Abstract
Perineural tumour spread refers to a contiguous neoplastic extension along a nerve. As it may be clinically silent, imaging plays a pivotal role in the evaluation and delineation of perineural infiltration in head and neck malignancies, which in turn affects treatment planning. This article focuses on the imaging features of perineural spread of head and neck tumours. The important potential perineural pathways and the possible underlying pathogenesis of this phenomenon are also reviewed.
Keywords: Perineural spread, head and neck tumours
Introduction
Perineural spread of head and neck tumours is a well-described phenomenon in the surgical and imaging literature. It implies direct neoplastic extension from a primary tumour using the nerve as a scaffold, and is of major clinical importance.
Perineural tumour spread carries a grave prognosis. It is associated with a nearly threefold increase in local recurrence and approximately 30% decrease in 5-year survival rate[1–4]. Perineural spread is now considered an independent prognostic indicator during tumour staging, as per the latest edition of the TNM classification of malignant tumours[5].
Up to 30–45% of patients with extensive perineural spread may remain asymptomatic, with normal nerve function at clinical examination[6,7]. Others present with subtle or non-specific clinical manifestations, which are often overlooked or misdiagnosed with benign conditions such as Bell palsy or trigeminal neuralgia[8,9].
Imaging therefore plays a critical role in the assessment of perineural spread in head and neck tumours. Precise radiologic identification and delineation of perineural malignancy may mean the difference between partial and complete surgical resection, or adequate and insufficient radiation treatment field[10].
Perineural tumour spread
Definition
Perineural tumour spread refers to extension of benign or malignant tumour along a nerve, which may reach a significant distance from the primary lesion and should be distinguished from perineural tumour invasion, which describes neoplastic infiltration of a nerve at the original tumour site. Strictly speaking, the term perineural spread should be used only for tumours infiltrating and extending along the perineurium, the condensed fascia surrounding each fascicle or bundle of nerve fibres, of a nerve. Nevertheless, this term is generally accepted to encompass neoplastic spread along any or all compartments within a nerve[11].
Pathogenesis
The precise mechanism of perineural tumour spread remains unclear. It is possible that the nerve simply presents a natural conduit of least resistance for the neoplastic cells to traverse. Intraneural lymphatics have been proposed as the route of dissemination. However, this theory is now firmly rejected as no lymphatic endothelial cell has ever been seen bordering the perineural tumour[12]. This has major therapeutic significance, as it implies that perineural tumour spread is contiguous. In the absence of tumour embolism through the perineural lymphatics, a complete surgical resection of an affected nerve should be curative[10].
The incidence of perineural tumour spread ranges from 2.5 to 5.0%, and may occur with a wide variety of head and neck malignancies[13]. Adenoid cystic carcinoma arising from the salivary gland is the malignancy most commonly associated with perineural spread, with a rate of up to 60%[14]. This may be related to a high expression of neural cell adhesion molecules (N-CAM), which are detected in approximately 93% of adenoid cystic carcinomas with perineural spread[15,16]. In practice, perineural spread is actually more frequently encountered in squamous cell carcinoma, a reflection of the much higher incidence of squamous cell carcinoma in head and neck malignancy[10]. Expression of N-CAM is also found in approximately 93% of squamous cell carcinomas with perineural spread[17].
The p75 neurotrophin receptor has also attracted much interest in the study of perineural tumour spread. Strong immunologic staining for p75 receptor has been shown in the desmoplastic variant of melanoma (which has a striking propensity for perineural spread) and a small series of adenoid cystic carcinomas[18,19]. During the development of the nervous system, it is the interaction between the nerve growth factor and the p75 receptor located on Schwann cells that stimulate the migration of Schwann cells to and along a nerve. It is possible that a similar mechanism mediates the malignant invasion and subsequent spread along a nerve.
Perineural pathways
Essentially, any nerve may serve as a conduit for the spread of head and neck tumours. The maxillary and mandibular divisions of the trigeminal nerve, and the facial nerve are most commonly implicated. The ophthalmic division of the trigeminal nerve and the hypoglossal nerve are less frequently involved; tumour infiltrations along the other cranial nerves have also been reported[6,20–22].
Perineural spread along the maxillary nerve may occur with cutaneous tumours arising from the facial region innervated by its peripheral branches, and oropharyngeal or sinonasal neoplasms. In addition, any skull base malignancies that infiltrate the pterygopalatine fossa may also extend intracranially through the foramen rotundum along the maxillary nerve[23].
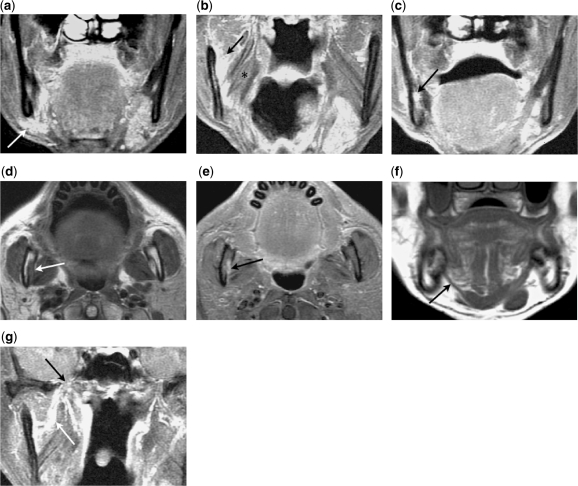
Mandibular nerve involvement is often seen in masticator space tumours and skull base cancers, such as nasopharyngeal carcinoma[24]. Malignancies of the submandibular gland, tongue and floor of the mouth may invade the lingual or inferior alveolar nerve, and track retrogradely to the mandibular nerve (Fig. 1).
Figure 1.
A 59-year-old male with previously resected adenoid cystic carcinoma of the right submandibular gland. (a) Coronal contrast-enhanced fat-suppressed T1-weighted image shows enhancing recurrent tumour in the right submandibular fossa (arrow). (b) Coronal contrast-enhanced fat-suppressed T1-weighted image shows retrograde tumour spread along the right lingual nerve to the mandibular nerve (arrows), which travels between the medial pterygoid (asterisk) and the mandible. (c) Coronal contrast-enhanced fat-suppressed T1-weighted image shows subsequent antegrade tumour spread along the right inferior alveolar nerve within the mandible (arrow). (d) Axial T1-weighted image shows perineural tumour along the right inferior alveolar nerve effacing the fat normally seen at the orifice of the mandibular foramen (arrow). (e) Axial contrast-enhanced fat-suppressed T1-weighted image shows the thickened and enhancing inferior alveolar nerve as it enters the mandibular foramen (arrow). The enhancement is more conspicuous due to nulling of the surrounding hyperintense fat signal. (f) Coronal T1-weighted image shows marked denervation atrophy of the right mylohyoid muscle (arrow), consistent with tumour involvement of the mylohyoid nerve, a branch of the mandibular nerve. (g) A coronal contrast-enhanced fat-suppressed T1-weighted image shows further perineural spread along the mandibular nerve (white arrow) toward the foramen ovale (black arrow).
Malignant neoplasms of the parotid gland may invade the facial nerve and spread along the intratemporal facial canal, as far as the fundus of the internal auditory canal. Less commonly, these tumours can track along the auriculotemporal nerve back to the mandibular nerve[25]. The facial nerve may also be involved by tumours in the pterygopalatine fossa, which extends along the vidian and greater superficial petrosal nerves to reach the geniculate ganglion[26].
Perineural tumours typically extend centripetally toward the brain, although centrifugal spread is not uncommonly seen (Figs. 2 and 3).
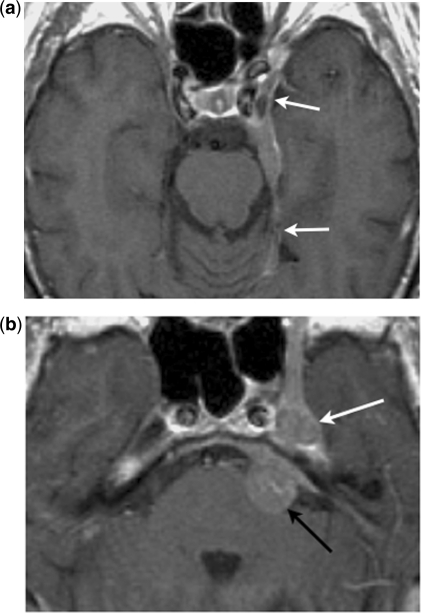
Figure 2.
Centripetal perineural spread. Axial contrast-enhanced T1-weighted images show (a) an en-plaque meningioma along the lateral wall of the left cavernous sinus and tentorial leaf (arrows), with (b) centripetal perineural spread to the trigeminal ganglion in the Meckel cave (white arrow) and the cisternal segment of the trigeminal nerve (black arrow).
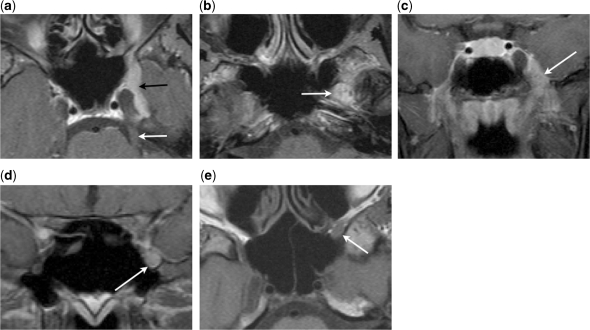
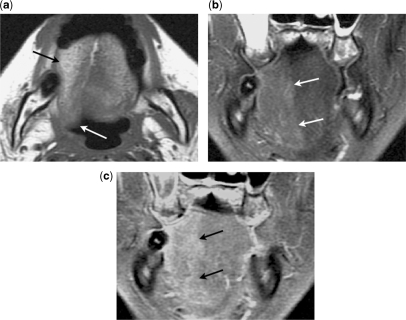
Figure 3.
Centrifugal perineural spread. (a) Axial contrast-enhanced fat-suppressed T1-weighted image shows a meningioma arising from the lateral wall of the left cavernous sinus (black arrow). No centripetal perineural spread to the cisternal segment of the left trigeminal nerve is seen (white arrow). (b) Axial and (c) coronal contrast-enhanced fat-suppressed T1-weighted images show centrifugal perineural tumour spread along the mandibular nerve through the foramen ovale (arrow). (d) Coronal contrast-enhanced fat-suppressed T1-weighted image shows centrifugal perineural tumour spread along the maxillary nerve through the foramen rotundum (arrow). (e) Axial T1-weighted image shows effacement of the normal fat tissue within the left pterygopalatine fossa by the perineural tumour along the maxillary nerve (arrow).
Imaging of perineural tumour spread
Imaging of perineural tumour spread is best accomplished with contrast-enhanced magnetic resonance (MR) imaging, due to its superior soft tissue contrast and less artefact from any dental hardware compared with computed tomography (CT)[27]. Multiplanar imaging is essential to fully evaluate the skull base. Coronal images are particularly important in delineating the mandibular nerve through the foramen ovale and the mastoid segment of the intratemporal facial nerve. Nemzek et al.[28] showed that the sensitivity of MR imaging in detecting perineural spread was as high as 95%.
Primary imaging features
Perineural tumour spread disrupts the blood-nerve barrier and results in increased permeability of the endoneurial capillaries[29]. This allows leakage and accumulation of iodinated or paramagnetic contrast agents that leads to nerve enhancement (before any appreciable nerve enlargement), which is more readily detected on MR imaging than CT[29,30].
Contrast-enhanced T1-weighted MR imaging with fat suppression is widely used to increase the conspicuity of the enhancing tumour infiltrated nerve by nulling the signal of the surrounding fat (Fig. 1e)[31,32]. However, when a frequency-selective fat-suppression technique is used, susceptibility artefacts, particularly around a prominent sphenoid sinus, may obscure the adjacent skull base foramina. Curtin[12] advocated the use of pre- and post-contrast high spatial resolution T1-weighted imaging. Maroldi et al.[11] preferred pre- and post-contrast isotropic high spatial resolution volumetric interpolated breath-hold sequence with fat saturation to fully evaluate the neural passages through the skull base foramina without artefacts.
As tumour cells proliferate along a nerve, the diameter of the nerve increases. This often results in obliteration of the perineural fat tissue at foraminal openings or pterygopalatine fossa, which may be appreciated on CT or non–fat-suppressed T1-weighted MR images (Figs. 1d and 3e). Further enlargement of the nerve may cause erosion or even destruction of the skull base foramina, well seen on bone-algorithm CT.
Not infrequently, a tumour-invaded nerve may appear to regain its normal size as it traverses a bone canal in the skull base, only to regain its enlarged macroscopic size on the opposite side of the foramen. This resurfacing phenomenon is now believed to merely reflect the macroscopic reappearance of a contiguous perineural tumour following confinement in a bone canal, rather than a true skip lesion that presumably disseminates via perineural lymphatic vessels[10].
Centripetal extension along the branches of the trigeminal nerve may eventually lead to tumour infiltration of the gasserian ganglion in the Meckel cave, and less frequently the cisternal segment of the trigeminal nerve.
Secondary imaging features
Perineural tumour spread along a motor nerve may lead to denervation atrophy of the innervated muscles. Animal models show that in the first 4 weeks of denervation, there is a relative decrease in intracellular water with a relative increase in extracellular water, although the total amount of tissue water remains unchanged[33]. Increased perfusion of the muscles is also evident[34,35]. With time, muscular atrophy associated with fatty infiltration takes place.
In the acute to subacute phases, T2-weighted MR images show hyperintense signals in the muscles simulating oedema. This is because the T2 of extracellular water is longer than the T2 of intracellular water. In addition, increased contrast enhancement is also seen in these muscles, due to the underlying increase in perfusion and accumulation of contrast medium in the extracellular space (Fig. 4).
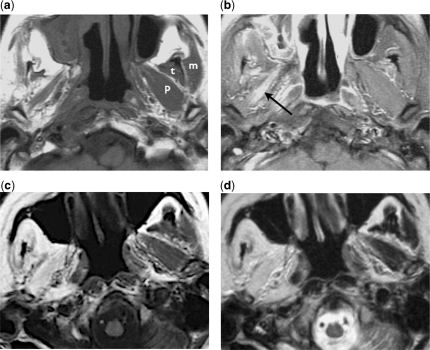
Figure 4.
Denervation atrophy of the masticator muscles. (a) Axial T1-weighted image of a patient with perineural tumour spread along the right mandibular nerve shows significant atrophy of the right masticator muscles, compared with the normal contralateral masseter (m), temporalis (t) and pterygoid (p) muscles. (b) Axial contrast-enhanced fat-suppressed T1-weighted image shows enhancement of the denervated muscle, notably the pterygoid muscles (arrow). (c) Axial T1-weighted and (d) axial T2-weighted images of another patient with similar perineural tumour involvement of the right mandibular nerve show marked fatty infiltration of the right masticator muscles without significant loss of the muscle bulks.
In the chronic phase, there is muscle atrophy with hyperintense signals on T1 and fast spin echo T2-weighted MR images due to fatty replacement (Figs. 4 and 5). This should be distinguished from direct tumour infiltration of the muscles, which often results in an increase (rather than decrease) in the size of the affected muscles. The MR signal changes in denervation atrophy are also more generalised and higher in intensity than malignant infiltration of the muscles.
Figure 5.
A 57-year-old male with nasopharyngeal carcinoma spreading along the right hypoglossal nerve resulting in subacute denervation atrophy of the ipsilateral tongue. (a) Axial T1-weighted image shows atrophy of the right side of the tongue with early fatty infiltration (black arrow) and posterior displacement (white arrow). (b) Coronal fat-suppressed T2-weighted image shows hyperintensity in the right side of the tongue due to relative increase in extracellular water (arrows). (c) Coronal contrast-enhanced fat-suppressed T1-weighted image shows enhancement of the right side of the tongue due to underlying increase in perfusion.
Denervation atrophy is most commonly seen in the masticator muscles (innervated by the mandibular nerve) and the tongue (innervated by the hypoglossal nerve). Hypoglossal denervation results in atrophy and fatty infiltration of the ipsilateral tongue, which may also be seen to drop posteriorly in the axial images of a supine patient[20] (Fig. 5). This posterior displacement of the denervated tongue may simulate an apparent increase in its longitudinal dimension mimicking a mass, and should be recognised for what it actually is[36]. Less commonly, denervation of the facial nerve may cause T2 hyperintense signals and contrast enhancement of the small muscles of facial expression[37].
Differential diagnosis
Enlargement and enhancement of a cranial nerve is not unique to perineural tumour spread. The differential diagnosis includes primary neural tumours such as schwannomas, invasive fungal infections such as aspergillosis or mucormysosis (in severely immunocompromised individuals) and meningeal inflammatory disorders such as sarcoidosis or histiocytosis[38]. Image-guided fine-needle aspiration of abnormal soft tissue along or adjacent to cranial nerves may provide further evaluations in selected cases[39].
Conclusion
Perineural spread is an important adverse prognostic indicator in the staging of head and neck malignancies, which influences the planning of the surgical approach and treatment regimen. Imaging plays a critical role in the assessment and delineation of perineural tumour spread. A comprehensive knowledge of the pertinent anatomy of the cranial nerves, and the typical imaging features of perineural tumour spread is therefore essential in the imaging of head and neck oncology.
References
- [1].Lee KJ, Abemayor E, Sayre J, Bhuta S, Kirsch C. Determination of perineural invasion preoperatively on radiographic images. Otolaryngol Head Neck Surg. 2008;139:275–80. doi: 10.1016/j.otohns.2008.04.026. doi:10.1016/j.otohns.2008.04.026. PMid:18656729. [DOI] [PubMed] [Google Scholar]
- [2].Rapidis AD, Givalos N, Gakipoulous H, et al. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol. 2005;41:328–35. doi: 10.1016/j.oraloncology.2004.12.004. doi:10.1016/j.oraloncology.2004.12.004. PMid:15743696. [DOI] [PubMed] [Google Scholar]
- [3].Fagan JJ, Collins B, Barnes L, D'Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:637–40. doi: 10.1001/archotol.124.6.637. [DOI] [PubMed] [Google Scholar]
- [4].Ampil FL, Hardin JC, Peskind SP, Stucker FJ. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34–8. doi: 10.1016/0278-2391(95)90496-4. doi:10.1016/0278-2391(95)90496-4. [DOI] [PubMed] [Google Scholar]
- [5].Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. 7th ed. Wiley-Blackwell, 2009. [Google Scholar]
- [6].Warden KF, Parmar H, Trobe JD. Perineural spread of cancer along the three trigeminal divisions. J Neuro-Ophthalmol. 2009;29:300–7. doi: 10.1097/WNO.0b013e3181b1b39a. doi:10.1097/WNO.0b013e3181b1b39a. PMid:19952904. [DOI] [PubMed] [Google Scholar]
- [7].Mendenhall WM, Parsons JT, Mendenhall NP, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 1989;11:301–8. doi: 10.1002/hed.2880110404. doi:10.1002/hed.2880110404. [DOI] [PubMed] [Google Scholar]
- [8].Catalano PJ, Sen C, Biller HR. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol. 1995;16:772–7. [PubMed] [Google Scholar]
- [9].Boerman RH, Maassen EM, Joosten H, et al. Trigeminal neuropathy secondary to perineural invasion of head and neck carcinomas. Neurology. 1999;53:213–6. doi: 10.1212/wnl.53.1.213. [DOI] [PubMed] [Google Scholar]
- [10].Parker GD, Harnsberger HR. Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics. 1991;11:383–99. doi: 10.1148/radiographics.11.3.1852933. [DOI] [PubMed] [Google Scholar]
- [11].Maroldi R, Farina D, Borghesi A, Marconi A, Gatti E. Perineural tumor spread. Neuroimag Clin N Am. 2008;18:413–29. doi: 10.1016/j.nic.2008.01.001. doi:10.1016/j.nic.2008.01.001. PMid:18466839. [DOI] [PubMed] [Google Scholar]
- [12].Curtin HD. Detection of perineural spread: fat suppression versus no fat suppression. AJNR Am J Neuroradiol. 2004;25:1–3. [PMC free article] [PubMed] [Google Scholar]
- [13].Fowler BZ, Crocker IR, Johnstone PA. Perineural spread of cutaneous malignancy to the brain: a review of literature and five patients treated with stereotactic radiotherapy. Cancer. 2005;103:2143–53. doi: 10.1002/cncr.21004. doi:10.1002/cncr.21004. PMid:15816051. [DOI] [PubMed] [Google Scholar]
- [14].Yousem DM, Gad K, Tufano RP. Resectability issues with head and neck cancer. AJNR Am J Neuroradiol. 2006;27:2024–36. [PMC free article] [PubMed] [Google Scholar]
- [15].Hutcheson JA, Vural E, Korourian S, Hanna E. Neural cell adhesion molecule expression in adenoid cystic carcinoma of the head and neck. Laryngoscope. 2000;110:946–8. doi: 10.1097/00005537-200006000-00011. doi:10.1097/00005537-200006000-00011. PMid:10852510. [DOI] [PubMed] [Google Scholar]
- [16].Gandour-Edwards R, Kapadia SB, Barnes L, Donald PJ, Janecka IP. Neural cell adhesion molecule in adenoid cystic carcinoma invading the skull base. Otolaryngol Head Neck Surg. 1997;117:453–8. doi: 10.1016/s0194-5998(97)70013-5. doi:10.1016/S0194-5998(97)70013-5. [DOI] [PubMed] [Google Scholar]
- [17].Vural E, Hutcheson JA, Korourian S, Kechelava S, Hanna E. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717–20. doi: 10.1016/S0194-5998(00)70203-8. doi:10.1016/S0194-5998(00)70203-8. [DOI] [PubMed] [Google Scholar]
- [18].Iwamoto S, Odland PB, Piepkorn M, Bothwell M. Evidence that the p75 neurotrophin receptor mediate perineural spread of desmoplastic melanoma. J Am Acad Dermatol. 1996;35:725–31. doi: 10.1016/s0190-9622(96)90728-8. doi:10.1016/S0190-9622(96)90728-8. [DOI] [PubMed] [Google Scholar]
- [19].Fanburg-Smith JC, Miettinen M. Low-affinity nerve growth factor receptor (p75) in dermatofibrosarcoma protuberans and other nonneural tumours: a study of 1,150 tumours and fetal and adult normal tissues. Hum Pathol. 2001;32:976–83. doi: 10.1053/hupa.2001.27602. doi:10.1053/hupa.2001.27602. PMid:11567228. [DOI] [PubMed] [Google Scholar]
- [20].Chong VF, Fan YF. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Eur Radiol. 1998;8:939–45. doi: 10.1007/s003300050492. doi:10.1007/s003300050492. PMid:9683697. [DOI] [PubMed] [Google Scholar]
- [21].Ginsberg LE, Eicher SA. Great auricular nerve anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21:568–71. [PMC free article] [PubMed] [Google Scholar]
- [22].Streams BN, Eaton JS, Zelac DE. Perineural spread of squamous cell carcinoma involving the spinal accessory nerve in an immunocompromised organ transplant recipient. Dermatol Surg. 2005;31:599–601. doi: 10.1111/j.1524-4725.2005.31173. doi:10.1111/j.1524-4725.2005.31173. [DOI] [PubMed] [Google Scholar]
- [23].Chong VFH, Fan YF. Pictorial essay: maxillary nerve involvement in nasopharyngeal carcinoma. Am J Roentgenol. 1996;167:1309–12. doi: 10.2214/ajr.167.5.8911202. [DOI] [PubMed] [Google Scholar]
- [24].Chong VF, Ong CK. Nasopharyngeal carcinoma. Eur J Radiol. 2008;66:437–47. doi: 10.1016/j.ejrad.2008.03.029. doi:10.1016/j.ejrad.2008.03.029. PMid:18485650. [DOI] [PubMed] [Google Scholar]
- [25].Schmalfuss IM, Tart RP, Mukherji S, Mancuso AA. Perineural tumor spread along the auriculotemporal nerve. AJNR Am J Neuroradiol. 2002;23:303–11. [PMC free article] [PubMed] [Google Scholar]
- [26].Ginsberg LE, De Monte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. AJNR Am J Neuroradiol. 1996;17:389–93. [PMC free article] [PubMed] [Google Scholar]
- [27].Liane FJ, Braun IF, Jensen ME, Nadel L, Som PM. Perineural tumour extension through the foramen ovale: evaluation with MR imaging. Radiology. 1990;174:65–71. doi: 10.1148/radiology.174.1.2152985. [DOI] [PubMed] [Google Scholar]
- [28].Nemzek WR, Hecht S, Gandour-Edwards R, Donald P, McKennan K. Perineural spread of head and neck tumors: how accurate is MR imaging. AJNR Am J Neuroradiol. 1998;19:701–6. [PMC free article] [PubMed] [Google Scholar]
- [29].Carter RL, Foster CS, Dinsdale EA, Pittam MR. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269–75. doi: 10.1136/jcp.36.3.269. doi:10.1136/jcp.36.3.269. PMid:6338053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ginsberg LE, DeMonte F. Imaging of perineural tumor spread from palatal carcinoma. AJNR Am J Neuroradiol. 1998;19:1417–22. [PMC free article] [PubMed] [Google Scholar]
- [31].Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradiol. 2004;25:5–11. [PMC free article] [PubMed] [Google Scholar]
- [32].Barakos JA, Dillon WP, Chew WM. Orbit, skull base, and pharynx: contrast-enhanced fat suppression MR imaging. Radiology. 1991;179:191–8. doi: 10.1148/radiology.179.1.2006277. [DOI] [PubMed] [Google Scholar]
- [33].Chong VF. Imaging the cranial nerves in cancer. Cancer Imaging. 2004;4:S1–5. doi: 10.1102/1470-7330.2004.0006. doi:10.1102/1470-7330.2004.0006. PMid:18215969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Russo CP, Smoker WR, Weissman JL. MR appearance of trigeminal and hypoglossal motor denervation. AJNR Am J Neuroradiol. 1997;18:1375–83. [PMC free article] [PubMed] [Google Scholar]
- [35].Davis SB, Matthews VP, Williams III DW. Masticator muscle enhancement in subacute denervation atrophy. AJNR Am J Neuroradiol. 1995;16:1292–4. [PMC free article] [PubMed] [Google Scholar]
- [36].Harnsberger HR, Dillon WP. Major motor atrophic patterns in the face and neck: CT evaluation. Radiology. 1985;155:665–70. doi: 10.1148/radiology.155.3.4001368. [DOI] [PubMed] [Google Scholar]
- [37].Fischbein NJ, Kaplan MJ, Jackler RK, Dillon WP. MR imaging in two cases of subacute denervation change in the muscles of facial expression. AJNR Am J Neuroradiol. 2001;22:880–4. [PMC free article] [PubMed] [Google Scholar]
- [38].Marsot-Dupuch K, Matozza F, Firat MM, Iyriboz AT, Chabolle F, Tubiana JM. Mandibular nerve: MR versus CT about 10 proved unusual tumors. Neuroradiology. 1990;32:492–6. doi: 10.1007/BF02426462. doi:10.1007/BF02426462. PMid:2287378. [DOI] [PubMed] [Google Scholar]
- [39].Barakos JA, Dillon WP. Lesions of the foramen ovale: CT-guided fine-needle aspiration. Radiology. 1992;182:573–5. doi: 10.1148/radiology.182.2.1732985. [DOI] [PubMed] [Google Scholar]