Abstract
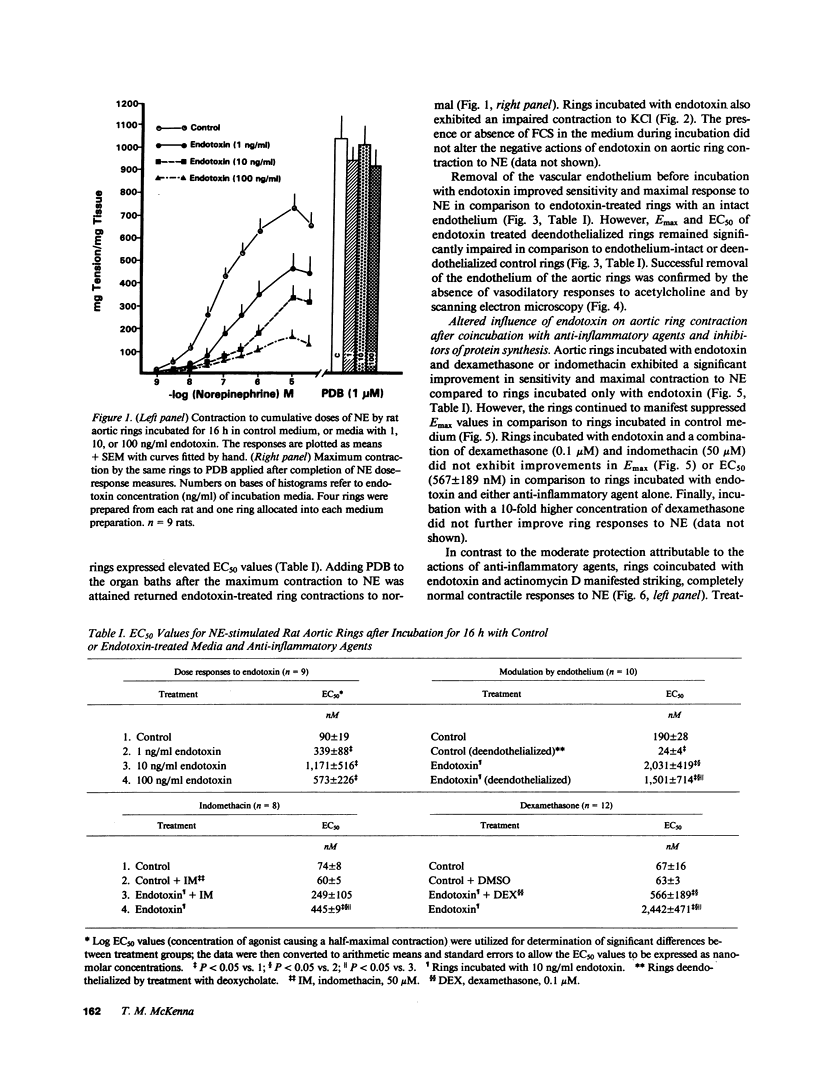
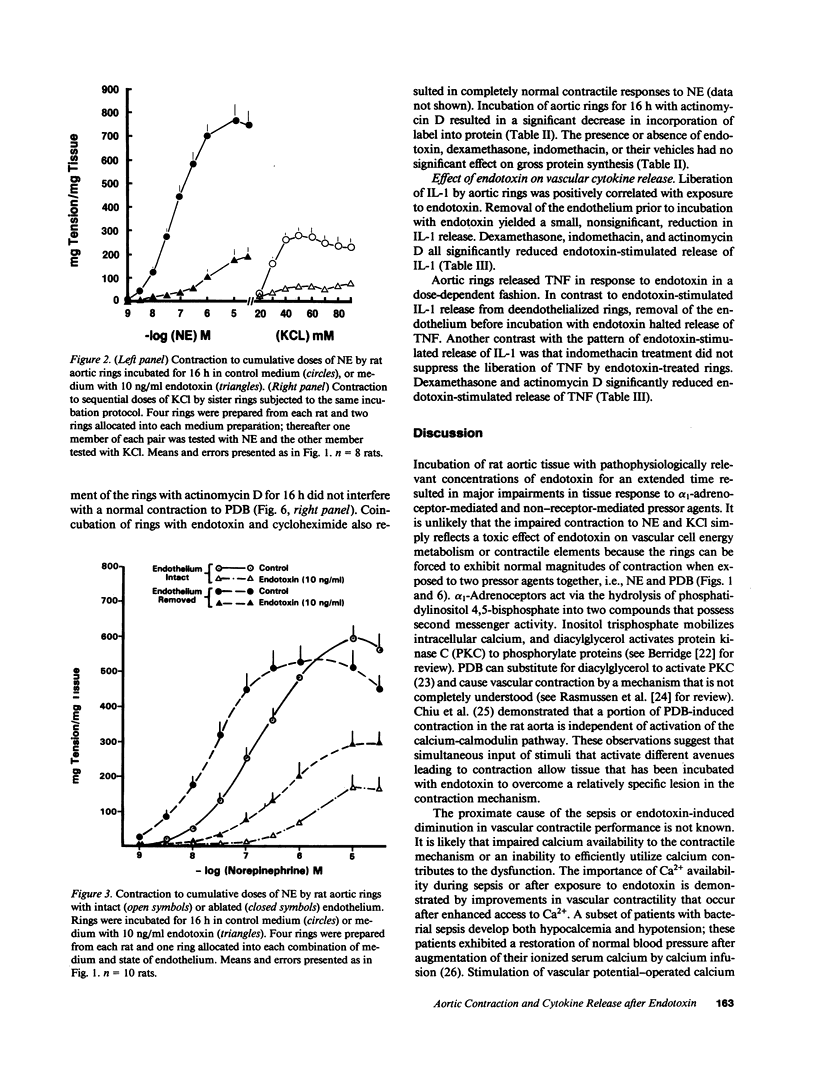
Treatment of volunteers or animals with endotoxin in vivo results in reduced vascular reactivity to catecholamines. Endotoxin also causes liberation of the vasoactive cytokines interleukin-1 (IL-1) and tumor necrosis factor (TNF) from vascular smooth muscle and endothelial cells in culture. This study tested whether defects in contractility could be induced in isolated vascular tissue by prolonged exposure to endotoxin (1-100 ng/ml) in vitro, and whether IL-1 and TNF release by blood vessels is altered during the establishment of endotoxin induced contractile dysfunction. A concentration of endotoxin as low as 1 ng/ml suppressed contractions to norepinephrine (NE) and KCl; aortic sensitivity to NE also decreased. The presence of serum constituents or an intact endothelium were not necessary for endotoxin-induced vascular suppression. Aortas incubated with endotoxin liberated IL-1 and TNF in a dose-dependent fashion. The addition of dexamethasone or indomethacin during the incubations generally suppressed release of the cytokines and improved tissue reactivity to NE. The endotoxin-induced diminished vascular contraction and augmented IL-1 and TNF liberation required de novo protein synthesis; tissue incubated with endotoxin plus actinomycin D was completely shielded from the influence of endotoxin on vascular reactivity to NE. The association between endotoxin-induced vascular cytokine release and diminished contraction suggests a possible role for cytokines derived from the vasculature in the regulation of contractile function.
Full text
PDF

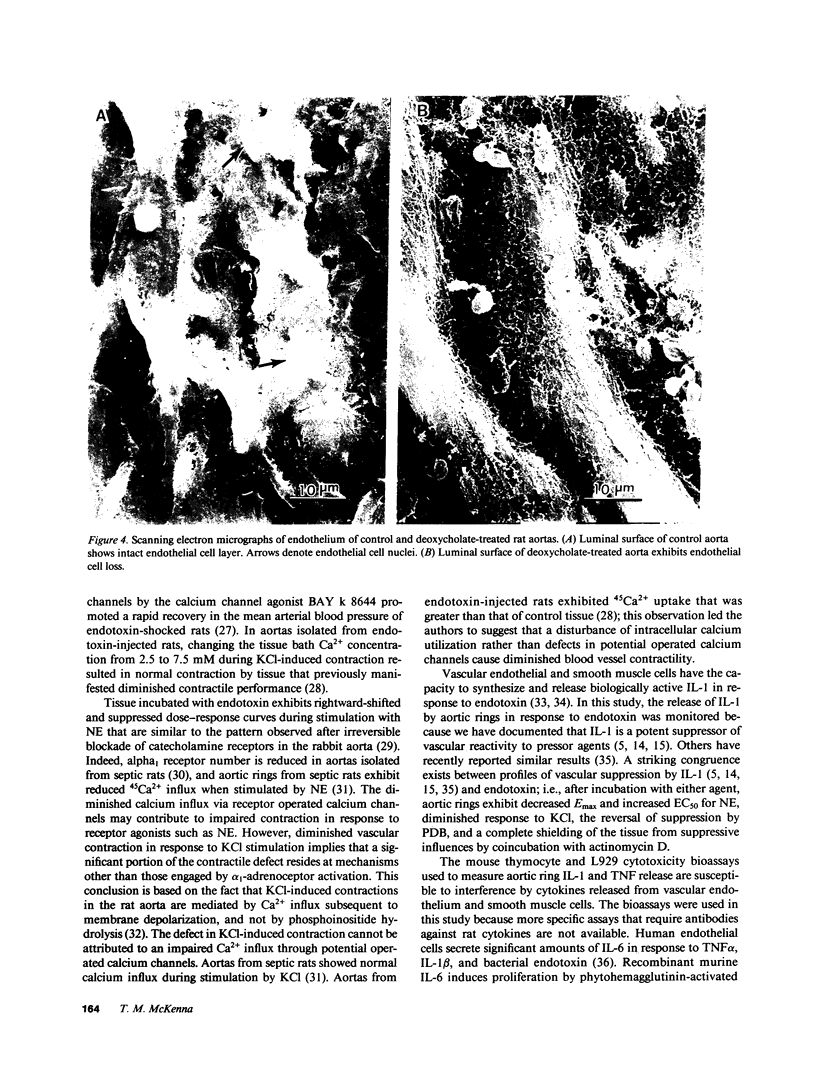
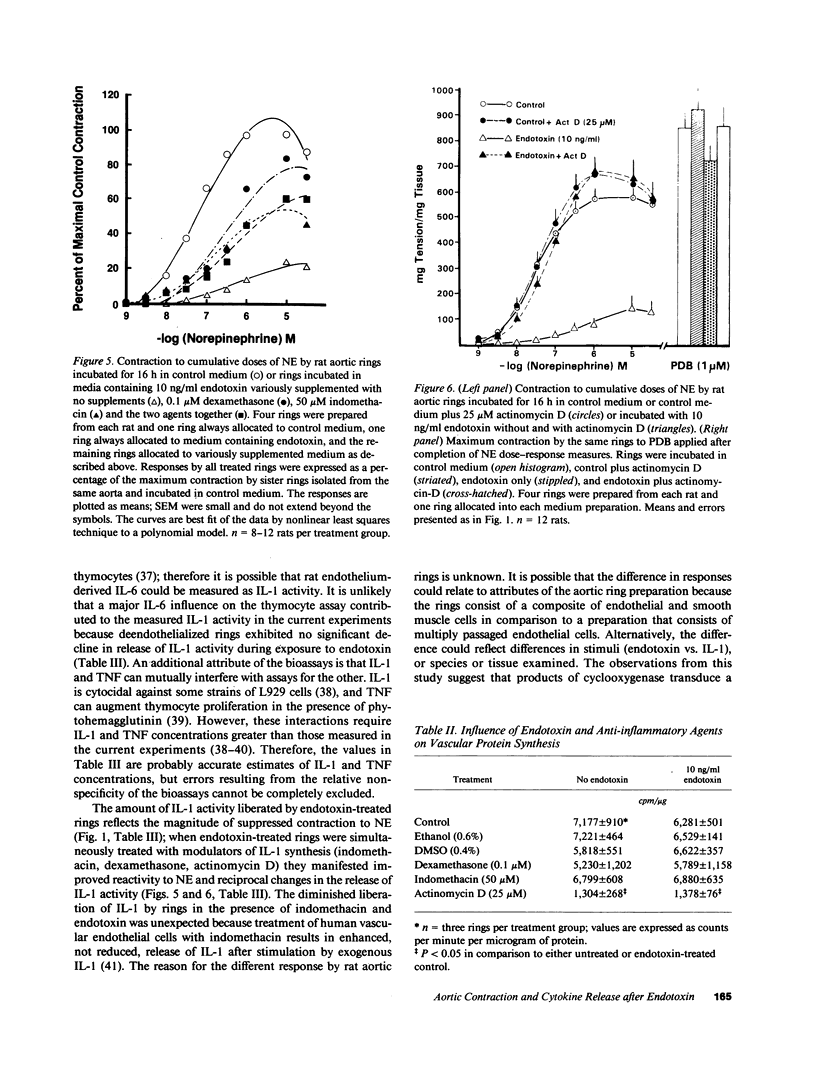



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Gebrewold A., Burton R. W. Failure of microscopic metarterioles to elicit vasodilator responses to acetylcholine, bradykinin, histamine and substance P after ischemic shock, endotoxemia and trauma: possible role of endothelial cells. Microcirc Endothelium Lymphatics. 1985 Apr;2(2):121–127. [PubMed] [Google Scholar]
- Bailey J. M., Makheja A. N., Pash J., Verma M. Corticosteroids suppress cyclooxygenase messenger RNA levels and prostanoid synthesis in cultured vascular cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1159–1163. doi: 10.1016/s0006-291x(88)80995-1. [DOI] [PubMed] [Google Scholar]
- Baker C. H., Wilmoth F. R., Sutton E. T. Reduced RBC versus plasma microvascular flow due to endotoxin. Circ Shock. 1986;20(2):127–139. [PubMed] [Google Scholar]
- Beasley D., Cohen R. A., Levinsky N. G. Interleukin 1 inhibits contraction of vascular smooth muscle. J Clin Invest. 1989 Jan;83(1):331–335. doi: 10.1172/JCI113879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989 Feb;159(2):195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Carcillo J. A., Litten R. Z., Suba E. A., Roth B. L. Alterations in rat aortic alpha 1-adrenoceptors and alpha 1-adrenergic stimulated phosphoinositide hydrolysis in intraperitoneal sepsis. Circ Shock. 1988 Nov;26(3):331–339. [PubMed] [Google Scholar]
- Chiu A. T., Bozarth J. M., Forsythe M. S., Timmermans P. B. Ca++ utilization in the constriction of rat aorta to stimulation of protein kinase C by phorbol dibutyrate. J Pharmacol Exp Ther. 1987 Sep;242(3):934–939. [PubMed] [Google Scholar]
- Chiu A. T., Bozarth J. M., Timmermans P. B. Relationship between phosphatidylinositol turnover and Ca++ mobilization induced by alpha-1 adrenoceptor stimulation in the rat aorta. J Pharmacol Exp Ther. 1987 Jan;240(1):123–127. [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F. Dibenamine blockade in strips of rabbit aorta and its use in differentiating receptors. J Pharmacol Exp Ther. 1954 Jul;111(3):265–284. [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M. P., Homer L. D., Fletcher J. R. Diminished pressor response to exogenous norepinephrine and angiotensin II in septic, unanesthetized rats: evidence for a prostaglandin-mediated effect. J Surg Res. 1985 Apr;38(4):335–342. doi: 10.1016/0022-4804(85)90046-0. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P., De la Lande I. S., Jellett L. B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther. 1972 May;181(2):339–345. [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- GREISMAN S. E., HORNIK R. B., CAROZZA F. A., Jr, WOODWARD T. E. THE ROLE OF ENDOTOXIN DURING TYPHOID FEVER AND TULAREMIA IN MAN. II. ALTERED CARDIOVASCULAR RESPONSES TO CATECHOLAMINES. J Clin Invest. 1964 May;43:986–999. doi: 10.1172/JCI104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld A. B., Nauta J. J., Thijs L. G. Peripheral vascular resistance in septic shock: its relation to outcome. Intensive Care Med. 1988;14(2):141–147. doi: 10.1007/BF00257468. [DOI] [PubMed] [Google Scholar]
- Groves A. C., Griffiths J., Leung F., Meek R. N. Plasma catecholamines in patients with serious postoperative infection. Ann Surg. 1973 Jul;178(1):102–107. doi: 10.1097/00000658-197307000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett G., Stünkel K. G., Schlumberger H. D. A method for the quantitation of interleukin-2 activity. J Immunol Methods. 1989 Feb 24;117(2):243–246. doi: 10.1016/0022-1759(89)90146-4. [DOI] [PubMed] [Google Scholar]
- Howells G. L., Chantry D., Feldmann M. Interleukin 1 (IL-1) and tumour necrosis factor synergise in the induction of IL-1 synthesis by human vascular endothelial cells. Immunol Lett. 1988 Oct;19(2):169–173. doi: 10.1016/0165-2478(88)90138-1. [DOI] [PubMed] [Google Scholar]
- Ives N., King J. W., Chernow B., Roth B. L. BAY k 8644, a calcium channel agonist, reverses hypotension in endotoxin-shocked rats. Eur J Pharmacol. 1986 Nov 4;130(3):169–175. doi: 10.1016/0014-2999(86)90265-7. [DOI] [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987 Dec 15;139(12):4129–4134. [PubMed] [Google Scholar]
- Lang C. H., Bagby G. J., Ferguson J. L., Spitzer J. J. Cardiac output and redistribution of organ blood flow in hypermetabolic sepsis. Am J Physiol. 1984 Mar;246(3 Pt 2):R331–R337. doi: 10.1152/ajpregu.1984.246.3.R331. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten R. Z., Carcillo J. A., Roth B. L. Alterations in bidirectional transmembrane calcium flux occur without changes in protein kinase C levels in rat aorta during sepsis. Circ Shock. 1988 Jun;25(2):123–130. [PubMed] [Google Scholar]
- McKenna T. M., Lueders J. E., Titius W. A. Monocyte-derived interleukin 1: effects on norepinephrine-stimulated aortic contraction and phosphoinositide turnover. Circ Shock. 1989 Jun;28(2):131–147. [PubMed] [Google Scholar]
- McKenna T. M., Reusch D. W., Simpkins C. O. Macrophage-conditioned medium and interleukin 1 suppress vascular contractility. Circ Shock. 1988 Jul;25(3):187–196. [PubMed] [Google Scholar]
- McKenna T. M., Titius W. A. Role of monokines in altering receptor and non-receptor mediated vascular contraction in sepsis. Prog Clin Biol Res. 1989;286:279–303. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- NAGLER A. L., THOMAS L., ZWEIFACH B. W. The role of epinephrine in the reactions produced by the endotoxins of gram-negative bacteria. II. The changes produced by endotoxin in the vascular reactivity to epinephrine, in the rat mesoappendix and the isolated, perfused rabbit ear. J Exp Med. 1956 Dec 1;104(6):881–896. doi: 10.1084/jem.104.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Aggarwal B. B., Oppenheim J. J. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985 Dec;135(6):3962–3968. [PubMed] [Google Scholar]
- Parker M. M., Parrillo J. E. Septic shock. Hemodynamics and pathogenesis. JAMA. 1983 Dec 23;250(24):3324–3327. [PubMed] [Google Scholar]
- Parratt J. R. Myocardial and circulatory effects of E. coli endotoxin; modification of responses to catecholamines. Br J Pharmacol. 1973 Jan;47(1):12–25. doi: 10.1111/j.1476-5381.1973.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson F. C., Dubczak J., Weary M., Bruszer G., Donohue G. Detection of endotoxin in the plasma of patients with gram-negative bacterial sepsis by the Limulus amoebocyte lysate assay. J Clin Microbiol. 1985 Jun;21(6):865–868. doi: 10.1128/jcm.21.6.865-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz K., Casey L., Fletcher J. R., Ramwell P. W. Vascular reactivity in endotoxin shock: effect of lidocaine or indomethacin pretreatment. Adv Shock Res. 1982;7:191–198. [PubMed] [Google Scholar]
- Ranges G. E., Zlotnik A., Espevik T., Dinarello C. A., Cerami A., Palladino M. A., Jr Tumor necrosis factor alpha/cachectin is a growth factor for thymocytes. Synergistic interactions with other cytokines. J Exp Med. 1988 Apr 1;167(4):1472–1478. doi: 10.1084/jem.167.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacker P. T., Samsel R. W. Oxygen delivery and uptake by peripheral tissues: physiology and pathophysiology. Crit Care Clin. 1989 Apr;5(2):255–269. [PubMed] [Google Scholar]
- Suda T., Hodgkin P., Lee F., Zlotnik A. Biological activity of recombinant murine interleukin-6 in interleukin-1 T cell assays. J Immunol Methods. 1989 Jun 21;120(2):173–178. doi: 10.1016/0022-1759(89)90239-1. [DOI] [PubMed] [Google Scholar]
- Townsend M. C., Hampton W. W., Haybron D. M., Schirmer W. J., Fry D. E. Effective organ blood flow and bioenergy status in murine peritonitis. Surgery. 1986 Aug;100(2):205–213. [PubMed] [Google Scholar]
- Wakabayashi I., Hatake K., Kakishita E., Nagai K. Diminution of contractile response of the aorta from endotoxin-injected rats. Eur J Pharmacol. 1987 Sep 2;141(1):117–122. doi: 10.1016/0014-2999(87)90417-1. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]
- Wessels B. C., Wells M. T., Gaffin S. L., Brock-Utne J. G., Gathiram P., Hinshaw L. B. Plasma endotoxin concentration in healthy primates and during E. coli-induced shock. Crit Care Med. 1988 Jun;16(6):601–605. doi: 10.1097/00003246-198806000-00007. [DOI] [PubMed] [Google Scholar]
- Whitworth P. W., Cryer H. M., Garrison R. N., Baumgarten T. E., Harris P. D. Hypoperfusion of the intestinal microcirculation without decreased cardiac output during live Escherichia coli sepsis in rats. Circ Shock. 1989 Feb;27(2):111–122. [PubMed] [Google Scholar]
- Zaloga G. P., Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987 Jul;107(1):36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Buller H. R., ten Cate J. W., Sturk A., Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988 Mar 19;1(8586):605–609. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]