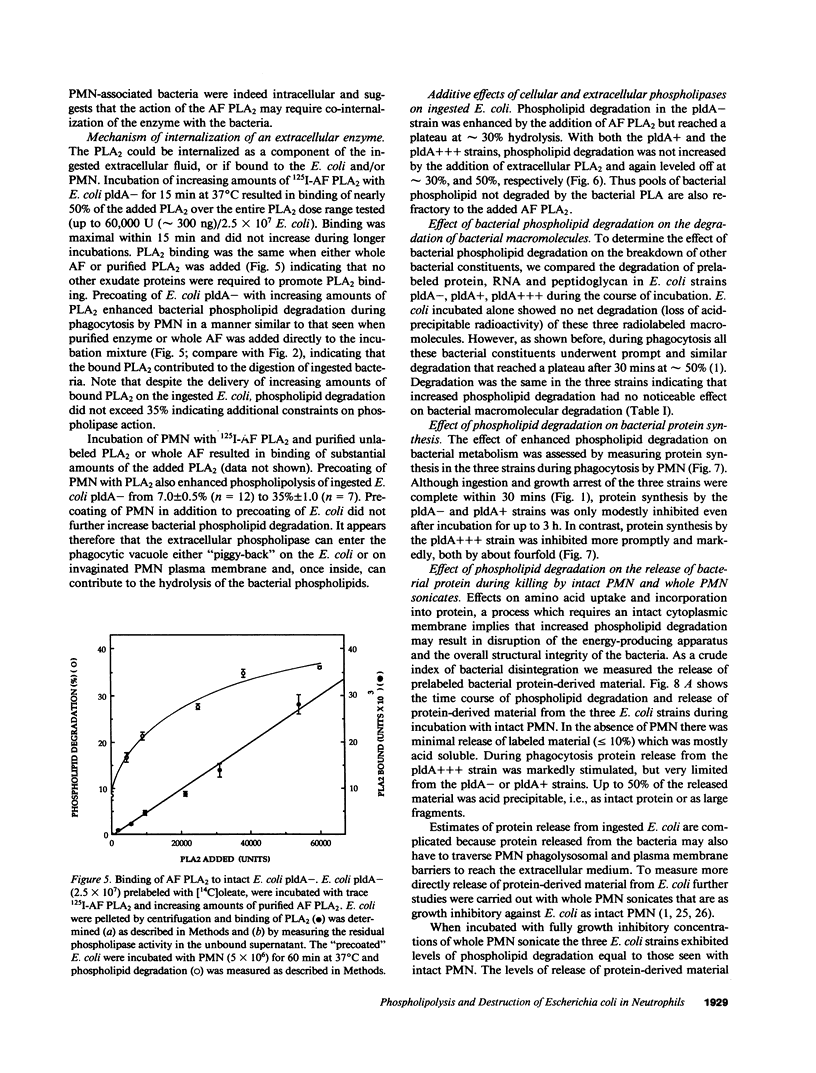
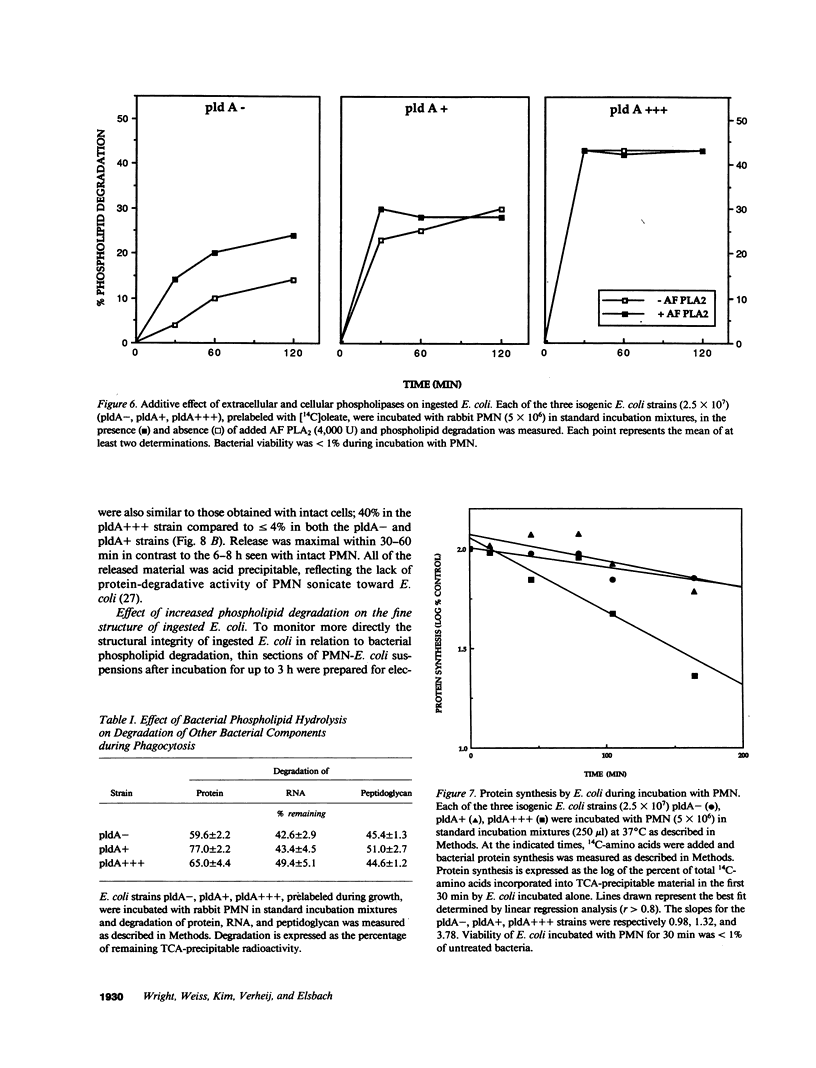
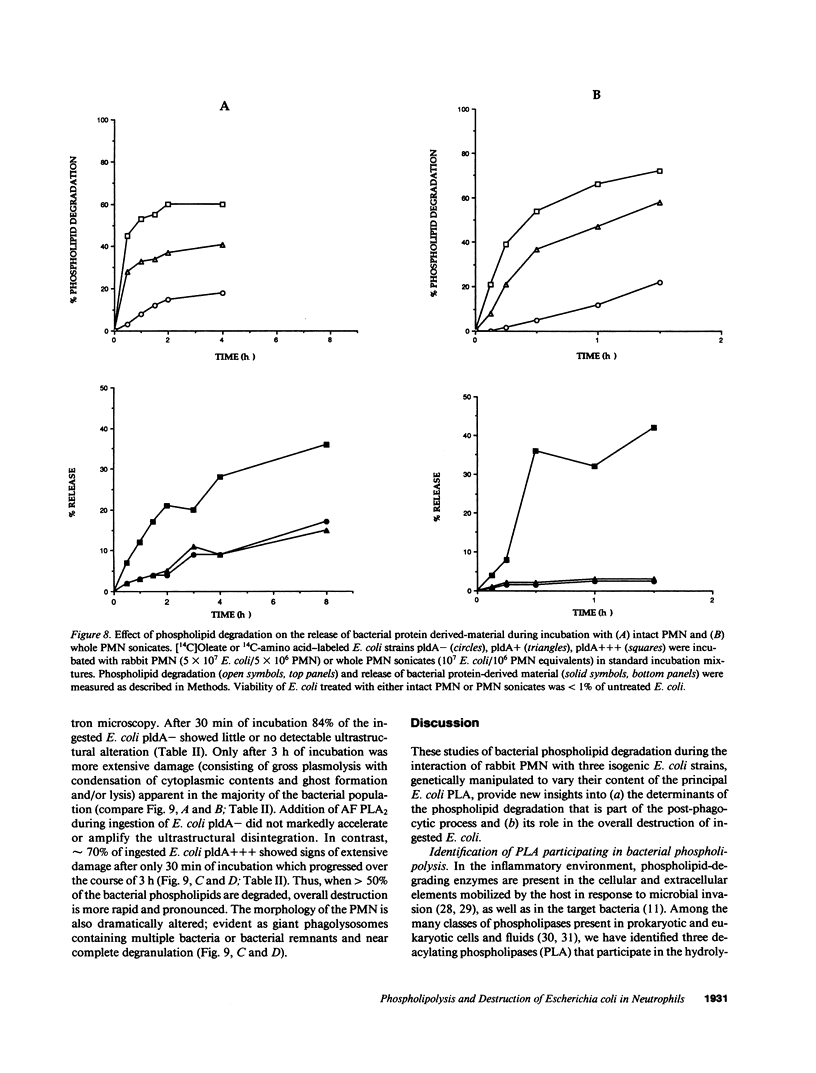
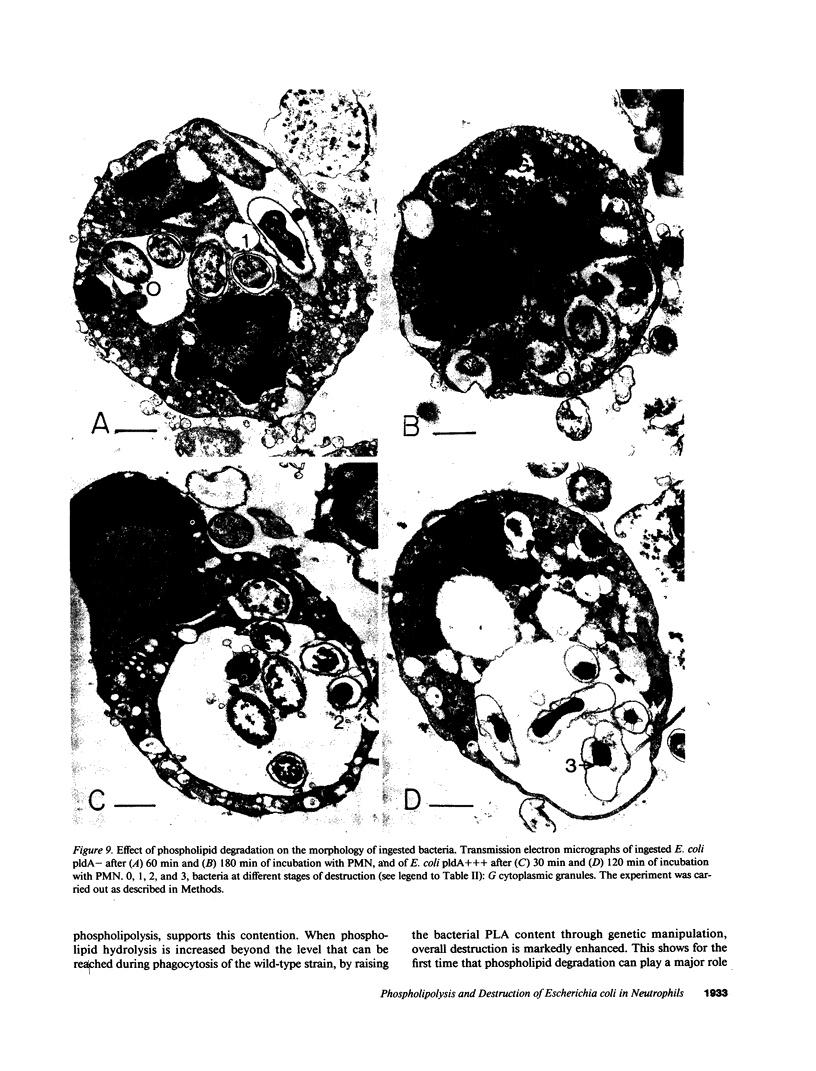
Escherichia coli ingested by PMN are promptly growth arrested but undergo limited destruction. We have studied bacterial phospholipid hydrolysis as a possible limiting factor in the disassembly of ingested E. coli, comparing the fates, during phagocytosis by rabbit peritoneal exudate PMN, of three isogenic strains, differing in their content of the pldA gene encoding the principal E. coli phospholipase A (PLA), i.e., pldA-, pldA+, pldA (the latter strain bearing the pldA gene in a multicopy plasmid resulting in a 20-fold increase in PLA content). Ingestion and growth inhibition (greater than 99% within 15 min) were the same for the three strains, but phospholipid degradation differed according to bacterial PLA content: pldA up to 60%, pldA+ up to 30%, and pldA- up to 20%. Since the pldA- strain has no activatable PLA, phospholipid degradation in this strain demonstrates the action of a PMN PLA. Added PLA2-rich ascitic fluid (AF) or purified AF PLA2 increased the rate and extent of degradation of the pldA- strain, provided the enzyme was added before ingestion was complete. 125I-AF-PLA2 binds to both E. coli and PMN and thus can enter the vacuole during phagocytosis. Although up to 50-fold more AF-PLA2 than the PLA2 content of the PMN could be loaded into the PMN in this way, degradation of pldA- E. coli did not exceed 30%. Increased phospholipid degradation had no effect on the degradation of bacterial macromolecules. In contrast, bacterial disassembly manifest as structural disorganization, release of bacterial protein derived material, and inhibition of protein synthesis were markedly enhanced when greater than 50% of prelabelled bacterial phospholipids were degraded. These findings reveal a link between envelope phospholipid degradation and overall bacterial destruction, suggesting therefore that factors limiting PLA action limit the destruction of E. coli ingested by PMN.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F. Sequential degranulation of the two types of polymorphonuclear leukocyte granules during phagocytosis of microorganisms. J Cell Biol. 1973 Aug;58(2):249–264. doi: 10.1083/jcb.58.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Nojima S. Lysophospholipase of Escherichia coli. J Biol Chem. 1975 Jul 10;250(13):5208–5214. [PubMed] [Google Scholar]
- Doi O., Nojima S. Nature of Escherichia coli mutants deficient in detergent-resistant and/or detergent-sensitive phospholipase A. J Biochem. 1976 Dec;80(6):1247–1258. doi: 10.1093/oxfordjournals.jbchem.a131396. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Beckerdite S., Pettis P., Franson R. Persistence of regulation of macromolecular synthesis by Escherichia coli during killing by disrupted rabbit granulocytes. Infect Immun. 1974 Apr;9(4):663–668. doi: 10.1128/iai.9.4.663-668.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P. Degradation of microorganisms by phagocytic cells. Rev Infect Dis. 1980 Jan-Feb;2(1):106–128. doi: 10.1093/clinids/2.1.106. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Kao L. The role of intramembrane Ca2+ in the hydrolysis of the phospholipids of Escherichia coli by Ca2+-dependent phospholipases. J Biol Chem. 1985 Feb 10;260(3):1618–1622. [PubMed] [Google Scholar]
- Elsbach P., Weiss J. Phagocytosis of bacteria and phospholipid degradation. Biochim Biophys Acta. 1988 Feb 24;947(1):29–52. doi: 10.1016/0304-4157(88)90018-4. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Weiss J., Blackburn P., Frangione B., Goni F., Elsbach P. Amino acid sequence of a basic Agkistrodon halys blomhoffii phospholipase A2. Possible role of NH2-terminal lysines in action on phospholipids of Escherichia coli. Biochemistry. 1986 Jul 29;25(15):4309–4314. doi: 10.1021/bi00363a020. [DOI] [PubMed] [Google Scholar]
- Forst S., Weiss J., Elsbach P., Maraganore J. M., Reardon I., Heinrikson R. L. Structural and functional properties of a phospholipase A2 purified from an inflammatory exudate. Biochemistry. 1986 Dec 30;25(26):8381–8385. doi: 10.1021/bi00374a008. [DOI] [PubMed] [Google Scholar]
- Forst S., Weiss J., Maraganore J. M., Heinrikson R. L., Elsbach P. Relation between binding and the action of phospholipases A2 on Escherichia coli exposed to the bactericidal/permeability-increasing protein of neutrophils. Biochim Biophys Acta. 1987 Aug 15;920(3):221–225. doi: 10.1016/0005-2760(87)90098-1. [DOI] [PubMed] [Google Scholar]
- Ginsburg I. The biochemistry of bacteriolysis: paradoxes, facts and myths. Microbiol Sci. 1988 May;5(5):137–142. [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956 May 1;103(5):589–611. doi: 10.1084/jem.103.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Chiba N., Kobayashi T., Kudo I., Inoue K., Ikeda H., Sekiguchi M., Nojima S. Characteristics of detergent-resistant phospholipase A overproduced in E. coli cells bearing its cloned structural gene. J Biochem. 1984 Dec;96(6):1645–1653. doi: 10.1093/oxfordjournals.jbchem.a134996. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Lugtenberg E. J., Peters R. Distribution of lipids in cytoplasmic and outer membranes of Escherichia coli K12. Biochim Biophys Acta. 1976 Jul 20;441(1):38–47. doi: 10.1016/0005-2760(76)90279-4. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Beckerdite S., Pettis P., Elsbach P. Phospholipid metabolism by phagocytic cells. VII. The degradation and utilization of phospholipids of various microbial species by rabbit granulocytes. Biochim Biophys Acta. 1972 Sep 7;280(1):45–56. [PubMed] [Google Scholar]
- Rozenberg-Arska M., Salters M. E., van Strijp J. A., Geuze J. J., Verhoef J. Electron microscopic study of phagocytosis of Escherichia coli by human polymorphonuclear leukocytes. Infect Immun. 1985 Dec;50(3):852–859. doi: 10.1128/iai.50.3.852-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., van Strijp J. A., Hoekstra W. P., Verhoef J. Effect of human polymorphonuclear and mononuclear leukocytes on chromosomal and plasmid DNA of Escherichia coli. Role of acid DNase. J Clin Invest. 1984 May;73(5):1254–1262. doi: 10.1172/JCI111327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Seilhamer J. J., Pruzanski W., Vadas P., Plant S., Miller J. A., Kloss J., Johnson L. K. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989 Apr 5;264(10):5335–5338. [PubMed] [Google Scholar]
- Victor M., Weiss J., Elsbach P. Heparin inhibits phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):295–299. doi: 10.1128/iai.32.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Determinants of the action of phospholipases A on the envelope phospholipids of Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):11010–11014. [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Determinants of the action of phospholipases A on the envelope phospholipids of Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):11010–11014. [PubMed] [Google Scholar]
- Weiss J., Elsbach P. The use of a phospholipase A-less Escherichia coli mutant to establish the action of granulocyte phospholipase A on bacterial phospholipids during killing by a highly purified granulocyte fraction. Biochim Biophys Acta. 1977 Apr 1;466(1):23–33. doi: 10.1016/0005-2736(77)90205-x. [DOI] [PubMed] [Google Scholar]
- Weiss J., Franson C., Schmeidler K., Elsbach P. Reversible envelope effects during and after killing of Escherichia coli w by a highly-purified rabbit polymorpho-nuclear leukocyte fraction. Biochim Biophys Acta. 1976 Jun 4;436(1):154–169. doi: 10.1016/0005-2736(76)90227-3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Kao L., Victor M., Elsbach P. Oxygen-independent intracellular and oxygen-dependent extracellular killing of Escherichia coli S15 by human polymorphonuclear leukocytes. J Clin Invest. 1985 Jul;76(1):206–212. doi: 10.1172/JCI111947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Kao L., Victor M., Elsbach P. Respiratory burst facilitates the digestion of Escherichia coli killed by polymorphonuclear leukocytes. Infect Immun. 1987 Sep;55(9):2142–2147. doi: 10.1128/iai.55.9.2142-2147.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Stendhal O., Elsbach P. Killing of gram-negative bacteria by polymorphonuclear leukocytes: role of an O2-independent bactericidal system. J Clin Invest. 1982 Apr;69(4):959–970. doi: 10.1172/JCI110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus P., van Die I., Bergmans H., Tommassen J., de Haas G. Molecular cloning of pldA, the structural gene for outer membrane phospholipase of E. coli K12. Mol Gen Genet. 1983;190(1):150–155. doi: 10.1007/BF00330338. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]