Abstract
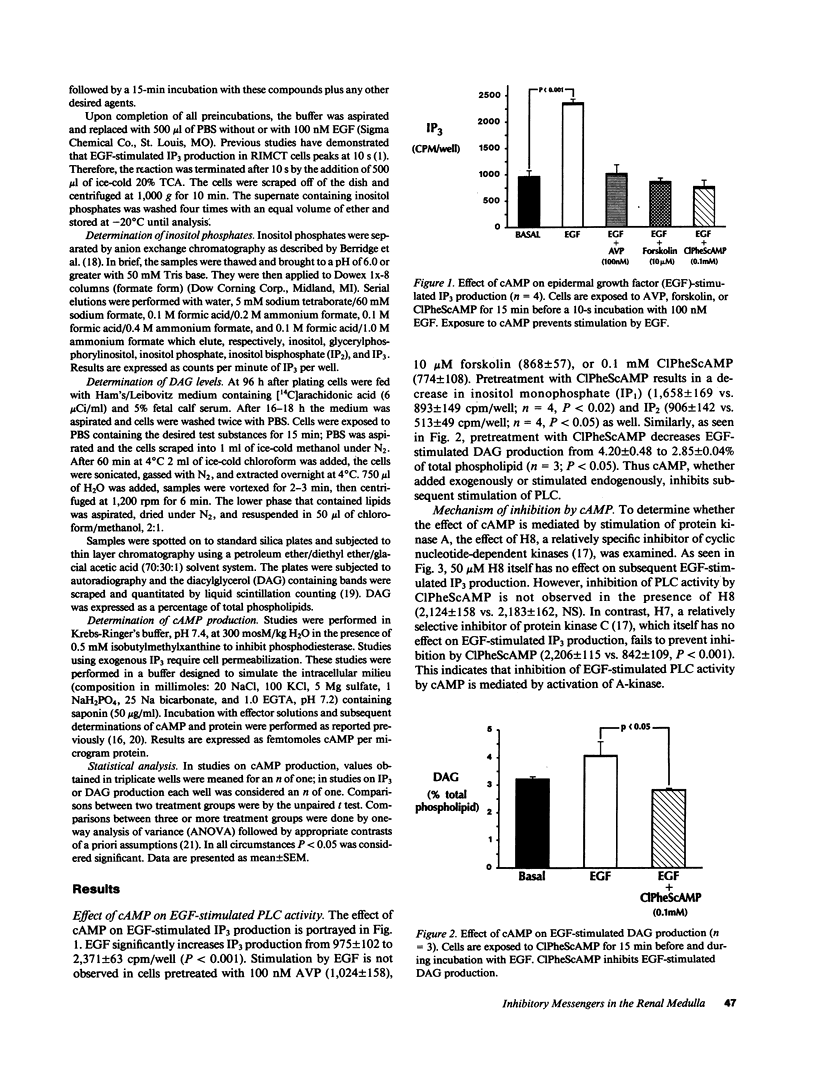
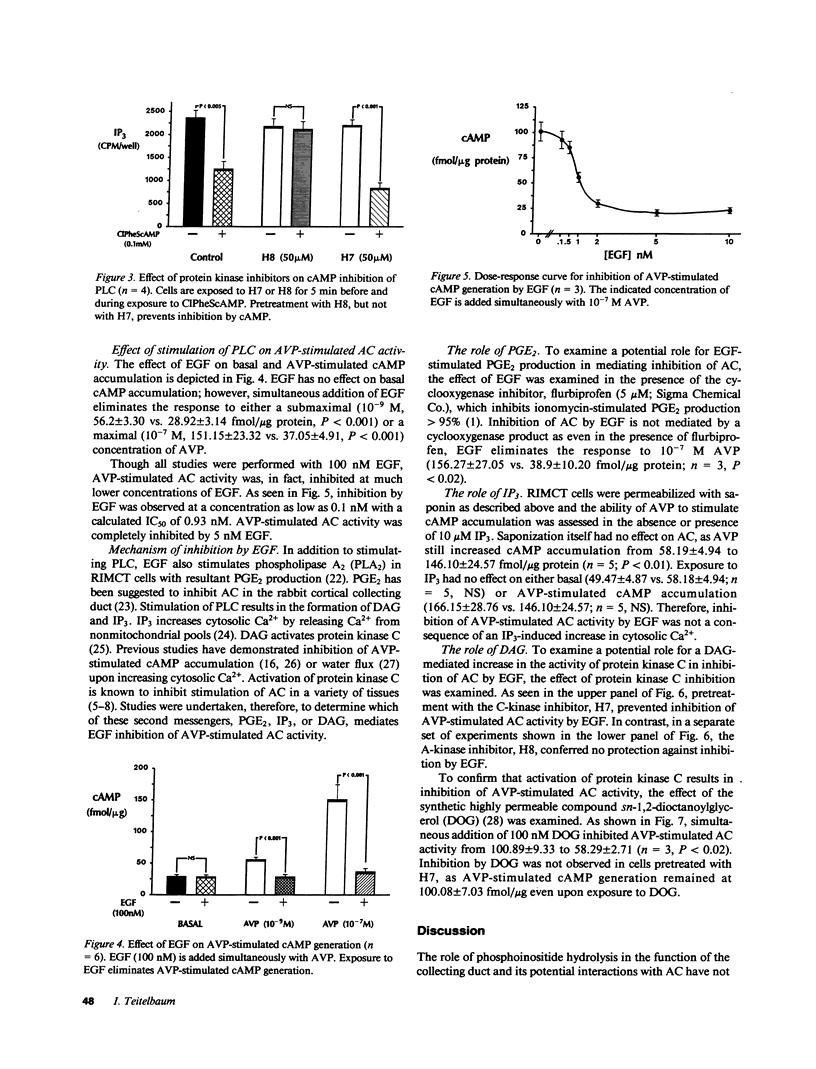
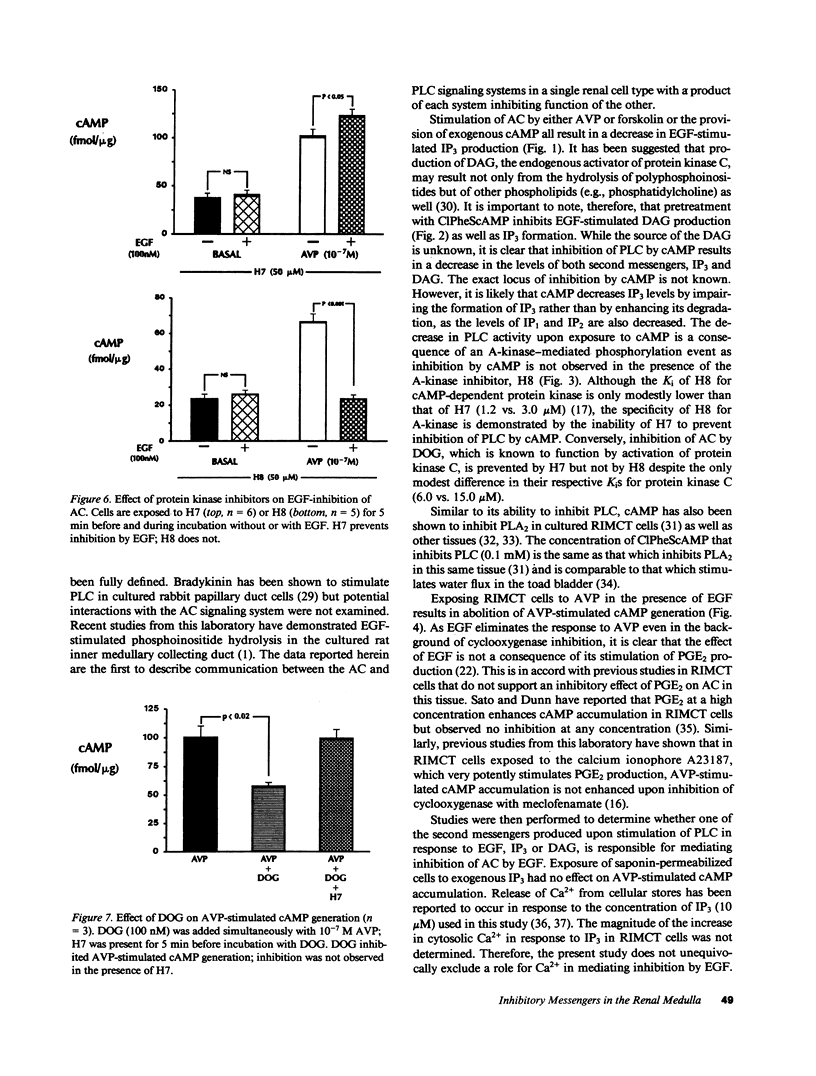
Studies were performed to examine interactions between the adenylyl cyclase (AC) and phospholipase C (PLC) signaling systems in cultured rat inner medullary collecting duct cells. Stimulation of AC by either arginine vasopressin (AVP) or forskolin or addition of exogenous cAMP inhibits epidermal growth factor (EGF)-stimulated PLC. This inhibition is mediated by activation of cAMP-dependent kinase as it is prevented by pretreatment with the A-kinase inhibitor, N-[2-(methylamino)ethyl]-5-isoquinoline-sulfonamide (H8) but not by the C-kinase inhibitor, 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (H7). Exposure to EGF eliminates AVP-stimulated cAMP generation. This is not mediated by a cyclooxygenase product as inhibition by EGF is observed even in the presence of the cyclooxygenase inhibitor, flurbiprofen. Inhibition by EGF is not due to an increase in inositol trisphosphate (IP3) as exposure of saponin-permeabilized cells to exogenous IP3 is without effect. Inhibition by EGF is prevented by pretreatment with the C-kinase inhibitor, H7, but not by the A-kinase inhibitor, H8. Exposure to the synthetic diacylglycerol (DAG), dioctanoylglycerol, also inhibits AVP-stimulated AC activity; therefore, inhibition by EGF is due to activation of protein kinase C. Thus, in cultured rat inner medullary collecting duct cells, cAMP and DAG function as mutually inhibitory second messengers with each impairing formation of the other.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando Y., Jacobson H. R., Breyer M. D. Phorbol myristate acetate, dioctanoylglycerol, and phosphatidic acid inhibit the hydroosmotic effect of vasopressin on rabbit cortical collecting tubule. J Clin Invest. 1987 Aug;80(2):590–593. doi: 10.1172/JCI113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner S. K., Farrar W. L. Inhibition of adenylate cyclase by IL 2 in human T lymphocytes is mediated by protein kinase C. Biochem Biophys Res Commun. 1987 May 29;145(1):176–182. doi: 10.1016/0006-291x(87)91303-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Breyer M. D., Jacobson H. R., Breyer J. A. Epidermal growth factor inhibits the hydroosmotic effect of vasopressin in the isolated perfused rabbit cortical collecting tubule. J Clin Invest. 1988 Oct;82(4):1313–1320. doi: 10.1172/JCI113732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambaut-Guerin A. M., Thomopoulos P. Protein kinase C potentiates isoproterenol-mediated cyclic AMP production without modifying the homologous desensitization process in J774 cells. Eur J Biochem. 1987 Dec 30;170(1-2):381–387. doi: 10.1111/j.1432-1033.1987.tb13711.x. [DOI] [PubMed] [Google Scholar]
- Chueh S. H., Gill D. L. Inositol 1,4,5-trisphosphate and guanine nucleotides activate calcium release from endoplasmic reticulum via distinct mechanisms. J Biol Chem. 1986 Oct 25;261(30):13883–13886. [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985 Feb 10;260(3):1562–1566. [PubMed] [Google Scholar]
- Della Bianca V., De Togni P., Grzeskowiak M., Vicentini L. M., Di Virgilio F. Cyclic AMP inhibition of phosphoinositide turnover in human neutrophils. Biochim Biophys Acta. 1986 May 29;886(3):441–447. doi: 10.1016/0167-4889(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Dunlop M., Metz S. A. A phospholipase D-like mechanism in pancreatic islet cells: stimulation by calcium ionophore, phorbol ester and sodium fluoride. Biochem Biophys Res Commun. 1989 Sep 15;163(2):922–928. doi: 10.1016/0006-291x(89)92310-3. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms of action of calcium-mobilizing agonists: some variations on a young theme. FASEB J. 1988 Aug;2(11):2670–2676. doi: 10.1096/fasebj.2.11.2456243. [DOI] [PubMed] [Google Scholar]
- Friedlander G., Amiel C. Protein kinase C activators and bradykinin selectively inhibit vasopressin-stimulated cAMP synthesis in MDCK cells. Biochim Biophys Acta. 1987 Jul 29;929(3):311–317. doi: 10.1016/0167-4889(87)90258-8. [DOI] [PubMed] [Google Scholar]
- Godfrey R. W., Manzi R. M., Gennaro D. E., Hoffstein S. T. Phospholipid and arachidonic acid metabolism in zymosan-stimulated human monocytes: modulation by cAMP. J Cell Physiol. 1987 Jun;131(3):384–392. doi: 10.1002/jcp.1041310310. [DOI] [PubMed] [Google Scholar]
- Hassid A. Inhibition of prostaglandin biosynthesis in renal (MDCK) cells by cAMP. Am J Physiol. 1983 May;244(5):C369–C376. doi: 10.1152/ajpcell.1983.244.5.C369. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., Daly J. W. Inhibition of receptor-mediated stimulation of cyclic AMP accumulation in neuroblastoma-hybrid NCB-20 cells by a phorbol ester. Biochim Biophys Acta. 1987 Sep 14;930(2):272–278. doi: 10.1016/0167-4889(87)90040-1. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Frindt G., Windhager E. E. Effect of peritubular [Ca] or ionomycin on hydrosmotic response of CCTs to ADH or cAMP. Am J Physiol. 1988 Feb;254(2 Pt 2):F240–F253. doi: 10.1152/ajprenal.1988.254.2.F240. [DOI] [PubMed] [Google Scholar]
- Magnaldo I., Pouysségur J., Paris S. Thrombin exerts a dual effect on stimulated adenylate cyclase in hamster fibroblasts, an inhibition via a GTP-binding protein and a potentiation via activation of protein kinase C. Biochem J. 1988 Aug 1;253(3):711–719. doi: 10.1042/bj2530711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Summers R. J. Inhibition by cAMP of the phosphoinositide response to alpha 1-adrenoceptor stimulation in rat kidney. Eur J Pharmacol. 1988 Apr 13;148(3):441–444. doi: 10.1016/0014-2999(88)90124-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Peuch L. P., Haiech J., Cavadore J. C. Phorbol ester modulation of cyclic AMP accumulation in a primary culture of rat aortic smooth muscle cells. J Pharmacol Exp Ther. 1988 Jun;245(3):1042–1047. [PubMed] [Google Scholar]
- Sato M., Dunn M. J. Interactions of vasopressin, prostaglandins, and cAMP in rat renal papillary collecting tubule cells in culture. Am J Physiol. 1984 Sep;247(3 Pt 2):F423–F433. doi: 10.1152/ajprenal.1984.247.3.F423. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., Levine S. D. Inhibition of vasopressin-stimulated water flow in toad bladder by phorbol myristate acetate, dioctanoylglycerol, and RHC-80267. Evidence for modulation of action of vasopressin by protein kinase C. J Clin Invest. 1985 Sep;76(3):1071–1078. doi: 10.1172/JCI112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D., Satriano J. A. Interactions of vasopressin, cAMP, and prostaglandins in toad urinary bladder. Am J Physiol. 1985 Mar;248(3 Pt 2):F454–F458. doi: 10.1152/ajprenal.1985.248.3.F454. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Morrison A. R. Bradykinin-induced changes in phosphatidyl inositol turnover in cultured rabbit papillary collecting tubule cells. J Clin Invest. 1985 Sep;76(3):978–984. doi: 10.1172/JCI112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Summers S. T., Walker J. M., Sando J. J., Cronin M. J. Phorbol esters increase adenylate cyclase activity and stability in pituitary membranes. Biochem Biophys Res Commun. 1988 Feb 29;151(1):16–24. doi: 10.1016/0006-291x(88)90553-0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Sano K., Nishizuka Y. Counteraction of calcium-activated, phospholipid-dependent protein kinase activation by adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in platelets. J Biochem. 1982 Jan;91(1):403–406. doi: 10.1093/oxfordjournals.jbchem.a133700. [DOI] [PubMed] [Google Scholar]
- Takaichi K., Kurokawa K. Inhibitory guanosine triphosphate-binding protein-mediated regulation of vasopressin action in isolated single medullary tubules of mouse kidney. J Clin Invest. 1988 Oct;82(4):1437–1444. doi: 10.1172/JCI113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I., Berl T. Effects of calcium on vasopressin-mediated cyclic adenosine monophosphate formation in cultured rat inner medullary collecting tubule cells. Evidence for the role of intracellular calcium. J Clin Invest. 1986 May;77(5):1574–1583. doi: 10.1172/JCI112473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I., Mansour J. N., Berl T. Effect of cAMP on prostaglandin E2 production in cultured rat inner medullary collecting tubule cells. Am J Physiol. 1986 Oct;251(4 Pt 2):F671–F677. doi: 10.1152/ajprenal.1986.251.4.F671. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A., Berl T. Adrenergic control of cAMP generation in rat inner medullary collecting tubule cells. Kidney Int. 1989 Feb;35(2):647–653. doi: 10.1038/ki.1989.34. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A., Berl T. Epidermal growth factor-stimulated phosphoinositide hydrolysis in cultured rat inner medullary collecting tubule cells. Regulation by G protein, calcium, and protein kinase C. J Clin Invest. 1990 Apr;85(4):1044–1050. doi: 10.1172/JCI114534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I. The epidermal growth factor receptor is coupled to a phospholipase A2-specific pertussis toxin-inhibitable guanine nucleotide-binding regulatory protein in cultured rat inner medullary collecting tubule cells. J Biol Chem. 1990 Mar 15;265(8):4218–4222. [PubMed] [Google Scholar]
- Williams K. A., Murphy W., Haslam R. J. Effects of activation of protein kinase C on the agonist-induced stimulation and inhibition of cyclic AMP formation in intact human platelets. Biochem J. 1987 May 1;243(3):667–678. doi: 10.1042/bj2430667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A., Kurokawa T., Une Y., Ishibashi S. Phorbol ester regulates stimulatory and inhibitory pathways of the hormone-sensitive adenylate cyclase system in rat reticulocytes. Eur J Pharmacol. 1988 Jul 7;151(2):167–175. doi: 10.1016/0014-2999(88)90797-2. [DOI] [PubMed] [Google Scholar]


