Abstract
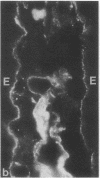
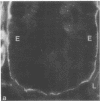
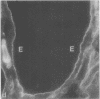
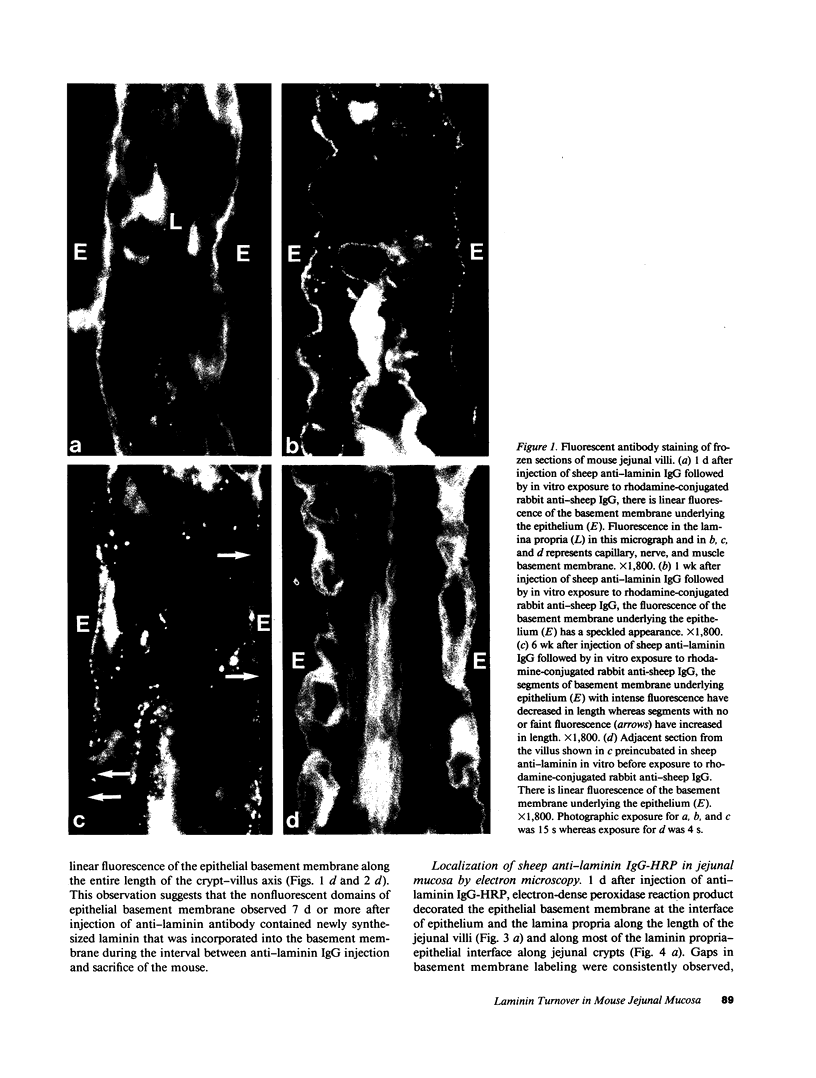
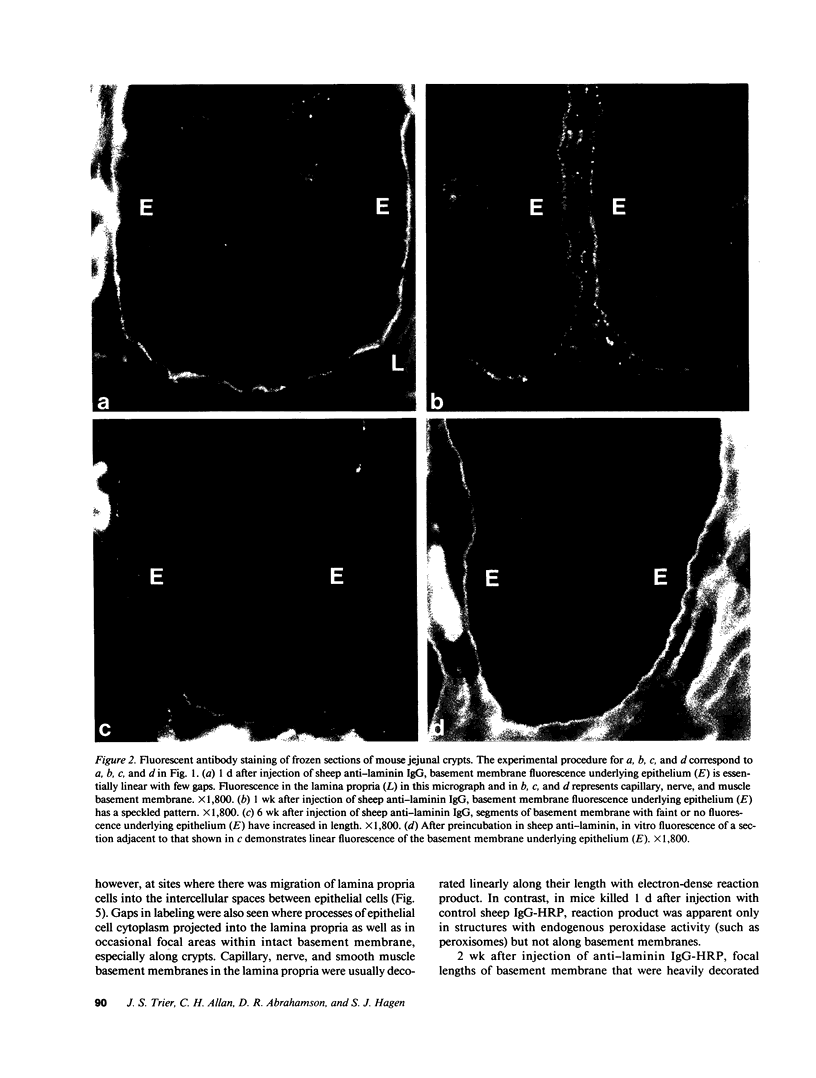
Little is known regarding turnover of the epithelial basement membrane in adult small intestine. Are components degraded and inserted along the length of the crypt-villus axis or selectively in the crypt region with subsequent migration of basement membrane from crypt to villus tip in concert with epithelium? We injected affinity-purified sheep anti-laminin IgG or sheep anti-laminin IgG complexed to horseradish peroxidase (HRP) into mice to label basement membrane laminin in vivo. Fluorescence microscopy revealed linear fluorescence along the length of the jejunal epithelial basement membrane 1 d after anti-laminin IgG injection. By 1 wk, small nonfluorescent domains were interposed between larger fluorescent domains. Over the next 5 wk the lengths of nonfluorescent domains increased progressively whereas those of fluorescent domains decreased. Additionally, electron microscopy revealed HRP reaction product along the length of the epithelial basement membrane after 1 d whereas unlabeled or lightly labeled domains that increased in length with time were observed interposed between heavily labeled domains by 2 and 4 wk along the entire crypt-villus axis. We conclude that laminin turnover occurs focally in the epithelial basement membrane of mouse jejunum along the crypt-villus axis over a period of weeks and that this basement membrane does not comigrate in concert with its overlying epithelium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Abrahamson D. R., Caulfield J. P. Proteinuria and structural alterations in rat glomerular basement membranes induced by intravenously injected anti-laminin immunoglobulin G. J Exp Med. 1982 Jul 1;156(1):128–145. doi: 10.1084/jem.156.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R., Irwin M. H., St John P. L., Perry E. W., Accavitti M. A., Heck L. W., Couchman J. R. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989 Dec;109(6 Pt 2):3477–3491. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol. 1985 Jun;100(6):1988–2000. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson D. R., Perry E. W. Evidence for splicing new basement membrane into old during glomerular development in newborn rat kidneys. J Cell Biol. 1986 Dec;103(6 Pt 1):2489–2498. doi: 10.1083/jcb.103.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M., Banerjee S. D., Koda J. E., Rapraeger A. C. Remodelling of the basement membrane: morphogenesis and maturation. Ciba Found Symp. 1984;108:179–196. doi: 10.1002/9780470720899.ch12. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Banerjee S. D. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol. 1982 Apr;90(2):291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Arenson D. M., Maher J. J., Roll F. J. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987 Mar;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins W. O., 3rd Human intestinal intraepithelial lymphocytes. Gut. 1986 Aug;27(8):972–985. doi: 10.1136/gut.27.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood G. L. Gastrointestinal epithelial renewal. Gastroenterology. 1977 May;72(5 Pt 1):962–975. [PubMed] [Google Scholar]
- Feil W., Wenzl E., Vattay P., Starlinger M., Sogukoglu T., Schiessel R. Repair of rabbit duodenal mucosa after acid injury in vivo and in vitro. Gastroenterology. 1987 Jun;92(6):1973–1986. doi: 10.1016/0016-5085(87)90632-9. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Risse G., von der Mark K. The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol. 1989 Aug;109(2):799–809. doi: 10.1083/jcb.109.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley M. A., Byers S. W., Suárez-Quian C. A., Kleinman H. K., Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985 Oct;101(4):1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen S. J., Trier J. S. Immunocytochemical localization of actin in epithelial cells of rat small intestine by light and electron microscopy. J Histochem Cytochem. 1988 Jul;36(7):717–727. doi: 10.1177/36.7.3290330. [DOI] [PubMed] [Google Scholar]
- Hahn U., Schuppan D., Hahn E. G., Merker H. J., Riecken E. O. Intestinal cells produce basement membrane proteins in vitro. Gut. 1987;28 (Suppl):143–151. doi: 10.1136/gut.28.suppl.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Jamieson J. D. Basement membrane as a spatial organizer of polarized epithelia. Exogenous basement membrane reorients pancreatic epithelial tumor cells in vitro. Am J Pathol. 1986 Jan;122(1):129–139. [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987 Jan;92(1):68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- Kedinger M., Haffen K., Simon-Assmann P. Intestinal tissue and cell cultures. Differentiation. 1987;36(1):71–85. doi: 10.1111/j.1432-0436.1987.tb00182.x. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Martin G. R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982 Oct;95(1):340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leardkamolkarn V., Abrahamson D. R. Binding of intravenously injected antibodies against laminin to developing and mature endocrine glands. Cell Tissue Res. 1988 Jan;251(1):171–181. doi: 10.1007/BF00215462. [DOI] [PubMed] [Google Scholar]
- Marsh M. N., Trier J. S. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. I. Structural features. Gastroenterology. 1974 Oct;67(4):622–635. [PubMed] [Google Scholar]
- Marsh M. N., Trier J. S. Morphology and cell proliferation of subepithelial fibroblasts in adult mouse jejunum. II. Radioautographic studies. Gastroenterology. 1974 Oct;67(4):636–645. [PubMed] [Google Scholar]
- Martinez-Hernandez A., Chung A. E. The ultrastructural localization of two basement membrane components: entactin and laminin in rat tissues. J Histochem Cytochem. 1984 Mar;32(3):289–298. doi: 10.1177/32.3.6198358. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Fink L. M., Pierce G. B. Removal of basement membrane in the involuting breast. Lab Invest. 1976 May;34(5):455–462. [PubMed] [Google Scholar]
- Mathan M., Hermos J. A., Trier J. S. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972 Mar;52(3):577–588. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClugage S. G., Low F. N. Microdissection by ultrasonication: porosity of the intestinal epithelial basal lamina. Am J Anat. 1984 Oct;171(2):207–216. doi: 10.1002/aja.1001710206. [DOI] [PubMed] [Google Scholar]
- McGuire P. G., Seeds N. W. The interaction of plasminogen activator with a reconstituted basement membrane matrix and extracellular macromolecules produced by cultured epithelial cells. J Cell Biochem. 1989 Jun;40(2):215–227. doi: 10.1002/jcb.240400210. [DOI] [PubMed] [Google Scholar]
- Moore R., Carlson S., Madara J. L. Rapid barrier restitution in an in vitro model of intestinal epithelial injury. Lab Invest. 1989 Feb;60(2):237–244. [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Neal J. V., Potten C. S. Description and basic cell kinetics of the murine pericryptal fibroblast sheath. Gut. 1981 Jan;22(1):19–24. doi: 10.1136/gut.22.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker F. G., Barnes E. N., Kaye G. I. The pericryptal fibroblast sheath. IV. Replication, migration, and differentiation of the subepithelial fibroblasts of the crypt and villus of the rabbit jejunum. Gastroenterology. 1974 Oct;67(4):607–621. [PubMed] [Google Scholar]
- Price R. G., Spiro R. G. Studies on the metabolism of the renal glomerular basement membrane. Turnover measurements in the rat with the use of radiolabeled amino acids. J Biol Chem. 1977 Dec 10;252(23):8597–8602. [PubMed] [Google Scholar]
- Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol. 1980 Apr;85(1):18–32. doi: 10.1083/jcb.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P., Bouziges F., Arnold C., Haffen K., Kedinger M. Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development. 1988 Feb;102(2):339–347. doi: 10.1242/dev.102.2.339. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P., Bouziges F., Vigny M., Kedinger M. Origin and deposition of basement membrane heparan sulfate proteoglycan in the developing intestine. J Cell Biol. 1989 Oct;109(4 Pt 1):1837–1848. doi: 10.1083/jcb.109.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Assmann P., Kedinger M., Haffen K. Immunocytochemical localization of extracellular-matrix proteins in relation to rat intestinal morphogenesis. Differentiation. 1986;32(1):59–66. doi: 10.1111/j.1432-0436.1986.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Gundersen D., Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. J Cell Biol. 1987 Apr;104(4):905–916. doi: 10.1083/jcb.104.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]