Abstract
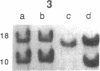
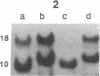
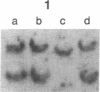
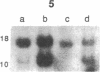
Clinically nonfunctioning pituitary adenomas are benign neoplasms comprising approximately 25-30% of pituitary tumors. Little is known about the pathogenesis of pituitary neoplasia. Clonal analysis allows one to make the important distinction between a polyclonal proliferation in response to a stimulatory factor versus a monoclonal expansion of a genetically aberrant cell. We investigated the clonal origin of pituitary tumors using X-linked restriction fragment length polymorphisms at the phosphoglycerate kinase and hypoxanthine phosphoribosyl-transferase genes. Restriction enzymes were used to distinguish maternal and paternal X-chromosomes, and combined with a methylation-sensitive restriction enzyme to analyze allelic X-inactivation patterns in six pituitary adenomas. All six tumors showed a monoclonal pattern of X-inactivation. These data indicate that nonfunctioning pituitary adenomas are unicellular in origin, a result consistent with the hypothesis that this tumor type is due to somatic mutation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arafah B. M. Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 1986 Jun;62(6):1173–1179. doi: 10.1210/jcem-62-6-1173. [DOI] [PubMed] [Google Scholar]
- Arnold A., Staunton C. E., Kim H. G., Gaz R. D., Kronenberg H. M. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med. 1988 Mar 17;318(11):658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Black P. M., Hsu D. W., Klibanski A., Kliman B., Jameson J. L., Ridgway E. C., Hedley-Whyte E. T., Zervas N. T. Hormone production in clinically nonfunctioning pituitary adenomas. J Neurosurg. 1987 Feb;66(2):244–250. doi: 10.3171/jns.1987.66.2.0244. [DOI] [PubMed] [Google Scholar]
- Brandi M. L., Marx S. J., Aurbach G. D., Fitzpatrick L. A. Familial multiple endocrine neoplasia type I: a new look at pathophysiology. Endocr Rev. 1987 Nov;8(4):391–405. doi: 10.1210/edrv-8-4-391. [DOI] [PubMed] [Google Scholar]
- DeStephano D. B., Lloyd R. V., Pike A. M., Wilson B. S. Pituitary adenomas. An immunohistochemical study of hormone production and chromogranin localization. Am J Pathol. 1984 Sep;116(3):464–472. [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Burke P. J., Schiffer C. A., Zehnbauer B. A., Vogelstein B. Differentiation of leukemia cells to polymorphonuclear leukocytes in patients with acute nonlymphocytic leukemia. N Engl J Med. 1986 Jul 3;315(1):15–24. doi: 10.1056/NEJM198607033150103. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Sagebiel R. W., Gartler S. M., Rimoin D. L. Multiple cell origin of hereditary neurofibromas. N Engl J Med. 1971 Feb 11;284(6):298–300. doi: 10.1056/NEJM197102112840604. [DOI] [PubMed] [Google Scholar]
- Friedman E., Sakaguchi K., Bale A. E., Falchetti A., Streeten E., Zimering M. B., Weinstein L. S., McBride W. O., Nakamura Y., Brandi M. L. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med. 1989 Jul 27;321(4):213–218. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Ziprkowski L., Krakowski A., Ezra R., Szeinberg A., Adam A. Glucose-6-phosphate dehydrogenase mosaicism as a tracer in the study of hereditary multiple trichoepithelioma. Am J Hum Genet. 1966 May;18(3):282–287. [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Klibanski A., Black P. M., Zervas N. T., Lindell C. M., Hsu D. W., Ridgway E. C., Habener J. F. Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J Clin Invest. 1987 Nov;80(5):1472–1478. doi: 10.1172/JCI113228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M., Habener J. F. Gonadotropin and thyrotropin alpha- and beta-subunit gene expression in normal and neoplastic tissues characterized using specific messenger ribonucleic acid hybridization probes. J Clin Endocrinol Metab. 1987 Feb;64(2):319–327. doi: 10.1210/jcem-64-2-319. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Esty A. C., Bernard H. U., Friedmann T. Isolation of a genomic clone partially encoding human hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5038–5041. doi: 10.1073/pnas.79.16.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanski A., Deutsch P. J., Jameson J. L., Ridgway E. C., Crowley W. F., Hsu D. W., Habener J. F., Black P. M. Luteinizing hormone-secreting pituitary tumor: biosynthetic characterization and clinical studies. J Clin Endocrinol Metab. 1987 Mar;64(3):536–542. doi: 10.1210/jcem-64-3-536. [DOI] [PubMed] [Google Scholar]
- Klibanski A. Nonsecreting pituitary tumors. Endocrinol Metab Clin North Am. 1987 Sep;16(3):793–804. [PubMed] [Google Scholar]
- Klibanski A., Ridgway E. C., Zervas N. T. Pure alpha subunit-secreting pituitary tumors. J Neurosurg. 1983 Oct;59(4):585–589. doi: 10.3171/jns.1983.59.4.0585. [DOI] [PubMed] [Google Scholar]
- Klibanski A., Shupnik M. A., Bikkal H. A., Black P. M., Kliman B., Zervas N. T. Dopaminergic regulation of alpha-subunit secretion and messenger ribonucleic acid levels in alpha-secreting pituitary tumors. J Clin Endocrinol Metab. 1988 Jan;66(1):96–102. doi: 10.1210/jcem-66-1-96. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Molitch M. E. Pathogenesis of pituitary tumors. Endocrinol Metab Clin North Am. 1987 Sep;16(3):503–527. [PubMed] [Google Scholar]
- Ridgway E. C., Klibanski A., Ladenson P. W., Clemmons D., Beitins I. Z., McArthur J. W., Martorana M. A., Zervas N. T. Pure alpha-secreting pituitary adenomas. N Engl J Med. 1981 May 21;304(21):1254–1259. doi: 10.1056/NEJM198105213042102. [DOI] [PubMed] [Google Scholar]
- Snyder P. J. Gonadotroph cell adenomas of the pituitary. Endocr Rev. 1985 Fall;6(4):552–563. doi: 10.1210/edrv-6-4-552. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Bouloux P., Wooding C., Chotai K., Broad P. M., Spurr N. K., Besser G. M., O'Riordan J. L. Association of parathyroid tumors in multiple endocrine neoplasia type 1 with loss of alleles on chromosome 11. N Engl J Med. 1989 Jul 27;321(4):218–224. doi: 10.1056/NEJM198907273210403. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Woodruff M. F. Tumor clonality and its biological significance. Adv Cancer Res. 1988;50:197–229. doi: 10.1016/s0065-230x(08)60438-8. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]