Abstract
Background
Impaired regulation of hepcidin in response to iron is the cause of genetic hemochromatosis associated with defects of HFE and transferrin receptor 2. However, the role of these proteins in the regulation of hepcidin expression is unclear.
Design and Methods
Hepcidin expression, SMAD and extracellular signal-regulated kinase (Erk) phosphorylation and furin expression were analyzed in hepatic HepG2 cells in which HFE and transferrin receptor 2 were down-regulated or expressed, or furin activity specifically inhibited. Furin expression was also analyzed in the liver of transferrin receptor 2 null mice.
Results
We showed that the silencing of HFE and transferrin receptor 2 reduced both Erk phosphorylation and furin expression, that the exogenous expression of the two enhanced the induction of phosphoErk1/2 and furin by holotransferrin, but that this did not occur when the pathogenic HFE mutant C282Y was expressed. Furin, phosphoErk1/2 and phosphoSMAD1/5/8 were down-regulated also in transferrin receptor 2-null mice. Treatment of HepG2 cells with an inhibitor of furin activity caused a strong suppression of hepcidin mRNA, probably due to the inhibition of bone morphogenic protein maturation.
Conclusions
The data indicate that transferrin receptor 2 and HFE are involved in holotransferrin-dependent signaling for the regulation of furin which involved Erk phosphorylation. Furin in turn may control hepcidin expression.
Keywords: hepcidin, TfR2, HFE, furin, iron homeostasis
Introduction
Hepcidin is a major regulator of systemic iron homeostasis since it controls the egress of iron from reticuloen-dothelial cells and from absorptive enterocytes by binding and inactivating the iron exporter ferroportin.1 It is expressed mainly in the liver, where its mRNA is abundant, and it responds to body iron status, inflammation, hypoxia and erythroid activity.2 The study of inherited defects of iron homeostasis showed that the expression of hepcidin in the liver is regulated in a positive way by hemojuvelin (HJV), transferrin receptor 2 (TfR2), HFE, bone morphogenic proteins (BMP), SMAD42–4 and by neogenin5 and in a negative way by matriptase-2.6–8 It has been established that the expression of hepcidin in the liver is under the control of the BMP/SMAD transduction pathway,3,9 which is strongly activated by HJV, a BMP co-receptor.10,11 The activity of HJV is under a complex post-translational regulation. Its level is proteolytically controlled by matriptase-2,7 while its secretion and processing into the soluble and inhibitory form is regulated by the expression of furin12 and by the interaction with neogenin.13 Most of the BMP tested, including BMP2, 4, 5, 6 and 9, are strong inducers of hepcidin in vitro,10,14 but BMP6 was found to be positively regulated by iron, and its absence in mice caused hepcidin down-regulation and severe iron overload.4,15 Less clear is the role of HFE and TfR2 which are responsible for the adult onset, less severe forms of hemochromatosis. They do not seem to be necessary for hepcidin induction by BMP,14 but TfR2 is thought to act as a sensor of body iron status. It binds holotransferrin with lower affinity than TfR1, its expression is liver-specific and its inactivation in mice leads to iron overload.16 Upon binding to holotransferrin, TfR2 activates the mitogen-activated protein kinase (MAPK) pathway by inducing the phosphorylation of extracellular signal-regulated kinase (Erk)-1/2.17 Even less clear is the role of HFE, which is known to form different complex types with TfR118 and with TfR219 which may be important for the holotransferrin-dependent hepcidin regulation. It has been proposed that the binding of holotransferrin to TfR1 causes a shift of HFE towards TfR2, which signals hepcidin regulation by unknown mechanisms.20,21 However, holotransferrin does not induce hepcidin in cultured hepatic cells, except in freshly prepared primary mouse hepatocytes,22 primary hepatocytes that have been treated with serum deprivation followed by supplementation,23 or in HepG2 cells transfected with HFE.20 The mechanism might involve Erk activation and signaling through the SMAD pathway.23 HFE/TfR2 double null mice had more severe iron loading than mice lacking either HFE or TfR2 suggesting that HFE and TfR2 regulate hepcidin through parallel pathways involving Erk1/2 and SMAD1/5/8.24
Furin is another partner of the hepcidin regulation system, since it is responsible for the processing of prohepcidin to produce the mature protein.25 Moreover, it mediates the formation of a soluble HJV form that competes with the membrane HJV to inhibit BMP activation.12,26 The oxygen- and iron-dependent regulation of furin expression, mediated by hypoxia-induced factor (HIF)-1α, was indicated as an important link between hypoxia and iron homeostasis, based on furin’s capacity to modulate hepcidin expression.12 Moreover, furin acts on the maturation of several key growth factors, including the large class of transforming growth factor (TGF)-β molecules, BMP4 and other BMP.27 Furin is also regulated by TGF-β1 in HepG2 cells through a mechanism that involves a cross-talk between Erk1/2 MAPK and SMAD2/3 pathways.28
In this study we investigated mechanisms of hepcidin regulation by down-regulating or over-expressing HFE and TfR2 and specifically inhibiting furin activity in hepatic HepG2 cells.
Design and Methods
Cell culture
HepG2 cells were cultured in minimal essential medium (PAA) with 10% fetal bovine serum (PAA), 40 μg/mL gentamicin and 1 mM L-glutamine. The cells were maintained at 37°C under 5% CO2. For studies of SMAD phosphorylation or activation 2×105 cells/well were seeded in 12-well plates, grown for 24 h and then for another 16 h in 0.5% fetal bovine serum. They were then incubated for 16 h with recombinant BMP2 (R&D Systems) (10–100 ng/mL) or for different periods (0.5, 1, 2, 4, 6, or 16 h) with BMP2 50 ng/mL. In other experiments dorsomorphin (5 μM) and U0126 (10 μM) were added 1 h before the addition of BMP2 50 ng/mL and the cells collected after 6 h. In the studies on the effect of the furin inhibitor dec-RVKR-chloromethylketone (CMK; Biomol International), 2×105 cells/well were seeded in the 12-well plates. After 24 h cells were incubated in serum-free medium for 16 h, followed by treatment with CMK (10, 50, or 100 ng/mL) for 16 h or with CMK 50 ng/mL for different periods of incubation (0.5, 1, 2, 4, 6, or 16 h). For the treatments with apotransferrin or holotransferrin the cells were seeded in 12-well plates (250,000 cells/well). After 24 h they were grown in serum-free medium for 16 h, and then treated with human holotransferrin or apotransferrin (Sigma) for 15–120 min.
HepG2 transfection
HepG2 cells (105 cells/well) were seeded in 12-well plates, transfected after 24 h with 100 pmol double-stranded small interfering RNA (siRNA) using Oligofectamine (Invitrogen, Paisley, UK) following the manufacturer’s instructions, grown for 72 h, and then harvested and analyzed. In some experiments the cells were treated with BMP2 50 ng/mL for 16 h before harvesting. The double-stranded siRNA specific for each gene were produced by Ambion (Ambion, Austin, TX, USA); the most effective and specific was selected for transfections. The sense sequences for each siRNA were the following: TfR2: 360 sense 5′GGAUGUCAACUAUGAGCCUtt3′; HJV: sense: 5′GUUUAGAGGUCAUGAAGGUtt3′;26 HFE: sense 5′CAGGAGAGAGUUGAACCUAATT3′; SiScramble (Scr) 10220776 from Qiagen (Qiagen-Xeragon) sense 5′-UUCUCCGAACGUGUCACGUtt. All experiments used Oligofectamine alone (mock) or with Scramble (Scr) as control.
HepG2 transfection with plasmids
Cells (105/well) were seeded in 12-well plates and transfected the following day with 1 μg of pCMV-Sport6-TfR2Hu (Open Biosystems, kind gift of Dr. Clara Camaschella), pcDNA3.1-HFEwt-Myc or pcDNA3.1-HFE282-Myc using Lipofectamine (Invitrogen, Paisley, UK) following the manufacturer’s instructions. The plasmids were transfected and the cells grown for 72 h. After 48 h cells were incubated in serum-free medium for 16 h, followed by treatment with human holotransferrin (30 μM) for 30 min and then harvested and analyzed. All experiments used the cells transfected with empty pcDNA3.1 as control (mock).
Immunoblot analysis
Cell extracts or liver homogenates were treated with Lysis Buffer (200 mM Tris-HCl pH8, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40 and 10% glycerol) containing a mixture of protease inhibitors (Sigma). For assays examining phosphorylated SMAD expression, 1 mM sodium orthovanadate (Sigma) and 1 mM sodium fluoride (Sigma) were added to the lysis buffer as phosphatase inhibitors. Cells lysates were analyzed by 10% sodium dodecylsulfate polyacrylaminde gel electrophoresis and, after transfer, the polyvinylidene fluoride filters were incubated for 16 h at 4°C with specific antibodies, washed, and further incubated for 1 h at 37°C with secondary peroxidase-labeled antibodies (anti-mouse IgG Dako, Glostrup, Denmark or anti-rabbit IgG Pierce). The primary antibodies used were rabbit anti-FtL anti-body (1:1000; Sigma); rabbit anti-phosphoSMAD1/5/8 antibody (1:1000; Cell Signaling technology), rabbit anti-SMAD1 antibody (1:1000; Cell Signaling technology), rabbit anti-furin antibody (1:1000; Santa Cruz Biotechnology), anti-phosphoErk1/2 antibody (1:1000; Cell Signaling technology), anti-Erk1 antibody (1:1000; Santa Cruz Biotechnology), mouse anti-Myc antibody (1:1000; Sigma), mouse anti-TfR2 antibody (1:1000; Santa Cruz Biotechnology), rabbit anti-actin antibody (1:1000; Sigma) and mouse anti-GAPDH antibody (1:10000; Sigma). Bound activity was revealed by an advanced enhanced chemiluminescence (ECL) kit (Amersham, Uppsala, Sweden) and detected using a KODAK Image Station 440CF (Kodak, Rochester, NY, USA). The same procedure was used for the analysis of pSMAD1/5/8, pErk1/2, furin and glyceraldehyde 3-phospohate dehydrogenase (GAPDH) in mouse livers.
RNA extraction and real-time reverse-transcriptase polymerase chain reaction
RNA was purified from cells using the guanidinium thio-cyanate–phenol–chloroform method (Tri reagent) according to the manufacturer’s instructions (Ambion, Austin, TX, USA). DNase-treated total RNA (1 μg) was used to synthesize the first strand of cDNA with the ImProm-II Reverse Transcription System (Promega), using oligodT as the primer. For real-time reverse-trasnscriptase polymerase chain reaction (RT-PCR) analysis, specific Assays-on-Demand products (20x) and TaqMan Master Mix (2x) from Applied Biosystems (Foster City, CA, USA) were used, according to the manufacturer’s instructions, and the reactions were run on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) in a final volume of 20 μL for 40 cycles. We analyzed the expression levels of hepcidin, TfR2, HFE, HJV and furin, normalizing the results to GAPDH or hypoxanthine phophoribosyltransferase-1 (HPRT1) levels in each sample.
Transferrin receptor 2 knockout mice
Mice with germinal inactivation of the TfR2 gene were used for the in vivo experiments.29 Livers from three 14-day old animals were isolated, immediately frozen, homogenized and used for the experiments. Aged-matched wild-type sibling pairs were used as normal controls. RNA was purified from livers in Tri Reagent solution according to the manufacturer’s instructions (Ambion). Total RNA was used to synthesize the first strand of cDNA with the Improm-II Reverse Transcription System (Promega), using oligodT as the primer. For RT-PCR analysis of hepcidin-1, furin and HPRT1 we used the following primers: hepcidin-1: forward TTGCGAT-ACCAATGCAGAAGAG, reverse TCTTCTGCTGTAAATGCT-GTAACAATT; furin: forward CCTTCTTCCGTGGGGTTAG reverse GCAGTTGCAGCTGTCATGTT and HPRT1: forward GCTTGCTGGTGAAAAGGACCTCTCGAAG reverse CCCT-GAAGTACTCATTATAGTCAAGGGCAT. The PCR were run for 25 cycles.
Statistical analysis
Values between mock and transfected/treated cells were compared using Student’s t-test for unpaired data. Differences were defined as statistically significant for P values less than 0.05.
Results
An initial analysis by real-time RT-PCR showed that the transcripts of hepcidin, TfR2, HJV, HFE and furin were expressed at detectable levels in the HepG2 cells. In basal conditions the amount of hepcidin mRNA was comparable to that of GAPDH, while that of TfR2, HJV, HFE and furin transcripts was about 1000-fold lower (Online Supplementary Figure S1A). We could detect endogenous furin by western blotting, but not TfR2, HFE and HJV, because of their low levels of expression and the insufficient affinity binding of the antibodies available to us. With the aim of studying the role of TfR2 and HFE in hepcidin regulation we developed methods for their down-regulation. We tested three different pre-designed siRNA for TfR2 and two for HFE and they were used at a concentration of 100 pmol/well for transfecting HepG2 cells. Analysis 72 h after transfection showed that the most efficient siRNA caused approximately 70% inhibition of the transcripts (Online Supplementary Figure S1B). The silenced cells reached confluence at the same time as the mock-transfected cells, indicating that none of the siRNA affected cell growth.
Silencing of transferrin receptor 2 and HFE in HepG2 cells
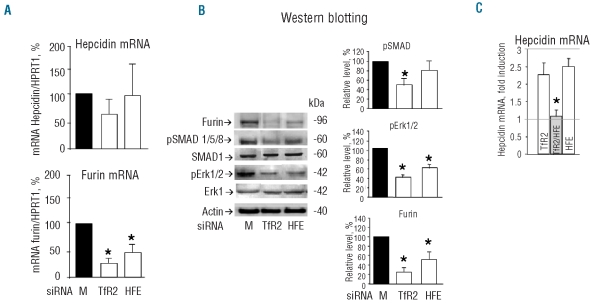
We first analyzed the effect of the silencing on the expression of hepcidin and of the proteins involved in the control of its expression. The silencing of TfR2 caused a minor and non-significant reduction of hepcidin mRNA, a significant reduction of SMAD1/5/8 phosphorylation (about 50%) and of furin expression (>70%), evident both from real time RT-PCR evaluation of the mRNA and by western blotting with anti-furin antibodies (Figure 1A and B). Since TfR2 is involved in MAPK signaling and phosphorylation of Erk1/2,17 we analyzed the level of phospho-Erk1/2 (also named p42/44), finding that it decreased by approximately 60% in the TfR2-silenced cells. Thus, TfR2 silencing modified Erk signaling and, to a lesser extent, also SMAD signaling, together with furin and hepcidin expression. The effect of HFE silencing was less potent: it did not cause an evident decrease in hepcidin mRNA, and the decrease in the level of pSMAD1/5/8 was minor and not statistically significant, while the decreases of furin mRNA and protein and of phosphor Erk1/2 level (~50% ) were statistically significant (Figure 1A and B). Silencing of the two together did not increase the inhibitions in a basal situation (data not shown). Hepcidin is strongly induced by BMP, which activate SMAD phosphorylation and signaling. In the conditions we used, BMP2 induced hepcidin expression in HepG2 cells by about 3-fold (Figure 1C); the induction was slightly, and non-significantly reduced by the silencing of TfR2 and of HFE. However, the silencing of the two together completely blunted the BMP2-dependent induction of hepcidin mRNA (Figure 1C). The similarity of the responses to TfR2 and HFE silencing was a first indication that the two act on the same pathways, which may involve furin expression.
Figure 1.
Effects of TfR2 and HFE silencing. HepG2 cells were transfected with the siRNA specific for TfR2 and HFE and analyzed after 72 h. (A) Levels of hepcidin and furin mRNA evaluated by real-time RT-PCR after the transfection, expressed as percentages of the levels of the mock transfected cells corrected for HPRT1 mRNA level. (B) Western blotting of total cell homogenates probed with the antibodies for furin, phosphorylated SMAD1/5/8 (pSMAD1/5/8), SMAD1, phosphorylated Erk1/2 (pErk1/2), total Erk1 and actin; the histograms show the densitometry values of the bands expressed as percentages of those of the mock transfected cells, and corrected for actin level. (C) Real time analysis of hepcidin mRNA level after transfection with TfR2 or HFE siRNA alone and in combination, and after 50 ng/mL BMP2 for 16 h. The histogram is expressed as the percentage of hepcidin mRNA of the mock transfected cells. Histograms of the densitometry and of qRT-PCR are the means and SD of at least three independent experiments. The horizontal lines indicate the hepcidin mRMA level in the basal and in the induced control cells. The asterisks indicate statistically significant difference (P<0.05) from the mock transfected controls.
Bone morphogenic protein 2 induces furin expression
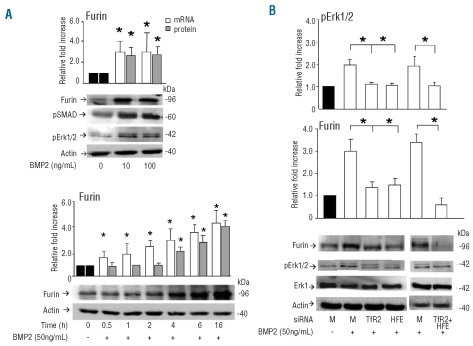
BMP are members of the TGF-β family and the two protein types are known to activate SMAD and MAPK signaling which affects a large number of genes. Furin expression in HepG2 cells was shown to be stimulated by TGF-β1 via a cross-talk between these two signaling pathways,28 but the effect of various BMP on its expression has not been described. In initial dose-response experiments we found that BMP2 at the concentration of 10 ng/mL not only induced SMAD1/5/8 and Erk1/2 phosphorylation, but also up-regulated furin protein and mRNA by about 2-fold, and that this effect did not increase further at the BMP2 concentration of 100 ng/mL (Figure 2A). Similar results were obtained using 10 ng/mL BMP6 (data not shown). Time-dependent studies showed that the induction of furin mRNA was fast, evident even after 30 min, and steadily increased for 16 h, while that of furin protein increased progressively in the period from 4 to 16 h (Figure 2A). We concluded that furin is actively regulated by various BMP in HepG2 cells, with a mechanism that may involve SMAD, Erk signaling or both. Next we analyzed the effect of TfR2 and HFE silencing on this induction. After transfection with the siRNA the cells were incubated for 16 h with 50 ng/mL BMP2 and analyzed. In the mock-transfected cells Erk1/2 phosphorylation and furin expression were up-regulated 2–3 fold, as expected, while in the cells silenced for TfR2 and HFE, pErk1/2 and furin were not induced (Figure 2B). The silencing of the two together had a more potent effect on furin expression, which was reduced below basal level (Figure 2B). These data suggest that furin is induced by BMP2 mainly via Erk signaling, since HFE silencing affects Erk, but not SMAD signaling (Figure 1B).
Figure 2.
Treatment of HepG2 cells with BMP2. (A) Upper: HepG2 cells were treated with different doses of BMP2 (10–100 ng/mL) for 16 h and analyzed for furin mRNA with real-time RT-PCR and for furin, phosphoErk1/2, pSMAD1/5/8 and actin with western blotting. Lower: time course of furin induction by BMP2. HepG2 cells were grown in 50 ng/mL BMP2 and analyzed at the indicated times for furin mRNA with real-time RT-PCR and for furin and actin with western blotting. Histograms of the densitometry and of qRT-PCR are expressed as fold increase relative to the cells untreated with BMP2, after normalization on actin level, or HPRT1. The histograms are mean and SD of three independent experiments. The asterisks indicate statistically significant difference (P<0.05) from the mock transfected controls. (B) HepG2 cells were transfected with siRNA for TfR2 and HFE alone or in combination (TfR2+HFE), then they were incubated with 50 ng/mL BMP2 for 16 h, and furin and pErk1/2 were analyzed by western blotting. Histograms of the densitometry expressed as percentage relative to the mock transfected cells and incubated with BMP2 after normalization on actin level. Means and SD of at least three independent experiments. The asterisks indicate statistically significant difference (P<0.05) from the mock transfected controls.
Expression of HFE and transferrin receptor 2
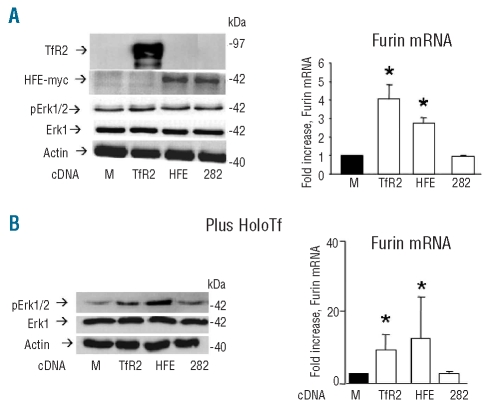
Once found that the down-regulation of TfR2 and HFE reduces Erk signaling and furin expression, it was important to verify whether the opposite occurs after exogenous expression of the two. We, therefore, transfected the cells with the cDNA of human TfR2, of myc-tagged human HFE or of its mutant C282Y. Western blotting with anti-TfR2 and with anti-myc antibodies confirmed that the transfections were efficient (Figure 3A). The level of pErk1/2 did not change appreciably, while the level of furin mRNA increased significantly 3- to 4-fold after transfection with TfR2 and HFE, but not with the HFE mutant C282Y (Figure 3A). Erk signaling was shown to be induced by holotransferrin binding to TfR2.17,23 We verified that this also occurs in HepG2 cells. Incubation with 30 μM human holotransferrin caused a transient induction of Erk1/2 phosphorylation that peaked after 30–45 min, while apotransferrin had no effect (Online Supplementary Figure S2). Furin mRNA increased 2–3 fold after 30 min incubation with holotransferrin (data not shown). To evaluate the effect of TfR2 and HFE on the signaling, 30 μM holotransferrin were then added to the transfected cells which were analyzed after 30 min. The treatment caused a higher increase of pErk1/2 in the cells transfected with TfR2 and HFE than in the mock-transfected cells, and the cells transfected with the HFE-C282Y behaved as the mock-transfected cells (Figure 3B). We also analyzed the level of furin mRNA: it increased in parallel with pErk1/2 in the cells transfected with TfR2 and HFE but not in those transfected with HFE-C282Y (Figure 3B). After addition of holotransferrin the tranfections increased furin transcript about 10-fold with respect to the mock-transfected cells (Figure 3B), and the increase was much higher than that in the cells before addition of holotransferrin (data not shown). These results indicate that both TfR2 and HFE (but not HFE-C282Y) participate in the signal transduction induced by holotransferrin.
Figure 3.
Effect of holotransferrin in cells expressing HFE and TfR2. (A) The HepG2 cells were transfected with cDNA for TfR2 (TfR2), myc-tagged HFE (HFE) and myc-tagged HFE mutant C282Y (282). Right: western blotting analysis for the expression of the transgene with antibodies for TfR2 and Myc-Tag, pErk1/2 and total Erk1. Left: real time analysis of furin mRNA level after transfection with cDNA for TfR2 (TfR2), myc-tagged HFE and myc-tagged HFE mutant C282Y. (B) The transfected cells were incubated for 30 min with 30 μM holo-transferrin (HoloTf) and analyzed for pErk1/2 and total Erk1 level by western blotting (right). Left: real-time RT-PCR evaluation of furin mRNA after 30 min of incubation with holotransferrin. Western blots are representative of three independent experiments, and the histograms are means of three experiments. The asterisks indicate statistically significant difference (P<0.05) from the mock transfected controls (M).
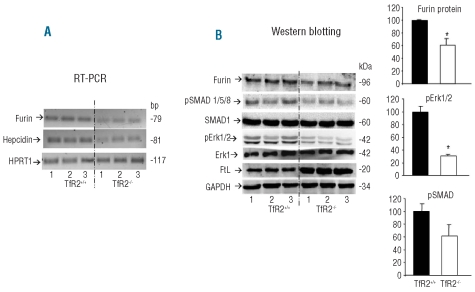
Furin expression in transferrin receptor 2 null-mice
Our data show that TfR2 down-regulation in HepG2 cells causes an inhibition of furin expression. To verify whether this occurs also in vivo, we analyzed the liver of 14-day old TfR2−/− mice recently described, which are characterized by liver iron overload and reduced expression of liver hepcidin.29 RT-PCR showed that the levels of furin and hepcidin mRNA were strongly reduced in the TfR2−/− mice (Figure 4A). The levels of furin protein, pErk1/2 and pSMAD1/5/8 were also reduced compared to those of the controls, while the L-ferritin level (FtL) was strongly increased (Figure 4B). Thus, in the TfR2−/− mice, liver iron overload was accompanied by the down-regulation of furin, pSMAD1/5/8 and pErk1/2 signaling.
Figure 4.
Furin expression in TfR2−/− mice. The livers of three wild type (TfR2+/+) and of three TfR2 knockout (TfR2−/−) 14-day old mice were analyzed. (A) RT-PCR analysis of furin, hepcidin and HRPT1 (as a control) mRNA. (B) Western blotting analysis of the liver extracts for furin, pSMA1/5/8, total SMAD1, pErk1/2, total Erk1 and ferritin light chain (FtL). GAPDH was used as a loading control. The histograms represent the means of two groups analyzed, TfR2+/+ and TfR2−/−. The asterisks indicate a statistically significant difference (P<0.05).
Inhibition of furin activity and expression
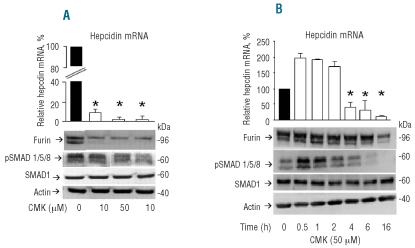
The data indicated that HFE and TfR2 are involved in Erk-MAPK signaling and that this is accompanied by the modulation of furin expression. Furin is a proconvertase with multiple roles in hepcidin expression, since it is responsible for the processing of prohepcidin to the mature hormone25 and for the cleavage of HJV to produce the inhibitory soluble HJV.12 Moreover furin is implicated in the processing of TGF-β and of BMP.30 To study its actual function in our cell model we initially treated HepG2 cells with the furin inhibitor CMK for 16 h. This, in the concentration range of 10–100 μM, caused a reduction of hepcidin mRNA of about 90–99% and strong reductions of SMAD phosphorylation and furin protein (Figure 5A). Time course experiments showed that the effect of CMK (50 μM) was biphasic with an initial up-regulation of pSMAD1/5/8 and of hepcidin mRNA followed by a progressive inhibition after 16 h (Figure 5B). The stimulation was faster for pSMAD1/5/8, which peaked at 30 min, while the hepcidin stimulation lasted up to 2 h (Figure 5B). Furin protein inhibition was evident only after 16 h of treatment. This biphasic pattern supports the hypothesis that furin activity has different targets that act in opposite ways on hepcidin expression.
Figure 5.
Inhibition of furin activity. (A) HepG2 cells were incubated for 16 h with the indicated concentrations of furin inhibitor CMK and then the level of hepcidin mRNA analyzed by qRT-PCR, and pSMAD1/5/8, SMAD1, actin and furin analyzed by western blotting. (B) Cells were exposed to 50 μM CMK for the indicated time and the levels of hepcidin mRNA and of pSMAD1/5/8, SMAD1 furin and actin were analyzed. Histograms of qRT-PCR are the means and SD of at least three independent experiments. The asterisks indicate statistically significant difference (P<0.05) from the untreated cells (0).
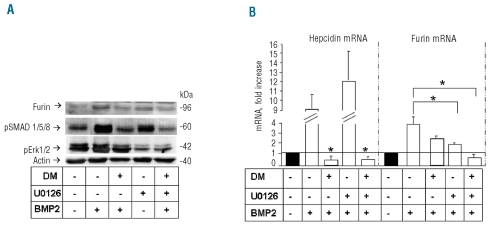
BMP activate various signaling pathways, among which those involving SMAD1/5/8 and Erk1/2 phosphorylation are thought to be the major ones. To identify the one involved in furin induction we applied two well known compounds, dorsomorphin, a specific inhibitor of type I BMP receptors and of SMAD1/5/8 phosphorylation,31 and U0126, a specific inhibitor of Erk phosphorylation.32 Figure 6A shows that dorsomorphin blocked pSMAD1/5/8 stimulation by BMP2, but did not modify the level of pErk1/2, as expected. U0126 reduced the level of pErk1/2 after BMP2 stimulation, and had no effect on pSMAD1/5/8. These treatments did not modify the level of total Erk or of SMAD1 (data not shown). Dorsomorphin suppressed hepcidin mRNA even after BMP2 induction, as expected, while U0126 had no evident effect (Figure 6B). More interestingly, furin induction by BMP2, both at the protein and mRNA levels, was inhibited by U0126, and, to a lesser extent by dorsomorphin, and the inhibition was stronger when the two were together (Figure 6A and B). These results confirm that hepcidin expression is regulated mainly by the BMP/SMAD pathway, and show that furin is regulated by a cross-talk between the SMAD and the Erk pathways.
Figure 6.
Treatment with dorsomorphin and U0126. HepG2 cells were grown for 6 h in the presence or absence of 50 ng/mL BMP2 with or without 5 μM dorsomorphin (DM) or 10 μM U0126. (A) Western blot analysis of furin, pSMAD1/5/8, pErk1/2 and actin. Representative of three independent experiments. (B) Evaluation of hepcidin, and furin mRNA by real time RT-PCR. Histograms are expressed as fold-increase relative to non-treated cells. Means of three independent experiments.
Discussion
Hepcidin expression in the liver and hepatic cell lines is mainly controlled by BMP signaling, with BMP6 probably being the major physiological activator,4,15 while BMP2 and BMP4 have been used in many cellular studies.14,33,34 The signaling includes the phosphorylation of SMAD1/5/8, which associate with SMAD4, the complex translocates to the nucleus for activation of the SMAD-binding elements of hepcidin promoter.35 The relationship between this pathway and the transferrin-dependent induction of hepcidin has not been fully elucidated. The expression of BMP6 is iron-regulated,36 but HFE null mice have inappropriately low levels of hepcidin and develop iron overload, although they have an adequate level of BMP6. This suggests that HFE (and possibly TfR2) acts upstream, or independently, of hepcidin induction by BMP6.37
To study the role of TfR2 and HFE we silenced them in HepG2 cells. The silencing of TfR2 caused a minor reduction of hepcidin expression and of SMAD1/5/8 phosphorylation, but strongly reduced the phosphorylation of Erk1/2 (Figure 1A and B). In hepatic cells, Erk signaling is activated by the BMP38 and also by holotransferrin binding TfR2,17,23 in a mechanism which is a sensor of transferrin saturation and body iron status. However, how this mechanism and signaling act in hepcidin regulation has not been clarified. We confirmed that this signaling is associated with TfR2 activity, since the level of pErk1/2 increased after addition of holotransferrin (Online Supplementary Figure S3), and even more in the cells transfected with TfR2 cDNA (Figure 3). Thus the induction of pErk1/2 seemed to be linked to TfR2 and to its binding to holotransferrin. Probably more interesting was the observation that pErk1/2 was directly associated with furin expression, both at the level of mRNA and protein. Our data, therefore, indicate that furin expression was suppressed by the silencing of TfR2, was induced by TfR2 transfection and further induced by holotransferrin. The linkage between Erk and furin is not surprising, since it has already been demonstrated that furin expression in HepG2 cells is regulated by TGF-β1 in a cross-talk between the Erk and the SMAD2/3 pathways.28 We found a similar cross-talk between Erk and SMAD1/5/8 pathways in HepG2 cells also after stimulation by BMP. The analysis of the TfR2−/− mice indicated that a relationship between TfR2 and furin also exists in vivo, since the levels of furin mRNA and protein were abnormally low in their livers, as were the levels of pErk1/2 and of pSMAD1/5/8 (Figure 4). The relationship between TfR2 activity and furin may be relevant in the regulation of hepcidin expression, since furin has already been shown to act as a regulator of hepcidin expression and to be modulated by HIF-α in an iron-dependent manner.12
We also analyzed the role of HFE, finding that its down-and up-regulation in HepG2 cells had an effect on pErk1/2 similar to that of TfR2, although slightly less robust. HFE silencing reduced pErk1/2 and furin, but did not modify pSMAD1/5/8 or hepcidin (Figure 1 A and B), and its exogenous expression enhanced the induction of furin and Erk signaling by holotransferrin (Figure 3). Of interest is that this activity was absent in the pathogenic mutant C282Y which, when expressed, did not modify pErk1/2 or furin expression (Figure 3). This supports the hypothesis that the activity in furin regulation is physiologically important in the control of hepcidin expression. Altogether these data indicate that TfR2 and HFE act on the same signaling pathways. HFE and TFR2 were shown to interact when expressed in the same cells,19 and the HFE-TFR2 complex was required for the transcriptional regulation of hepcidin by holotransferrin in hepatic cells.20 Thus, it is conceivable that the two cooperate in a complex mechanism which affect hepcidin expression. Indeed we found that the silencing of the two together had a stronger inhibitory effect on the inhibition of the stimulation by BMP2 on furin and on hepcidin expression (Figures 1C and 2B).
We showed that furin expression was stimulated by BMP2 in a dose-dependent and time-dependent manner (Figure 2A). The induction was abolished by the silencing of TfR2 and HFE, and also by U0126, a specific inhibitor of Erk phosphorylation32 (Figures 2B and 6). This confirms that furin in HepG2 cells is regulated by the MEK/Erk1/2 MAPK cascade, as previously indicated.28 However, also dorsomorphin, a pSMAD inhibitor, reduced furin expression, although slightly and particularly when added together with U0126 (Figure 6). This indicates that furin is also regulated by BMP in a cross-talk between the SMAD1/5/8 and Erk1/2 pathways, similar to the TGF-β1 induction that acts on pErk1/2 and on pSMAD2/3 for the regulation of furin expression.28 It should be noted that the regulation of furin differs largely from that of hepcidin, which is highly sensitive to inhibition by dorsomorphin but not by U0126 (Figure 6B).
Once established that TfR2 and HFE act on furin expression, the role of furin in the regulation of hepcidin remains to be assessed. Furin was indicated as a regulator of hepcidin because of its capacity to process HJV and transform it from a membrane-bound BMP-co-receptor into a soluble antagonist.12 However, furin is also responsible for the processing of prohepcidin into the mature protein.25 Moreover, furin was shown to be involved in the bioactivation of multiple growth/cell differentiation-related factors, which include TGF-β, BMP4, BMP2 and probably most of the BMP family members.27,30 Thus the effect of furin on hepcidin expression may be complex, resulting in activation or inhibition depending on the conditions. And this is what we observed: the suppression of furin activity by the specific proteolytic inhibitor CMK fully suppressed the expression of hepcidin mRNA after 16 h of incubation (Figure 5). The effect was specific, since the levels of HJV, TfR2 and HFE mRNA were not affected by CMK (data not shown), although furin itself was down-regulated (Figure 5). However, the kinetics of CMK treatment was biphasic, with an initial stimulation of hepcidin mRNA and pSMAD1/5/8 followed by gradual suppression that was complete at 16 h (Figure 5B). This can be interpreted by an early effect in which the suppression of furin activity inhibited the production of soluble HJV, resulting in the activation of HJV/BMP/SMAD signaling. In the late phase the absence of furin activity reduced some essential processes probably upstream of HJV signaling. The likely candidate is the processing of BMP members, the production of which is essential for hepcidin expression. That furin-dependent release of soluble HJV is inhibited by CMK had already been demonstrated in a cellular system in which exogenous HJV was expressed;12 unfortunately the tools are not presently available to evaluate the level of endogenous mHJV and sHJV. Likewise, an experimental demonstration that low furin level/activity results in the accumulation of non-functional BMP cannot be approached at present because of the lack of adequate tools.
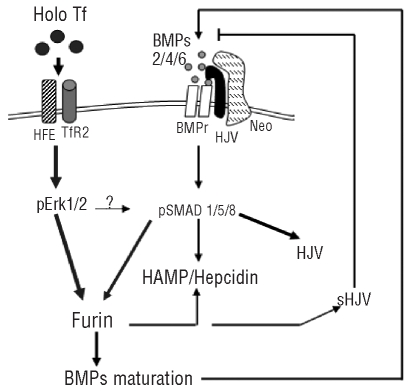
From these data we propose that furin’s multiple roles in processing hepcidin, HJV and BMP members participate in the regulation of hepcidin expression, as summarized in Figure 7. The in vivo data are consistent with this model, since furin and pErk1/2 were down-regulated in TfR2−/− mice, and furin mRNA level was reported to be abnormally low in the liver of subjects with HFE hemochromatosis.39 Moreover, mice in which HFE, TfR2 and both were deleted had lower levels of pErk1/2 in the liver.24 We realize that the model cannot be tested in HepG2 cells, since they do not respond to holotransferrin with hepcidin induction. This was attributed to HFE deficit,20 but we did not observe hepcidin up-regulation when we over-expressed HFE or TfR2 (data not shown). Furin is involved in the processing of key molecules for cellular growth and differentiation processes, and its inactivation is lethal to embryos.40 However the conditional inactivation of furin in the liver did not produce a severe phenotype and all the tested putative targets of furin activity were processed, although to variable degrees.41 Liver functionality was also fully preserved, except for occasional mild congestion, but liver iron load was not analyzed.
Figure 7.
Proposed scheme of the signaling pathway by TfR2 and HFE. Holotransferrin by binding to TfR2 in a complex with HFE, induces Erk1/2 phosphorylation. This, in turn, induces furin expression possibly acting also on the SMAD1/5/8 pathway. Furin participates in the maturation of hepcidin, and of the BMP, which induce hepcidin expression. It also produces the soluble form of HJV, which has an inhibitory effect on hepcidin expression.
In conclusion, the present data indicate that HFE and TfR2 co-operate for holotransferrin sensing which results in furin regulation. The lack of this sensing by the C282Y mutants of HFE may contribute to the development of HFE hemochromatosis. We propose that the iron-dependent (or holotransferrin-dependent) signaling involving TfR2 and HFE acts via the MAPK/Erk pathway which cross-talks with the main BMP/HJV/SMAD pathway. This regulates furin expression, whose role in the maturation of BMP members may be important in the control of hepcidin expression.
Acknowledgments
we are grateful to Dr Clara Camaschella for the generous gift of plasmid pCMV-Sport6-TfR2Hu.
Footnotes
Funding: the work was partially supported by Euroiron1 grant 200-037296, by Telethon-Italy grant GGP05141 and by Murst-Cofin-2006 to PA.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Ganz T. Hepcidin–a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18(2):171–82. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112(2):219–30. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–7. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang AS, West AP, Jr, Wyman AE, Bjorkman PJ, Enns CA. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J Biol Chem. 2005;280(40):33885–94. doi: 10.1074/jbc.M506207200. [DOI] [PubMed] [Google Scholar]
- 6.Du X, She E, Gelbart T, Truksa J, Lee P, Xia Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–92. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–11. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melis MA, Cau M, Congiu R, Sole G, Barella S, Cao A, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93(10):1473–9. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 9.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–9. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 10.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–9. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Domenico I, Ward DM, Kaplan J. Hepcidin regulation: ironing out the details. J Clin Invest. 2007;117(7):1755–8. doi: 10.1172/JCI32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924–31. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Zhou LJ, Zhou Z, Xie JX, Jung JU, Liu Y, et al. Neogenin inhibits HJV secretion and regulates BMP induced hepcidin expression and iron homeostasis. Blood. 2010;115(15):3136–45. doi: 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci USA. 2006;103(27):10289–93. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–81. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata H, Fleming RE, Gui D, Moon SY, Saitoh T, O’Kelly J, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105(1):376–81. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 17.Calzolari A, Raggi C, Deaglio S, Sposi NM, Stafsnes M, Fecchi K, et al. TfR2 localizes in lipid raft domains and is released in exosomes to activate signal transduction along the MAPK pathway. J Cell Sci. 2006;119(Pt 21):4486–98. doi: 10.1242/jcs.03228. [DOI] [PubMed] [Google Scholar]
- 18.Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci USA. 1998;95(4):1472–7. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–8. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9(3):217–27. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7(3):205–14. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110(6):2182–9. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramey G, Deschemin JC, Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94(6):765–72. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50(6):1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 25.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40(1):132–8. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106(8):2884–9. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 27.Constam DB, Robertson EJ. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol. 1999;144(1):139–49. doi: 10.1083/jcb.144.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanchette F, Rivard N, Rudd P, Grondin F, Attisano L, Dubois CM. Cross-talk between the p42/p44 MAP kinase and Smad pathways in transforming growth factor beta 1-induced furin gene transactivation. J Biol Chem. 2001;276(36):33986–94. doi: 10.1074/jbc.M100093200. [DOI] [PubMed] [Google Scholar]
- 29.Roetto A, Di Cunto F, Pellegrino RM, Hirsch E, Azzolino OD, Bondi A, et al. Comparison of three Tfr2-deficient murine models suggests distinct functions for TFR2 alpha and beta isoforms in different tissues. Blood. 2010;115(16):3382–9. doi: 10.1182/blood-2009-09-240960. [DOI] [PubMed] [Google Scholar]
- 30.Degnin C, Jean F, Thomas G, Christian JL. Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol Biol Cell. 2004;15(11):5012–20. doi: 10.1091/mbc.E04-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 33.Nemeth E. Iron regulation and erythropoiesis. Curr Opin Hematol. 2008;15(3):169–75. doi: 10.1097/MOH.0b013e3282f73335. [DOI] [PubMed] [Google Scholar]
- 34.Milet J, Dehais V, Bourgain C, Jouanolle AM, Mosser A, Perrin M, et al. Common variants in the BMP2, BMP4, and HJV genes of the hepcidin regulation pathway modulate HFE hemochromatosis penetrance. Am J Hum Genet. 2007;81(4):799–807. doi: 10.1086/520001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truksa J, Lee P, Beutler E. Two BMP responsive elements, STAT, and bZIP/HNF4/COUP motifs of the hepcidin promoter are critical for BMP, SMAD1, and HJV responsiveness. Blood. 2009;113(3):688–95. doi: 10.1182/blood-2008-05-160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–9. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 37.Kautz L, Meynard D, Besson-Fournier C, Darnaud V, Al Saati T, Coppin H, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114(12):2515–20. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 38.Nohe A, Keating E, Knaus P, Petersen N. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16(3):291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Barisani DPS, Pansa A, Meneveri R, Trombini P, Salvioni A, Mariani R, Piperno A. Furin expression is decreased in the liver of HFE-hemochtomatosis patients. 2009 International Bioiron Society Meeting; Porto, Portugal 7–11 June 2009; Abstract 189. [Google Scholar]
- 40.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20(12):1954–63. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 41.Roebroek AJ, Taylor NA, Louagie E, Pauli I, Smeijers L, Snellinx A, et al. Limited redundancy of the proprotein convertase furin in mouse liver. J Biol Chem. 2004;279(51):53442–50. doi: 10.1074/jbc.M407152200. [DOI] [PubMed] [Google Scholar]