Abstract
Because of their paternal antigens, the fetus and placenta may be considered an allograft in the maternal host. Local properties of the maternal-fetal interface, the placenta and decidua basalis, are important in preventing maternal immunologic rejection of the fetoplacental allograft. However, the exact nature of these local properties remains a fundamental unsolved problem in immunology. We now report that three macrophage functions were inhibited by the substratum formed by monolayers of decidual stromal cells via a novel pathway. Solid-phase inhibitors blocked macrophage adhesion, spreading, and lysis of tumor necrosis factor-alpha-resistant P815 mastocytoma tumor cells. Inhibition was not solely attributable to an inability of macrophages to adhere to decidual substratum because there were differences in macrophage functions on this surface versus polyhema where no adherence occurred. Because macrophages play a central role in cell-mediated immunity, including allograft rejection, inhibiting their function in the decidua basalis may help prevent maternal antifetal responses.
Full text
PDF

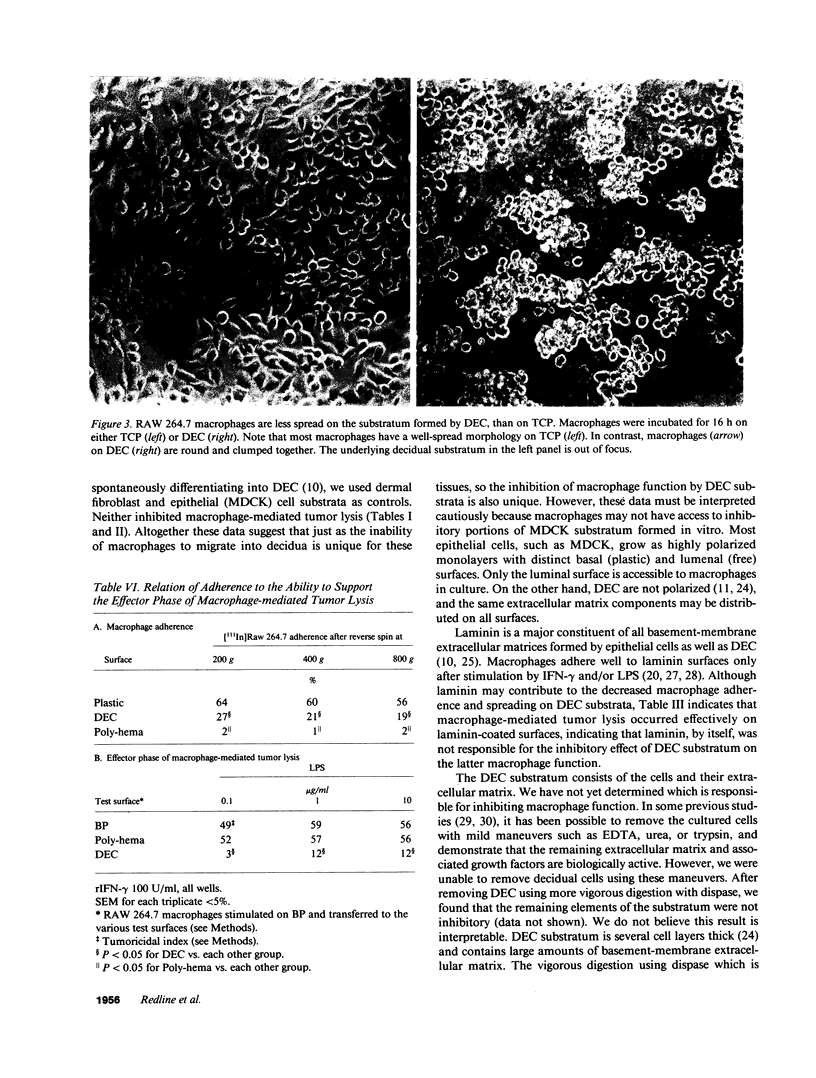


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Gifford G. E. Cell-associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987 Feb 1;138(3):957–962. [PubMed] [Google Scholar]
- Friedman A., Beller D. I. The effect of adherence on the in vitro induction of cytocidal activity by macrophages. Immunology. 1987 Aug;61(4):469–474. [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Giavazzi R., Liotta L., Hart I. Laminin inhibits the adhesion of a murine tumor of macrophage origin. Exp Cell Res. 1982 Aug;140(2):315–322. doi: 10.1016/0014-4827(82)90120-3. [DOI] [PubMed] [Google Scholar]
- Gorecka-Tisera A. M., Snowdowne K. W., Borle A. B. Implications of a rise in cytosolic free calcium in the activation of RAW-264 macrophages for tumor cell killing. Cell Immunol. 1986 Jul;100(2):411–421. doi: 10.1016/0008-8749(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Gonzalez R., Fujii D. K. Are factors originating from serum, plasma, or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol. 1983 Feb;114(2):191–202. doi: 10.1002/jcp.1041140208. [DOI] [PubMed] [Google Scholar]
- Harrell R. A., Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Suppression of the respiratory burst of human monocytes by a synthetic peptide homologous to envelope proteins of human and animal retroviruses. J Immunol. 1986 May 15;136(10):3517–3520. [PubMed] [Google Scholar]
- Head J. R., Drake B. L., Zuckermann F. A. Major histocompatibility antigens on trophoblast and their regulation: implications in the maternal-fetal relationship. Am J Reprod Immunol Microbiol. 1987 Sep;15(1):12–18. doi: 10.1111/j.1600-0897.1987.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Hunziker R. D., Wegmann T. G. Placental immunoregulation. Crit Rev Immunol. 1986;6(3):245–285. [PubMed] [Google Scholar]
- Inaba K., Kitaura M., Kato T., Watanabe Y., Kawade Y., Muramatsu S. Contrasting effect of alpha/beta- and gamma-interferons on expression of macrophage Ia antigens. J Exp Med. 1986 Apr 1;163(4):1030–1035. doi: 10.1084/jem.163.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., Marino P. A., Schreiber R. D., Adams D. O. Sequential activation of murine mononuclear phagocytes for tumor cytolysis: differential expression of markers by macrophages in the several stages of development. J Immunol. 1983 Aug;131(2):1038–1043. [PubMed] [Google Scholar]
- Johnson W. J., Whisnant C. C., Adams D. O. The binding of BCG-activated macrophages to tumor targets stimulates secretion of cytolytic factor. J Immunol. 1981 Nov;127(5):1787–1792. [PubMed] [Google Scholar]
- Levi-Schaffer F., Dayton E. T., Austen K. F., Hein A., Caulfield J. P., Gravallese P. M., Liu F. T., Stevens R. L. Mouse bone marrow-derived mast cells cocultured with fibroblasts. Morphology and stimulation-induced release of histamine, leukotriene B4, leukotriene C4, and prostaglandin D2. J Immunol. 1987 Nov 15;139(10):3431–3441. [PubMed] [Google Scholar]
- Lu C. Y., Lemay P. A., Lombardi M. J. Inhibition of antigen-specific activation of an L3T4+ T cell line by cyclosporine with maintenance of macrophage-mediated antigen presentation. Transplantation. 1988 Jan;45(1):187–194. doi: 10.1097/00007890-198801000-00039. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Lombardi M. J., Shea C. M., Dustin L. B. High strength binding of P815 mastocytoma cells is not necessary for their lysis by macrophages which have been primed and triggered in vitro. J Immunol. 1988 Aug 15;141(4):1083–1090. [PubMed] [Google Scholar]
- Lu C. Y., Redline R. W., Shea C. M., Dustin L. B., McKay D. B. Pregnancy as a natural model of allograft tolerance. Interactions between adherent macrophages and trophoblast populations. Transplantation. 1989 Nov;48(5):848–855. doi: 10.1097/00007890-198911000-00025. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Ettensohn C. A. Cell adhesion in morphogenesis. Annu Rev Cell Biol. 1987;3:319–345. doi: 10.1146/annurev.cb.03.110187.001535. [DOI] [PubMed] [Google Scholar]
- Mercurio A. M., Shaw L. M. Macrophage interactions with laminin: PMA selectively induces the adherence and spreading of mouse macrophages on a laminin substratum. J Cell Biol. 1988 Nov;107(5):1873–1880. doi: 10.1083/jcb.107.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D. Metabolism of receptor-bound and matrix-bound basic fibroblast growth factor by bovine capillary endothelial cells. J Cell Biol. 1988 Aug;107(2):753–759. doi: 10.1083/jcb.107.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J. D., Kleinfeld R. G., Morrow H. A. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat. 1983 Mar;166(3):271–298. doi: 10.1002/aja.1001660304. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Friedrich F., Lang F. Electrical properties of Madin-Darby-canine-kidney cells. Effects of extracellular sodium and calcium. Pflugers Arch. 1986 Sep;407(3):258–263. doi: 10.1007/BF00585300. [DOI] [PubMed] [Google Scholar]
- Perri R. T., Vercellotti G., McCarthy J., Vessella R. L., Furcht L. T. Laminin selectively enhances monocyte-macrophage-mediated tumoricidal activity. J Lab Clin Med. 1985 Jan;105(1):30–35. [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Localization of fetal major histocompatibility complex antigens and maternal leukocytes in murine placenta. Implications for maternal-fetal immunological relationship. Lab Invest. 1989 Jul;61(1):27–36. [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Role of local immunosuppression in murine fetoplacental listeriosis. J Clin Invest. 1987 Apr;79(4):1234–1241. doi: 10.1172/JCI112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline R. W., Lu C. Y. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J Immunol. 1988 Jun 1;140(11):3947–3955. [PubMed] [Google Scholar]
- Redline R. W., Shea C. M., Papaioannou V. E., Lu C. Y. Defective anti-listerial responses in deciduoma of pseudopregnant mice. Am J Pathol. 1988 Dec;133(3):485–497. [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Sananès N., Weiller S., Baulieu E. E., Le Goascogne C. In vitro decidualization of rat endometrial cells. Endocrinology. 1978 Jul;103(1):86–95. doi: 10.1210/endo-103-1-86. [DOI] [PubMed] [Google Scholar]
- Shaw L. M., Mercurio A. M. Interferon gamma and lipopolysaccharide promote macrophage adherence to basement membrane glycoproteins. J Exp Med. 1989 Jan 1;169(1):303–308. doi: 10.1084/jem.169.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Macrophage deactivation. Altered kinetic properties of superoxide-producing enzyme after exposure to tumor cell-conditioned medium. J Exp Med. 1986 Oct 1;164(4):1319–1331. doi: 10.1084/jem.164.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Damjanov A., Weiss J., Liotta L. A., Damjanov I. Mouse endometrial stromal cells produce basement-membrane components. Differentiation. 1986;32(1):49–58. doi: 10.1111/j.1432-0436.1986.tb00555.x. [DOI] [PubMed] [Google Scholar]