Abstract
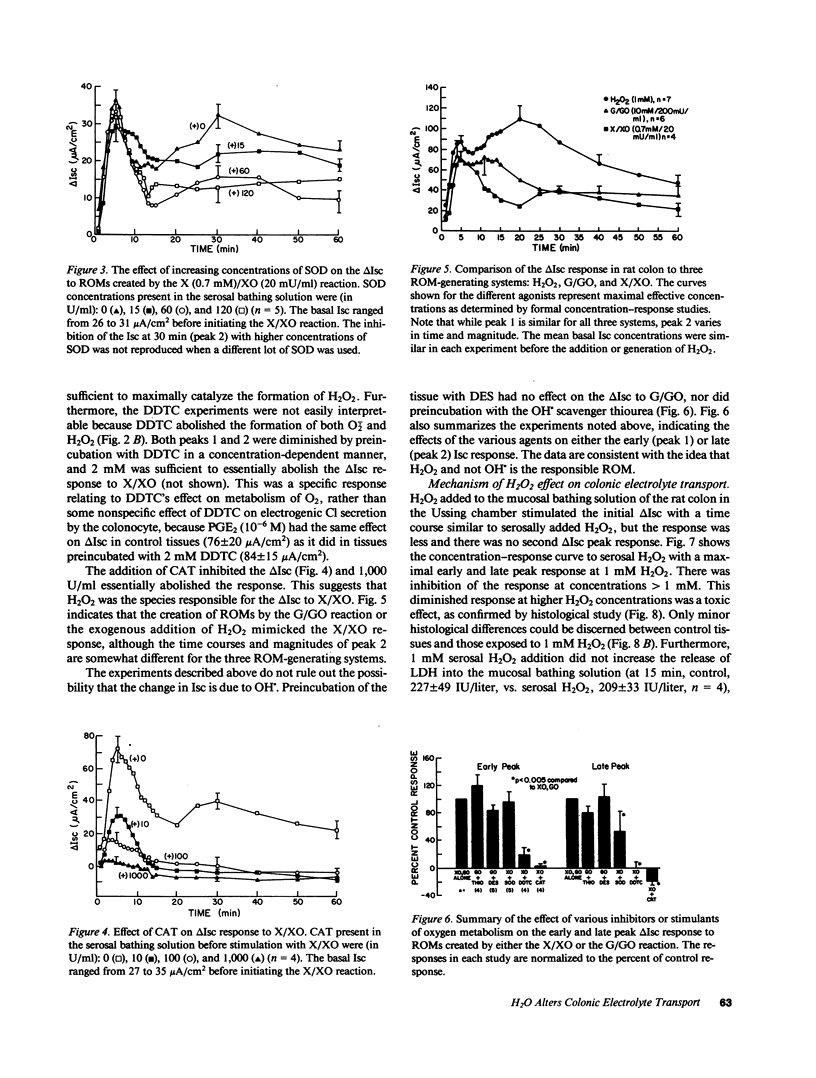
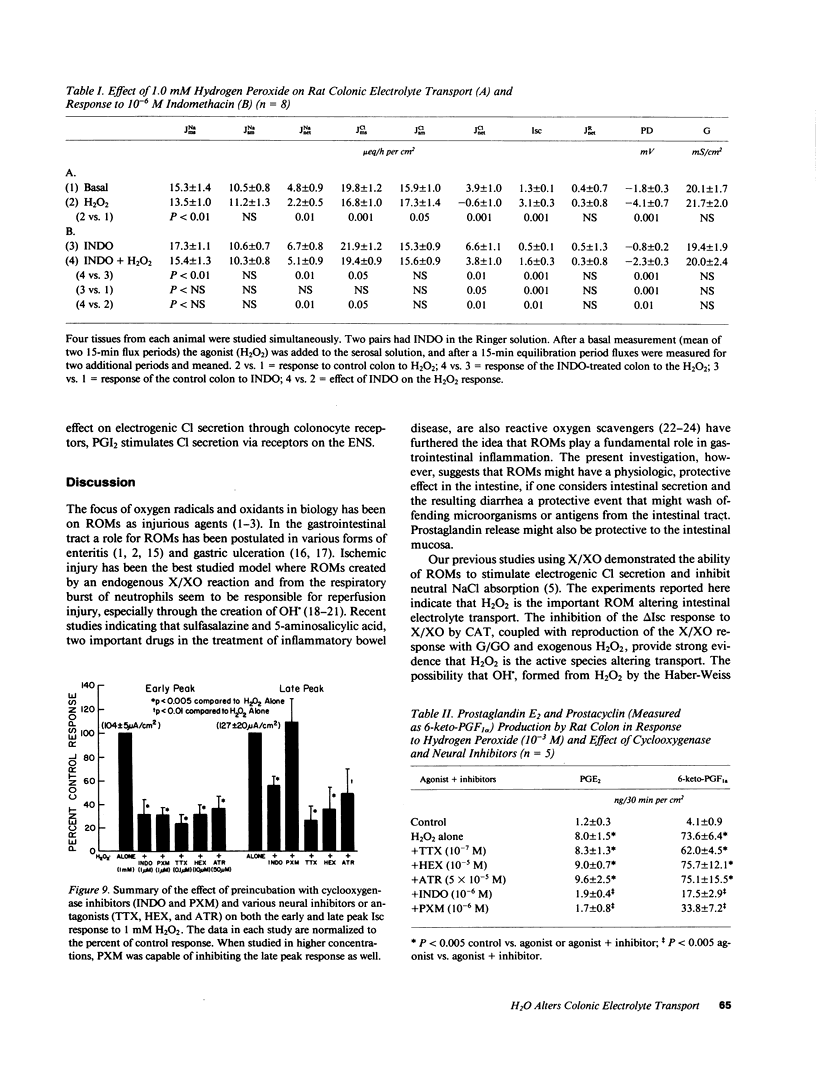
The changes in short circuit current (electrogenic Cl- secretion) of rat colon brought about by xanthine/xanthine oxidase in the Ussing chamber were inhibited by catalase and diethyldithiocarbamate, but not by superoxide dismutase. These results, the reproduction of the response with glucose/glucose oxidase and with exogenous H2O2, and the lack of effect of preincubation with deferoxamine or thiourea implicate H2O2, and not O2- or OH., as the important reactive oxygen metabolite altering intestinal electrolyte transport. 1 mM H2O2 stimulated colonic PGE2 and PGI2 production 8- and 15-fold, respectively, inhibited neutral NaCl absorption, and stimulated biphasic electrogenic Cl secretion with little effect on enterocyte lactic dehydrogenase release, epithelial conductance, or histology. Cl- secretion was reduced by cyclooxygenase inhibition. Also, the Cl- secretion, but not the increase in prostaglandin production, was reduced by enteric nervous system blockade with tetrodotoxin, hexamethonium, or atropine. Thus, H2O2 appears to alter electrolyte transport by releasing prostaglandins that activate the enteric nervous system. The change in short circuit current in response to Iloprost, but not PGE2, was blocked by tetrodotoxin. Therefore, PGI2 may be the mediator of the H2O2 response. H2O2 produced in nontoxic concentrations in the inflamed gut could have significant physiologic effects on intestinal water and electrolyte transport.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeken W., Northwood I., Beliveau C., Gump D. Phagocytes in cell suspensions of human colon mucosa. Gut. 1987 Aug;28(8):976–980. doi: 10.1136/gut.28.8.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H. J., Rawlins C. L. Electrolyte transport across isolated large intestinal mucosa. Am J Physiol. 1973 Nov;225(5):1232–1239. doi: 10.1152/ajplegacy.1973.225.5.1232. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Henderson W. R. Ultrastructure of mast cell degranulation induced by eosinophil peroxidase: Use of diaminobenzidine cytochemistry by scanning electron microscopy. J Histochem Cytochem. 1984 Mar;32(3):337–341. doi: 10.1177/32.3.6420461. [DOI] [PubMed] [Google Scholar]
- Coble B. I., Lindroth M., Molin L., Stendahl O. Histamine release from mast cells during phagocytosis and interaction with activated neutrophils. Int Arch Allergy Appl Immunol. 1984;75(1):32–37. doi: 10.1159/000233586. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest. 1986 Mar;77(3):850–859. doi: 10.1172/JCI112382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., Saito R., DeRubertis F. R. Actions of sulfasalazine and 5-aminosalicylic acid as reactive oxygen scavengers in the suppression of bile acid-induced increases in colonic epithelial cell loss and proliferative activity. Gastroenterology. 1987 Jun;92(6):1998–2008. doi: 10.1016/0016-5085(87)90635-4. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Diener M., Bridges R. J., Knobloch S. F., Rummel W. Neuronally mediated and direct effects of prostaglandins on ion transport in rat colon descendens. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jan;337(1):74–78. doi: 10.1007/BF00169480. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Goerg K. J., Roux M., Wanitschke R., Meyer zum Büschenfelde K. H. Die Bedeutung des Meissnerschen Plexus für die sekretorische Funktion der Colonschleimhaut. Z Gastroenterol. 1986 Sep;24 (Suppl 3):70–78. [PubMed] [Google Scholar]
- Granström E., Kindahl H. Radioimmunoassay of prostaglandins and thromboxanes. Adv Prostaglandin Thromboxane Res. 1978;5:119–210. [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Callahan K. S. Role of hydrogen peroxide in the neutrophil-mediated release of prostacyclin from cultured endothelial cells. J Clin Invest. 1984 Aug;74(2):442–448. doi: 10.1172/JCI111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Cook H. W., Lands W. E. Prostaglandin biosynthesis can be triggered by lipid peroxides. Arch Biochem Biophys. 1979 Apr 1;193(2):340–345. doi: 10.1016/0003-9861(79)90038-9. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Lands W. E. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem. 1980 Jul 10;255(13):6253–6261. [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Granger D. N. A role for iron in oxidant-mediated ischemic injury to intestinal microvasculature. Am J Physiol. 1987 Jul;253(1 Pt 1):G49–G53. doi: 10.1152/ajpgi.1987.253.1.G49. [DOI] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Smith S. M., Grisham M. B., Manci E. A. 5-Aminosalicylic acid protects against ischemia/reperfusion-induced gastric bleeding in the rat. Gastroenterology. 1988 Mar;94(3):733–738. doi: 10.1016/0016-5085(88)90247-8. [DOI] [PubMed] [Google Scholar]
- Lawson L. D., Powell D. W. Bradykinin-stimulated eicosanoid synthesis and secretion by rabbit ileal components. Am J Physiol. 1987 Jun;252(6 Pt 1):G783–G790. doi: 10.1152/ajpgi.1987.252.6.G783. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Whatley R. E., Cain P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988 Dec;82(6):2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. J., Lands W. E. In vitro formation of activators for prostaglandin synthesis by neutrophils and macrophages from humans and guinea pigs. J Lab Clin Med. 1986 Dec;108(6):525–534. [PubMed] [Google Scholar]
- Matalon S., Beckman J. S., Duffey M. E., Freeman B. A. Oxidant inhibition of epithelial active sodium transport. Free Radic Biol Med. 1989;6(6):557–564. doi: 10.1016/0891-5849(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of sulphasalazine and its metabolites on the generation of reactive oxygen species. Gut. 1987 Feb;28(2):190–195. doi: 10.1136/gut.28.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C. E. Glutathione modulates toxic oxygen metabolite injury of canine chief cell monolayers in primary culture. Am J Physiol. 1988 Jan;254(1 Pt 1):G49–G56. doi: 10.1152/ajpgi.1988.254.1.G49. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Winter M., Shasby S. S. Oxidants and conductance of cultured epithelial cell monolayers: inositol phospholipid hydrolysis. Am J Physiol. 1988 Dec;255(6 Pt 1):C781–C788. doi: 10.1152/ajpcell.1988.255.6.C781. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Holm-Rutili L., Perry M. A., Grisham M. B., Arfors K. E., Granger D. N., Kvietys P. R. Role of neutrophils in hemorrhagic shock-induced gastric mucosal injury in the rat. Gastroenterology. 1987 Sep;93(3):466–471. doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Molin L., Lindroth M. Granulocyte-mediated release of histamine from mast cells. Effect of myeloperoxidase and its inhibition by antiinflammatory sulfone compounds. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):277–284. doi: 10.1159/000233335. [DOI] [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Test S. T., Weiss S. J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984 Jan 10;259(1):399–405. [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Turk J., Needleman P. A mechanism for the hydroperoxide-mediated inactivation of prostacyclin synthetase. Blood. 1979 Jun;53(6):1191–1196. [PubMed] [Google Scholar]
- Welsh M. J., Shasby D. M., Husted R. M. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985 Sep;76(3):1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]





