Abstract
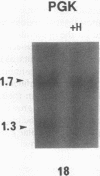
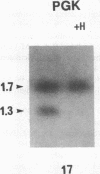
We determined clonality of thyroid tumors from female patients who had restriction fragment length polymorphisms (RFLP) in the X chromosome genes hypoxanthine phosphoribosyltransferase (HPRT) or phosphoglycerate kinase (PGK). We screened normal thyroid tissue from 59 female patients; of the informative cases 14 were heterozygous for a Bgl I site on PGK and 4 were heterozygous for a Bam HI site on HPRT. In monoclonal tumors, one of the polymorphic alleles was selectively digested after additional digestion with Hpa II, a methylation sensitive enzyme, whereas in polyclonal tissue both were decreased to a similar extent. Normal thyroid tissue from all patients showed a polyclonal pattern. Of the 18 tumors studied, 12 were solitary thyroid nodules, and 6 were obtained from multinodular goiters (MNG). The following were monoclonal: 6/6 follicular adenomas, 2/2 follicular carcinomas, and 1/1 anaplastic carcinoma. Two of the three papillary carcinomas showed intermediate patterns, possibly due to contaminating effects of stromal tissue present in most of these neoplasms. Of the six nodules from MNG, four were polyclonal. The two largest gave a distinct monoclonal pattern. Most solitary thyroid tumors are monoclonal, supporting a somatic cell mutation model of thyroid neoplasm formation. Nodules from MNG are largely hyperplastic, although monoclonal neoplasms do occasionally arise within these glands. The specific somatic mutations leading to clonal expansion and determination of tumor phenotype are presently unknown.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Staunton C. E., Kim H. G., Gaz R. D., Kronenberg H. M. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med. 1988 Mar 17;318(11):658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gartler S. M., Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J., Sagebiel R. W., Gartler S. M., Rimoin D. L. Multiple cell origin of hereditary neurofibromas. N Engl J Med. 1971 Feb 11;284(6):298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Fusco A., Grieco M., Santoro M., Berlingieri M. T., Pilotti S., Pierotti M. A., Della Porta G., Vecchio G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987 Jul 9;328(6126):170–172. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gerber H., Studer H., Conti A., Engler H., Kohler H., Haeberli A. Reaccumulation of thyroglobulin and colloid in rat and mouse thyroid follicles during intense thyrotropin stimulation. A clue to the pathogenesis of colloid goiters. J Clin Invest. 1981 Nov;68(5):1338–1347. doi: 10.1172/JCI110381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Axelman J., Beggs A. H. Effect of ageing on reactivation of the human X-linked HPRT locus. Nature. 1988 Sep 1;335(6185):93–96. doi: 10.1038/335093a0. [DOI] [PubMed] [Google Scholar]
- Peter H. J., Gerber H., Studer H., Becker D. V., Peterson M. E. Autonomy of growth and of iodine metabolism in hyperthyroid feline goiters transplanted onto nude mice. J Clin Invest. 1987 Aug;80(2):491–498. doi: 10.1172/JCI113097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H. J., Studer H., Groscurth P. Autonomous growth, but not autonomous function, in embryonic human thyroids: a clue to understanding autonomous goiter growth? J Clin Endocrinol Metab. 1988 May;66(5):968–973. doi: 10.1210/jcem-66-5-968. [DOI] [PubMed] [Google Scholar]
- Rojeski M. T., Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985 Aug 15;313(7):428–436. doi: 10.1056/NEJM198508153130707. [DOI] [PubMed] [Google Scholar]
- Ron E., Griffel B., Liban E., Modan B. Histopathologic reproducibility of thyroid disease in an epidemiologic study. Cancer. 1986 Mar 1;57(5):1056–1059. doi: 10.1002/1097-0142(19860301)57:5<1056::aid-cncr2820570531>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Saxén E., Franssila K., Bjarnason O., Normann T., Ringertz N. Observer variation in histologic classification of thyroid cancer. Acta Pathol Microbiol Scand A. 1978 Nov;86A(6):483–486. doi: 10.1111/j.1699-0463.1978.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studer H., Peter H. J., Gerber H. Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev. 1989 May;10(2):125–135. doi: 10.1210/edrv-10-2-125. [DOI] [PubMed] [Google Scholar]
- Thomas G. A., Williams D., Williams E. D. The clonal origin of thyroid nodules and adenomas. Am J Pathol. 1989 Jan;134(1):141–147. [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]