Abstract
Objective
Adequate management of increased intracranial pressure (ICP) is critical in patients with traumatic brain injury (TBI), and decompressive craniectomy is widely used to treat refractory increased ICP. The authors reviewed and analyzed complications following decompressive craniectomy for the management of TBI.
Methods
A total of 89 consecutive patients who underwent decompressive craniectomy for TBI between February 2004 and February 2009 were reviewed retrospectively. Incidence rates of complications secondary to decompressive craniectomy were determined, and analyses were performed to identify clinical factors associated with the development of complications and the poor outcome.
Results
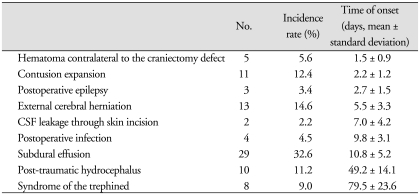
Complications secondary to decompressive craniectomy occurred in 48 of the 89 (53.9%) patients. Furthermore, these complications occurred in a sequential fashion at specific times after surgical intervention; cerebral contusion expansion (2.2 ± 1.2 days), newly appearing subdural or epidural hematoma contralateral to the craniectomy defect (1.5 ± 0.9 days), epilepsy (2.7 ± 1.5 days), cerebrospinal fluid leakage through the scalp incision (7.0 ± 4.2 days), and external cerebral herniation (5.5 ± 3.3 days). Subdural effusion (10.8 ± 5.2 days) and postoperative infection (9.8 ± 3.1 days) developed between one and four weeks postoperatively. Trephined and post-traumatic hydrocephalus syndromes developed after one month postoperatively (at 79.5 ± 23.6 and 49.2 ± 14.1 days, respectively).
Conclusion
A poor GCS score (≤ 8) and an age of ≥ 65 were found to be related to the occurrence of one of the above-mentioned complications. These results should help neurosurgeons anticipate these complications, to adopt management strategies that reduce the risks of complications, and to improve clinical outcomes.
Keywords: Decompressive craniectomy, Traumatic brain injury, Complication
INTRODUCTION
Traumatic head injury is an important cause of intracranial hypertension1). The aims of the management of traumatic intracranial hypertension are the control of intracranial pressure (ICP), and the maintenance of cerebral perfusion pressure (CPP) and cerebral blood flow (CBF) to prevent cerebral ischemia. Severe intracranial hypertension does not respond to medical management in 10 to 15% of head injured patients1,16,23) and decompressive craniectomy appears to be a reasonable alternative treatment in these patients, but the long-term functional outcomes of this method when used to treat an elevated ICP (ICP > 20 mmHg) remain unknown. Nevertheless, several authors have reported a gradual decrease in mortality and an increase in the proportion of patients that achieve a good outcome after decompressive craniectomy1) and others have concluded that surgical decompression may improve oxygen delivery to brain tissue, cerebral compliance, CPP, and CBF, and reduce swelling, and thus, improve clinical outcomes3,8,19,26,28). The surgical method is relatively straightforward, but postoperative complications can severely affect clinical outcome. Here, we retrospectively analyzed complications following decompressive craniectomy in patients with traumatic brain injury (TBI) and sought to identify the clinical risk factors related to poor outcome.
MATERIALS AND METHODS
Study characteristics
We retrospectively investigated the medical records on patients with TBI who underwent decompressive craniectomy between February 2004 and February 2009.
Surgical indications and procedure
Surgical indications for decompressive craniectomy were included : 1) the appearance of unilateral or bilateral brain swelling and a midline shift of at least 5 mm on CT scans with a poor initial GCS score (≤ 8); 2) neurological worsening (a decrease of GCS score of ≥ 2 points) and aggravation of pupillary response to light during initial medical therapy; 3) bilateral fixed pupils with an intact brain stem reflex; 4) therapy-resistant increases in ICP to > 25 mmHg in patients undergoing ICP monitoring; 5) a swollen brain despite hematoma evacuation. Surgical decompression was not performed in any patient without brain stem reflex.
Patients received decompressive craniectomy, hematoma removal, augmentative duraplasty, and decompressive lobectomy if necessary. Decompressive craniectomy was performed by removing a large portion of the frontotemporoparietal cranium (> 12 cm) for lesions confined to one cerebral hemisphere. Patients with bifrontal or anterior cranial fossa lesions underwent bilateral frontal craniectomy from the anterior cranial fossa to the coronal suture. After craniectomy, epidural hematoma (EDH) and subdural hematoma (SDH) were evacuated when present. Brain parenchymal hemorrhagic contusion was removed in cases with persistent and significant brain swelling after craniectomy and hematoma evacuation.
Diagnosis of complications
Several complications occurred after decompressive craniectomy, such as, contusion expansion, postoperative epilepsy, external cerebral herniation, intracranial hematoma contralateral to the craniectomy defect, subdural effusion, CSF leakage through the skin incision, postoperative infection, post-traumatic hydrocephalus, and syndrome of the trephined.
The following criteria were used to diagnose these complications following decompressive craniectomy. Contusion expansion was defined as an enlarged non-hemorrhagic or hemorrhagic contusion ipsilateral or contralateral to the decompressed hemisphere on serial cranial CT scans. Subdural effusion was defined as a newly appearing subdural fluid collection on serial cranial CT scans. External cerebral herniation was defined as more than 1.5 cm of herniated brain tissue through the craniectomy in the center of the skull defect, as previously described by Yang et al.26). Post-operative epilepsy was defined as one or more postoperative epilepsy attack. Post-traumatic hydrocephalus was defined as; 1) ventricular dilation not due to brain atrophy; 2) onset within 6 months after surgery; and 3) neurological deterioration. Syndrome of the trephined was defined as the following profile; 1) newly appearing symptoms including headache, irritability, memory problems, mood disturbances, or neurological deficits following decompressive craniectomy; with 2) a sunken parenchymal contour on the skull defect side by brain CT.
Assessment of neurological outcomes
Neurological outcomes were evaluated using the Glasgow outcome scale (GOS) as follows : 1) death; 2) vegetative state; 3) severe disability; 4) moderate disability; and 5) good recovery. Clinical outcomes were analyzed with respect to initial GCS score, age, pupillary response to light, and the midline shift by cranial CT scan at admission. In addition, we compared the incidences of complications among different patient subgroups to identify risk factors affecting complications.
Statistical analysis
Data were analyzed using SPSS 14.0 for Windows (SPSS, Inc., Chicago, IL, USA). Pearson's chi-square test, Fisher's exact test and logistic regression analysis were used to calculate the significances of differences. Statistical significance was accepted for p values of < 0.05.
RESULTS
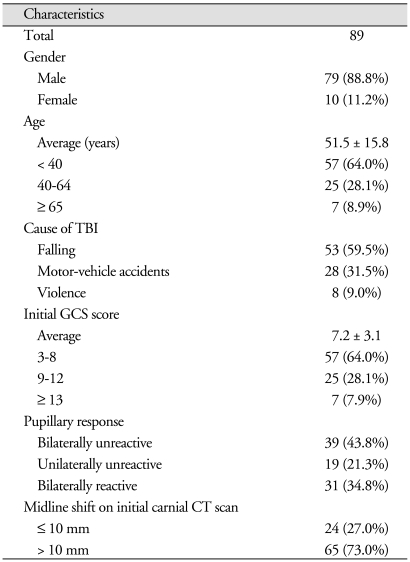
Eighty-nine patients (79 men and 10 women), mean age of 51.4 years old (range, 4-82 years old), underwent decompressive craniectomy. The most common cause of head injury was fall (n = 53, 59.5%), and other causes were motor-vehicle accidents (n = 28, 31.5%) and violence (n = 8, 9%). The mean preoperative GCS score was 7/15 (range, 3-14). Bilaterally dilated unreactive pupils were seen in 39 patients (43.8%), a dilated unreactive pupil was seen in 19 patients (21.3%), and bilaterally reactive pupils were seen in 31 patients (34.8%). The midline shift of ≤ 10 mm on initial cranial CT scans was seen in 24 patients (27.0%) (Table 1).
Table 1.
Complications associated with decompressive craniectomy for TBI
Complications associated with decompressive craniectomy occurred in 48 of the 89 patients (53.9%), and 21 (23.6%) patients developed more than one complication. Each complication following decompressive craniectomy showed a tendency to occur within a specific time window after surgery (Table 1). We divided complications into three subgroups according to onset time after surgery; early (within 1 week), late (from day 8 to 30) and delayed (> 1 month).
Early complications
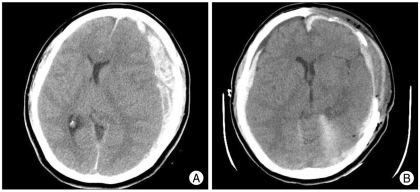
Hematoma contralateral to the decompressive craniectomy defect occurred in 5 patients and was the earliest complication to develop (1.5 ± 0.9 days) (Fig. 1). Reoperation was performed in 2 patients due to significant mass effect and neurological deterioration.
Fig. 1.
A : Initial brain CT image showing left traumatic subdural hematoma. B : Brain CT after decompressive craniectomy showing a new hematoma contralateral to the craniectomy defect.
Contusion expansion occurred in 11 of 48 patients (2.2 ± 1.2 days) and 4 of these underwent hematoma evacuation to reduce ICP. Furthermore, despite preventive antiepileptic medication, post-traumatic epilepsy occurred in 3 patients (2.7 ± 1.5 days).
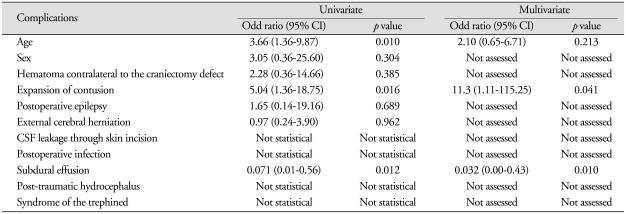
External cerebral herniation occurred in 13 patients (5.5 ± 3.3 days) and CSF leakage in 2 patients (7.0 ± 4.2 days). These complications resolved spontaneously (Fig. 2).
Fig. 2.
Brain CT image demonstrating external cerebral herniation.
Late complications (from postoperative 8 to 30 days)
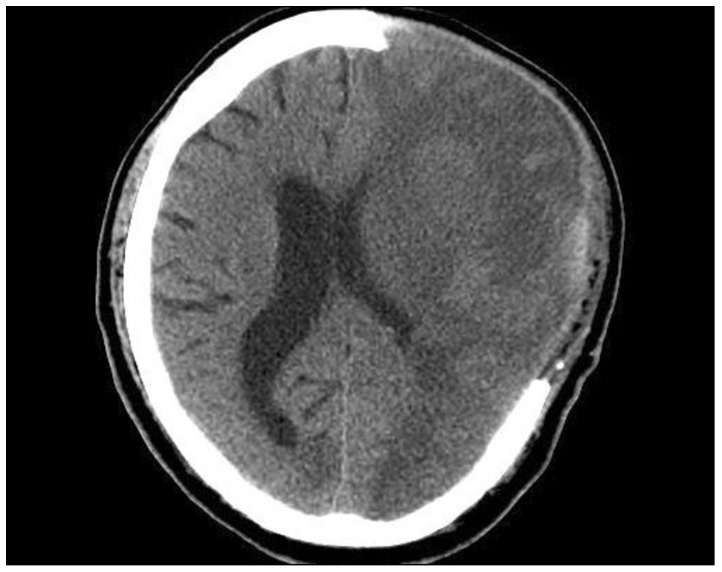
Postoperative infection occurred in 4 patients (9.8 ± 3.1 days) and was treated by antibiotics. Subdural effusion occurred in 29 patients within 15 days (10.8 ± 5.2 days) (Fig. 3) and was the most common complication encountered. Most of these lesions resolved spontaneously. However, surgery was necessary on one case because of the neurological deficit.
Fig. 3.
Brain CT image of postoperative subdural effusion.
Delayed complications
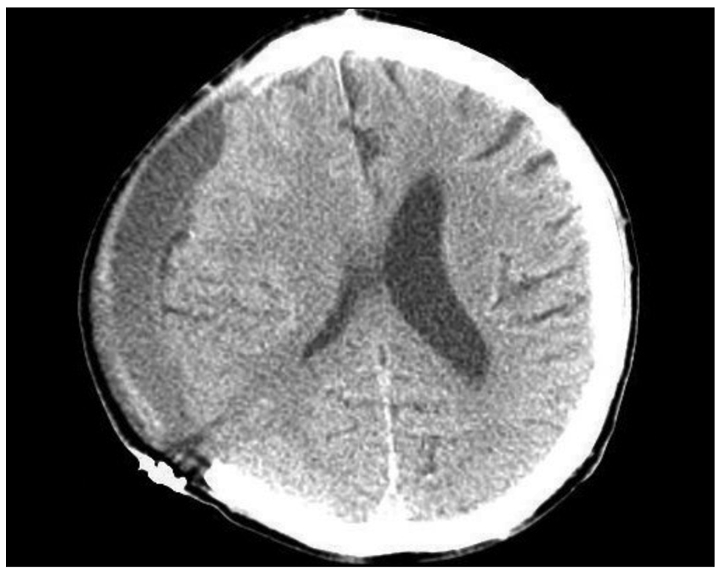
Post-traumatic hydrocephalus occurred in 10 patients (49.2 ± 14.1 days) (Fig. 4) and a venticuloperitoneal (VP) shunt was placed in all. Syndrome of the trephined occurred in 8 patients (79.5 ± 23.6 days) (Fig. 5), and these patients improved after cranioplasty.
Fig. 4.
Brain MR image showing post-traumatic hydrocephalus.
Fig. 5.
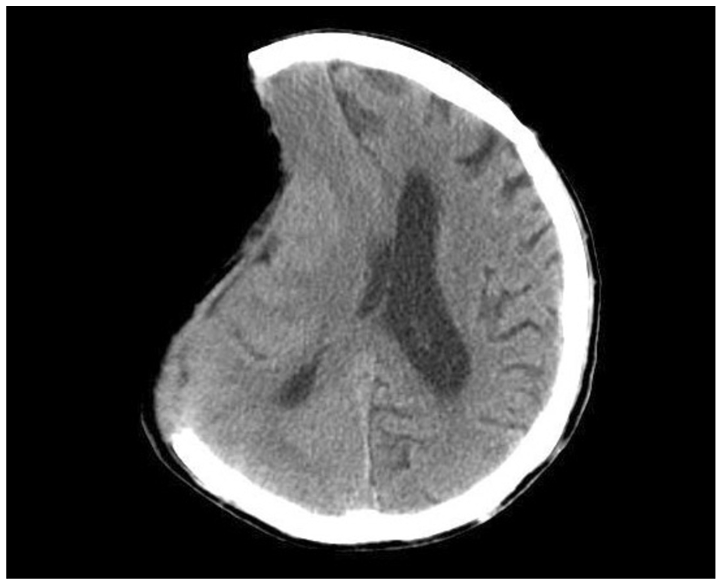
Brain CT image showing a sinking scalp flap.
Relations between complications and clinical factors
Patients were divided into three groups according to initial GCS score; group 1 (GCS 3-8), group 2 (9-12) and group 3 (≥ 13). Group 1 contained 57 (64.0%) patients, group 2 contained 22 (28.1%) and group 3 contained 7 (7.9%). We also divided patients into three groups by age; group I (< 40 years old) contained 57 (64.0%), group II (40-64 years old) 25 (28.1%) and group III (≥ 65 years old) 7 (8.9%) patients (Table 2). It was found that patients with lower GCS score at ad-mission (p = 0.048) and older patients (≥ 65; p = 0.042) had significantly higher complication incidence rates. Furthermore, 41 patients without complications achieved functional recovery (GOS 4-5) after surgery (p = 0.048).
Table 2.
Clinical characteristics of patients with TBI
Clinical outcomes
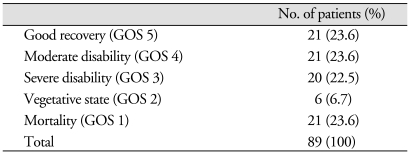
The clinical outcomes were determined using GOS score. Final outcomes were evaluated at postoperative 6 months or at death. Twenty-one of 89 patients who underwent decompressive craniectomy died within 1 month of surgery (GOS 1). Of these, 15 patients died of intractable intracranial hypertension and six patients succumbed to other medical problems. Of the 68 survivors after decompressive craniectomy, 42 patients achieved functional recovery (GOS 4 & 5) and 26 non-functional recovery (GOS 2 & 3) (Table 3). Our analysis showed that patients with the poor GCS score (≥ 8) at admission (p < 0.001), older patients (≥ 65, p = 0.010), patients with the midline shift exceeding 10 mm (p < 0.05) and patients with bilaterally unreactive pupils (p < 0.005) showed worse clinical outcome rates.
Table 3.
Clinical outcome at postoperative 6 months
We performed univariate and multivariate analysis to examine relations between postoperative outcomes and postoperative complications. Complications with a p value of < 0.1 by univariate analysis were subjected to multivariate analysis. Contusion expansion (p = 0.010) and age (p = 0.016) were found to be significantly predictive of postoperative death, and subdural effusion (p = 0.012) was found to be significantly associated with low risk of postoperative death. However, multivariate analysis revealed that only contusion expansion and subdural effusion were significantly associated with clinical outcome (Table 4).
Table 4.
The relation between postoperative outcome and postoperative complications (univariate & multivariate analysis)
Complications with a p value of < 0.1 by univariate analysis were subjected to multivariate analysis
DISCUSSION
Traumatic brain injury is one of the most common causes of death among young people in the industrialized countries6,24). Marked ICP elevation, which causes cerebral ischemia and secondary brain damage, is difficult to treat by medical management alone6,9,20,21) and thus, in these cases, neurosurgeons generally perform decompressive craniectomy to reduce ICP and prevent additional brain damage. Furthermore, many studies on decompressive craniectomy have shown that it improves clinical outcomes1,2,7,11,13,14,17,18,25). Nevertheless, there is no prospective controlled randomized trial to establish that decompressive craniectomy for TBI actually improve quality of life29) and long-term functional outcomes following this procedure for intractable intracranial hypertension have not been proved.
The purpose of treating traumatic brain injury is to prevent secondary injury and the establishment of a vicious cycle. However, decompressive craniectomy can be accompanied by several complications, which can adversely affect clinical outcome. The majority of these complications develop from normal pathophysiologic changes in ICP, CSF circulation and CBF following the removal of a large portion of skull. Several surgical complications were examined in the present study, whereas the majority of previous studies have concentrated on the management of increased ICP and clinical outcome, and relatively few have detailed the complications of decompressive craniectomy1,15,27,29,30). In previous studies, the most common complication of decompressive craniectomy for TBI was subdural hygroma, and the majority of patients affected resolved spontaneously1,15,27,29,30) which concurs with our findings.
Early complications
Contusion expansion or a new intracranial hematoma could develop after decompressive craniectomy contralateral or remote to the decompressed hemisphere. This complication may occur because of a reduction or loss of the tamponade effect10,22,27) and may develop early after decompression27,29). Appropriate management based on close monitoring and early detection is the key to proper management.
The mechanism of postoperative epilepsy is still not fully understood, but graded increases in hyperexcitability and a reduced epileptogenic threshold have been suggested to be potential causes of seizure5). Prophylactic antiepileptics may prevent postoperative epilepsy, and we adopted this management strategy. As has been reported previously29), 3 of our patients (3.4%) developed postoperative epilepsy. However, this complication disappeared in all after increasing the dosage and/or adding other antiepileptics.
The mechanism of external cerebral herniation has been attributed to brain edema27). External cerebral herniation may cause cortical vein compression and cause cortical laceration resulting in venous infarction of herniated brain tissue27,29) and cortical damage. Large craniectomies with augmentative duraplasty allow the brain to expand outward without constriction, and thus, minimize the risk of venous infarction27,29). In the present study, this complication occurred in 13 patients (14.6%) although craniectomy of larger than 12 cm in diameter had been performed. However, surgical intervention, such as, decompressive lobectomy, was not used in any patient because of the lack of postoperative neurological deterioration, medically responsive ICP, and a CPP of larger than 70 mmHg. Furthermore, external cerebral herniation disappeared in all with time without surgical intervention. In addition, our analysis revealed that external cerebral herniation is unlikely to affect postoperative outcome, especially when a large craniectomy is performed.
CSF leakage occurred in 2 patients (2.2%) through small wound dehiscence, but was resolved by simple suture placement.
Late complications
Subdural effusion is known to be caused by traumatic rupture of the dura-arachnoid interface and a resultant change in the dynamics of the CSF circulation4,27,29,30). In a recent report29), subdural effusion occurred at 13.3 ± 9 days postoperatively and was the most common complication after decompressive craniectomy, which concurs with our findings. In our series, this complication resolved spontaneously in 28 of the 29 patients affected; the other patient had progressive neurological deterioration but recovered after surgical intervention.
Postoperative CNS infection can increase morbidiy and mortality. S. epidermidis which is a major inhabitant of the skin has been reported to be the pathogen most commonly associated with postoperative infections and31). As previously described, clean skin preparation and prophylactic antibiotics are important for preventing these infections31). In the present study, 4 patients developed this complication and all responded to antibiotics.
Delayed complications
Posttraumatic hydrocephalus develops when CSF flow is perturbed and the CSF circulation fails to normalize27,29). In the present study, 10 patients (11.2%) developed this complication and all underwent a VP shunt operation.
The syndrome of the trephined was first described by Grant and Norcross in 1939, who detailed its symptoms of headache, seizures, mood swings and behavioral disturbances12). After decompressive craniectomy, the scalp above the bone defect sinks because of a lack of bone support, which transmits atmospheric pressure directly to the brain, reduces the subarachnoid space, and exerts pressure on the underlying cortex, which perturbs CSF circulation and cerebral blood flow. Cranioplasty is beneficial in cases with syndrome of the trephined, and early cranioplasty after decompressive craniectomy has been recommended27,29,30). In the present study, 8 patients (9.0%) developed this complication and symptoms in all were much improved after cranioplasty.
In the present study, multivariate analysis showed patients without complications were more likely to achieve functional recovery (GOS 4-5) after surgery, and that the development of contusion expansion significantly indicated worse outcome. Therefore, it is important to prevent these complications and to detect them at the earliest opportunity after decompressive craniectomy.
The retrospective, non-randomized, non-controlled nature of this study, and the small number of patients recruited limit our ability to draw firm conclusions. Nevertheless, this study does show that complications following decompressive craniectomy have specific onset times and that some complications can affect postoperative outcome.
CONCLUSION
Complications following decompressive craniectomy for TBI were found to occur at specific times, and the poor GCS score (≤ 8) and the older age (≥ 65) were found to be related to the occurrence of these complications. In addition, the lack of complications was found to be significantly associated with functional recovery (GOS 4-5) after surgery, and contusion expansion was found to affect postoperative outcome significantly. These results should allow neurosurgeons to better anticipate when complications are likely to develop and adopt to appropriate management strategies.
References
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–479. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.Albanèse J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury : Evaluation of the effects at one year. Crit Care Med. 2003;31:2535–2538. doi: 10.1097/01.CCM.0000089927.67396.F3. [DOI] [PubMed] [Google Scholar]
- 3.Boret H, Fesselet J, Meaudre E, Gaillard PE, Cantais E. Cerebral microdialysis and P(ti)O2 for neuro-monitoring before decompressive craniectomy. Acta Anaesthesiol Scand. 2006;50:252–254. doi: 10.1111/j.1399-6576.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 4.Carvi Y Nievas MN, Hollerhage HG. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF-circulation disorders. Neurol Res. 2006;28:139–144. doi: 10.1179/016164106X98008. [DOI] [PubMed] [Google Scholar]
- 5.Chen JW, Ruff RL, Eavery R, Wasterlain CG. Posttraumatic epilepsy and treatment. J Rehabil Res Dev. 2009;46:685–696. doi: 10.1682/jrrd.2008.09.0130. [DOI] [PubMed] [Google Scholar]
- 6.Chibbaro S, Tacconi L. Role of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure. Our experience with 48 cases. Surg Neurol. 2007;68:632–638. doi: 10.1016/j.surneu.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 7.Cooper PR, Rovit RL, Ransohoff J. Hemicraniectomy in the treatment of acute subdural hematoma : a re-appraisal. Surg Neurol. 1976;5:25–28. [PubMed] [Google Scholar]
- 8.Danish SF, Barone D, Lega BC, Stein SC. Quality of life after hemicraniectomy for traumatic brain injury in adults. A review of the literature. Neurosurg Focus. 2009;26:E2. doi: 10.3171/2009.3.FOCUS945. [DOI] [PubMed] [Google Scholar]
- 9.DeWitt DS, Jenkins LW, Prough DS. Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz. 1995;3:376–383. [PubMed] [Google Scholar]
- 10.Flint AC, Manley GT, Gean AD, Hemphill JC, 3rd, Rosenthal G. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;25:503–512. doi: 10.1089/neu.2007.0442. [DOI] [PubMed] [Google Scholar]
- 11.Gaab MR, Rittierodt M, Lorenz M, Heissler HE. Traumatic brain swelling and operative decompression : a prospective investigation. Acta Neurochir Suppl (Wien) 1990;51:326–328. doi: 10.1007/978-3-7091-9115-6_110. [DOI] [PubMed] [Google Scholar]
- 12.Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488–512. doi: 10.1097/00000658-193910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling : indications and results. J Neurosurg. 1999;90:187–196. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 14.Huang AP, Tu YK, Tsai YH, Chen YS, Hong WC, Yang CC, et al. Decompressive craniectomy as the primary surgical intervention for hemorrhagic contusion. J Neurotrauma. 2008;25:1347–1354. doi: 10.1089/neu.2008.0625. [DOI] [PubMed] [Google Scholar]
- 15.Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury : a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623–628. doi: 10.1089/neu.2005.22.623. [DOI] [PubMed] [Google Scholar]
- 16.Juul N, Morris GF, Marshall SB, Marshall LF The Executive Committee of the International Selfotel Trial. Intracranial hypertension and cerebral perfusion pressure : influence on neurological deterioration and outcome in severe head injury. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 17.Kim KH. Predictors for functional recovery and mortality of surgically treated traumatic acute subdural hematoma in 256 patients. J Korean Neurosurg Soc. 2009;45:143–150. doi: 10.3340/jkns.2009.45.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjellberg RN, Prieto A., Jr Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488–493. doi: 10.3171/jns.1971.34.4.0488. [DOI] [PubMed] [Google Scholar]
- 19.Kontopoulos V, Foroglou N, Patsalas J, Magras J, Foroglou G, Yiannakou-Pephtoulidou M, et al. Decompressive craniectomy for the management of patients with refractory hypertension : should it be reconsidered. Acta Neurochir Wien. 2002;144:791–796. doi: 10.1007/s00701-002-0948-z. [DOI] [PubMed] [Google Scholar]
- 20.Kunze E, Meixensberger J, Janka M, Sorensen N, Roosen K. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998;71:16–18. doi: 10.1007/978-3-7091-6475-4_5. [DOI] [PubMed] [Google Scholar]
- 21.Langfitt TW, Tannanbaum HM, Kassell NF. The etiology of acute brain swelling following experimental head injury. J Neurosurg. 1966;24:47–56. doi: 10.3171/jns.1966.24.1.0047. [DOI] [PubMed] [Google Scholar]
- 22.Makino H, Yamaura A. Assessment of outcome following large decompressive craniectomy in management of serious cerebral contusion. A review of 207 cases. Acta Neurochir Suppl (Wien) 1979;28:193–194. doi: 10.1007/978-3-7091-4088-8_46. [DOI] [PubMed] [Google Scholar]
- 23.Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I : the significance of intracranial pressure monitoring. J Neurosurg. 1979;50:20–25. doi: 10.3171/jns.1979.50.1.0020. [DOI] [PubMed] [Google Scholar]
- 24.Martins ET, Linhares MN, Sousa DS, Schroeder HK, Meinerz J, Rigo LA, et al. Mortality in severe traumatic brain injury : a multivariated analysis of 748 Brazilian patients from Florianópolis City. J Trauma. 2009;67:85–90. doi: 10.1097/TA.0b013e318187acee. [DOI] [PubMed] [Google Scholar]
- 25.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–92. doi: 10.1097/00006123-199707000-00018. discussion 92-94. [DOI] [PubMed] [Google Scholar]
- 26.Sahuquillo J, Arikan F. Decompressive craniectomy for the treatment of refractory high intracranial pressure in traumatic brain injury. Cochrane Database Syst Rev. 2006:CD003983. doi: 10.1002/14651858.CD003983.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Stiver SI. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26:E7. doi: 10.3171/2009.4.FOCUS0965. [DOI] [PubMed] [Google Scholar]
- 28.Yamakami I, Yamaura A. Effects of decompressive craniectomy on regional cerebral blood flow in severe head trauma patients. Neurol Med Chir (Tokyo) 1993;33:616–620. doi: 10.2176/nmc.33.616. [DOI] [PubMed] [Google Scholar]
- 29.Yang XF, Wen L, Shen F, Li G, Lou R, Liu WG, et al. Surgical complications secondary to decompressive craniectomy in patients with a head injury : a series of 108 consecutive cases. Acta Neurochir Wien. 2008;150:1241–1247. doi: 10.1007/s00701-008-0145-9. discussion 1248. [DOI] [PubMed] [Google Scholar]
- 30.Yang XJ, Hong GL, Su SB, Yang SY. Complications induced by decompressive craniectomies after traumatic brain injury. Chin J Traumatol. 2003;6:99–103. [PubMed] [Google Scholar]
- 31.Zunt JR. Infections of the central nervous system in the neurosurgical patient. Handb Clin Neurol. 2010;96:125–141. doi: 10.1016/S0072-9752(09)96009-2. [DOI] [PubMed] [Google Scholar]