Abstract
Background
Allocation of cadaver kidneys by graded human leukocyte antigen (HLA) compatibility scoring arguably has had little effect on overall survival while prejudicing the transplant candidacy of African-American and other hard to match populations. Consequently, matching has been proposed of deduced amino acid residues of the individual HLA molecules shared by cross-reactive antigen groups (CREGs). We have examined the circumstances under which compatibility with either method impacted graft survival.
Methods
Using Cox proportional hazards regression modeling, we studied the relationship between levels of conventional HLA mismatch and other donor and recipient factors on primary cadaver kidney survival between 1981 and 1995 at the University of Pittsburgh (n=1,780) and in the United Network for Organ Sharing (UNOS) Scientific Registry during 1991–1995 (n=31,291). The results were compared with those obtained by the matching of amino acid residues that identified CREG-compatible cases with as many as four (but not five and six) HLA mismatches.
Results
With more than one HLA mismatch (>85% of patients in both series), most of the survival advantage of a zero mismatch was lost. None of the HLA loci were “weak.” In the UNOS (but not Pittsburgh) category of one-HLA mismatch (n=1334), a subgroup of CREG-matched recipients (35.3%) had better graft survival than the remaining 64.7%, who were CREG-mismatched. There was no advantage of a CREG match in the two- to four-HLA incompatibility tiers. Better graft survival with tacrolimus was observed in both the Pittsburgh and UNOS series.
Conclusions
Obligatory national sharing of cadaver kidneys is justifiable only for zero-HLA-mismatched kidneys. The potential value of CREG matching observed in the one-HLA-mismatched recipients of the UNOS (but not the Pittsburgh) experience deserves further study.
The use of human leukocyte antigen (HLA*) matching as the dominant factor for the allocation of cadaver kidneys has been criticized on grounds that it is poorly predictive of graft survival except when perfect and diverts the organs away from African-American and other hard to match recipients (1–5). Before undertaking reforms in organ distribution, it is necessary to have precise knowledge about the extent to which degrees of HLA matching influence prognosis. Thus, the primary aim of the present study was to examine the effect of prospective HLA matching on primary cadaver graft survival in patients compiled in the United Network for Organ Sharing (UNOS) database for the years 1991–1995 and in a separate 15-year (1981–1995) longitudinal study at the University of Pittsburgh. A secondary objective was to explore whether cross-reactive antigen group (CREG) matching, which is a refinement of conventional histocompatibility determination (6–10), might offer a better chance of allograft survival to a broader range of recipients.
PATIENTS AND METHODS
Study subjects
A total of 2723 patients underwent kidney transplantation at the University of Pittsburgh between 1981 and 1995 and had complete follow-up to the end of 1996. Of that group of patients, 1897 received primary cadaveric allografts under immune suppression based on cyclosporine (n = 1217), tacrolimus (n=649), or azathioprine (n=31). Patients treated with azathioprine as the baseline immunosuppressant were excluded from analysis, as well as 86 patients in whom none of the HLA antigens were identified (“double blanks”) at one or more of the A, B, or DR loci, in either the donor or the recipient. Therefore, the analyses were based upon survival of 1780 allografts, 1142 under cyclosporine and 638 under tacrolimus.
The HLA and other data collected in these cases were compared with those from 31,291 primary cadaver transplantations reported from 243 centers to the UNOS Scientific Renal Transplant Registry during the period from 1991 through 1995. Donor and recipient serologic typing of the HLA-A, -B, and -DR loci was routinely obtained throughout the period of both studies. All “splits” (11) were reviewed individually and categorized appropriately. The degree of incompatibility was expressed in each case as zero to six HLA mismatches. It has been reported (12) and was confirmed in this study (data not shown) that such results accurately reflect the compatibility expressed by the six- to zero-HLA match scale. To achieve statistical independence between the two study cohorts, all Pittsburgh patients reported to the registry were excluded from the UNOS analysis.
Kidney allocation
From the beginning of the 15-year Pittsburgh study, six-antigen-matched allografts were targeted selectively to cadaver kidney recipients. This restricted use of HLA matching was the centerpiece of the computerized “point system” introduced in western Pennsylvania in 1986 for the equitable allocation of cadaver kidneys (13). Although credit was given to lesser degrees of matching and to logistic and urgency of need factors, time waiting on dialysis was shown in extensive field trials during 1986–1987 to be the single most important determinant for organ allocation in the vast majority of cases (13). The Pittsburgh point system was adopted by UNOS for nationwide use beginning in November 1987, but variances at local, regional, and national levels had subordinated all other allocation credits (including waiting time) to gradations of HLA matching by the time of the 1991–1995 UNOS case accrual. During this period, A locus matching was deemphasized by UNOS directive (14).
CREGs
Although the genes at the HLA-A, -B, and -DR loci are highly polymorphic (greater than 250 known specificities), their respective class I and II protein products have minimal sequence variability over a range of single and regional amino acid residue positions. Because these different residues share antigenic determinants, they constitute a basis for common (public) groups (also called CREGs) (15) against which anti-HLA antibodies are largely directed (16). The inference is that donor-recipient compatibility might be achieved by avoiding donor mismatches of these residues rather than of the complete HLA molecules. A computer program (kindly provided by Dr. Steve Takemoto [9]) was therefore used to convert conventional donor and recipient HLA phenotypes in both the Pittsburgh and UNOS data to 10 groups of amino acid residues representing class I public determmants and 10 broadly defined class II antigens (9). The presence of any residue(s) in the donor but not in the recipient was by definition a CREG mismatch.
Statistical analysis
The relationship between the level of HLA mismatch and rate of graft survival was assessed with Cox proportional hazards regression modeling (program 2L of the BMDP package [17]), which takes into account varying lengths of patient follow-up. Regression modeling was also used in the secondary analysis of the relationship between CREG match and graft survival at various levels of HLA incompatibility. The results were summarized as adjusted hazard ratios (HRs) with corresponding 95% confidence intervals. The estimated HR (or relative hazard) is a measure of association that can be interpreted as a relative risk.
The analysis strategy consisted of initially fitting a model with only the level of HLA mismatch. This yielded unadjusted HRs, estimating the relative risk of individual one- to six-HLA mismatches versus zero mismatches. Adjusted HRs were obtained subsequently by modeling all the variables simultaneously. A final “parsimonious” model was arrived at by eliminating all nonconfounders from the previous model using a backward selection approach. The “change-in-estimate” criterion was used to measure the level of confounding among the factors in the model (18). A factor was considered to be a confounder if it produced more than a 10% change in any of the HLA-related HRs of interest. The resulting model contained the HLA factor in addition to all possible confounders detected according to this criterion.
An assumption underlying the Cox regression analysis is that the hazard rates are proportional. This assumption was examined by plotting the graft survival curves on a log-log scale. Because the curves were parallel, the proportional hazards modeling approach was regarded as appropriate. Although a model-free approach could have been used, the number of subcategories over which the data would have been dispersed would have yielded imprecise estimates of graft survival rates. Regression modeling is desirable because it preserves precision while simultaneously controlling for many potentially confounding factors, as well as allowing for the efficient detection of statistical interactions.
Differences between levels of HLA mismatch were tested for statistical significance using a likelihood ratio test (17). Graft survival rates and half-lives (beyond 1 year) were estimated from the regression model. The half-life values were estimated according to the formula HL=2loge2/(logeS1–logeS2), where S1 and S2 are the predicted survival rates from the regression model at 1 and 3 years, respectively. Adjusted graft survival curves were obtained from the regression model by using the mean value for the covariate being adjusted (19). Because half-lives customarily have been calculated taking into account only actual data from 1 year onward (20), this conventional method was also used for the primary UNOS analysis with side-by-side presentation of the latter results in parentheses (Fig. 1). Because the results were undistinguishable statistically from those with the Cox regression methodology, the conventional half-life analyses will not be discussed further.
Fig. 1.
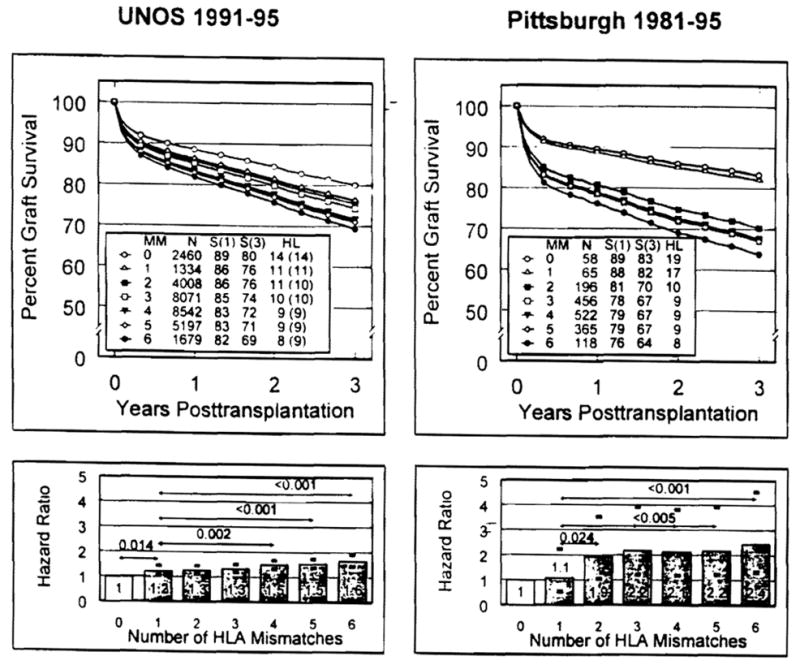
Comparisons of predicted graft survival curves (top panels) and HRs (bottom panels) by numbers of HLA -A, -B, -DR-mismatched primary cadaver renal transplantations reported to the UNOS (left panels) and University of Pittsburgh (right panels) databases. The estimates of rates of graft survival (S), half-lives (HL) beyond 1 year after transplantation, and HRs were computed for each degree of HLA mismatch (one to six antigens) after adjusting for the potential confounding effects of the regimen of immune suppression (i.e., cyclosporine vs. tacrolimus). The HL in parentheses for the UNOS cases was computed with the most widely used formula (see Patients and Methods). The differences in HRs (95% confidence limits given by darkened error bars shown in the bottom panels) between HLA groups and the one-antigen-mismatched group were significant when indicated by P-value (listed above arrow line depicting comparison).
Missing covariate data were replaced with mode values for categorical variables and median values for continuous variables. Less than 2% of values were missing for any covariate in the Pittsburgh data, except for duration of cold ischemia (30% missing) and body mass index (42% missing). Collection of data on these two covariates was not started until January 1986 and January 1987, respectively. In UNOS, less than 5% of values were missing for any covariate. The effect of the imputation procedure was assessed by comparing models fit with imputed values to models in which patients with missing data were excluded. None of the analyses yielded appreciably different results. All continuous variables were categorized to obviate linearity assumptions using categories suggested previously in the kidney transplant literature (21, 22).
RESULTS
Donor and Recipient Characteristics
The University of Pittsburgh and UNOS registry cases were similar across all but two factors (Table 1). The first exception was a cold ischemia time that was nearly 10 hr longer in the Pittsburgh data, exceeding 36 hr for 26% of the allografts. Second, 96.8% of recipients in the UNOS registry were treated with cyclosporine compared with 64.2% in Pittsburgh, where cyclosporine was largely replaced by tacrolimus from November 1989 onward (23). Although dependence on prednisone was much reduced in the tacrolimus cohort (23, 24), the principles of management were the same as had been delineated previously with cyclosporine (25).
Table 1.
Baseline characteristics of kidney transplant recipients and donorsa
| Factors | Pittsburgh 1981–1995 (n=1780) | UNOS 1991–1995 (n=31291) |
|---|---|---|
| Recipient factors | ||
| Age (years) | ||
| Mean ± SD | 41.8 ± 15.1 | 44.0 ± 14.0 |
| 0–17 | 5.8 | 3.7 |
| 18–39 | 39.4 | 32.7 |
| 40–54 | 31.5 | 38.7 |
| 55+ | 23.3 | 24.9 |
| Gender | ||
| Female | 37.5 | 39.9 |
| Male | 62.5 | 61.1 |
| Race | ||
| Caucasian | 78.9 | 59.0 |
| African-American | 14.7 | 25.9 |
| Other | 6.4 | 15.1 |
| BMIa (kg/m2) | ||
| Mean ± SD | 23.9 ± 4.2 | 24.7 ± 5.2 |
| 0–20 | 12.2 | 19.7 |
| 21–30 | 81.1 | 68.0 |
| 31+ | 6.7 | 12.3 |
| Diabetes | ||
| No | 77.6 | 79.3 |
| Yes | 22.4 | 20.7 |
| Peak PRAa (%) | ||
| Mean ± SD | 15.5 ± 25.0 | 12.2 ± 22.1 |
| 0 | 23.1 | 35.0 |
| 1–10 | 45.6 | 38.8 |
| 11–50 | 20.2 | 18.5 |
| 51+ | 11.1 | 7.7 |
| Immunosuppression | ||
| Cyclosporine | 64.2 | 96.8 |
| Tacrolimus | 35.8 | 3.2 |
| Donor factors | ||
| Age (years) | ||
| Mean ± SD | 27.9 ± 18.0 | 32.9 ± 17.0 |
| 0–17 | 30.3 | 21.6 |
| 18–39 | 43.1 | 41.8 |
| 40–54 | 16.3 | 23.9 |
| 55+ | 10.3 | 12.8 |
| Gender | ||
| Female | 36.3 | 38.4 |
| Male | 63.7 | 61.6 |
| Race | ||
| Caucasian | 90.9 | 78.7 |
| African-American | 6.3 | 11.1 |
| Other | 2.8 | 10.3 |
| Cold ischemia time (hr) | ||
| Mean ± SD | 32.2 ± 9.1 | 22.8 ± 9.6 |
| 0–23 | 14.8 | 57.6 |
| 24–35 | 59.2 | 32.8 |
| 36–47 | 18.4 | 8.0 |
| 48+ | 7.6 | 1.6 |
| Transplant factors | ||
| ABO compatibility | ||
| Nonidentical | 3.0 | 5.1 |
| Identical | 97.0 | 94.9 |
| HLA-ABDR mismatches | ||
| Mean ± SD | 3.6 ± 1.4 | 3.3 ± 1.5 |
| 0 | 3.3 | 7.9 |
| 1 | 3.7 | 4.3 |
| 2 | 11.0 | 12.8 |
| 3 | 25.6 | 25.8 |
| 4 | 29.3 | 27.3 |
| 5 | 20.5 | 16.6 |
| 6 | 6.6 | 5.4 |
| Transplantation era | ||
| Before 1988 | 47.7 | 0.0 |
| January 1, 1988 onward | 52.3 | 100.0 |
BMI, body mass index; PRA, panel-reactive antibody.
Primary Analysis
Pittsburgh series
Of the 1780 Pittsburgh recipients undergoing primary cadaver kidney transplantation between 1981 and 1995, 936 experienced graft failure. The median time to failure or end of study (December 1996) was 3.83 years among all patients. For the 936 who experienced graft failure, the median time to failure was 1.83 years. The adjusted HRs measuring the effect of HLA mismatching and all other factors on the (relative) risk of graft failure arising from the proportional hazards regression models are shown in Table 2.
Table 2.
Adjusted HRs from Cox proportional hazards regression analyses
| Baseline factors | Pittsburgh 1981–1995 (n=1780) HR(95% CI) | UNOS 1991–1995 (n=31291) HR(95% CI) |
|---|---|---|
| HLA-A,B,DR mismatches | ||
| 1 vs. 0 | 1.04 (0.50–2.17) | 1.22 (1.04–1.43) |
| 2 vs. 0 | 1.85 (1.00–3.42) | 1.28 (1.12–1.44) |
| 3 vs. 0 | 2.08 (1.15–3.75) | 1.31 (1.17–1.47) |
| 4 vs. 0 | 2.06 (1.14–3.71) | 1.46 (1.30–1.63) |
| 5 vs. 0 | 2.08 (1.15–3.78) | 1.49 (1.32–1.68) |
| 6 vs. 0 | 2.38 (1.28–4.44) | 1.60 (1.39–1.85) |
| Immunosuppressive therapy | ||
| Tacrolimus vs. cyclosporine | 0.67 (0.54–0.84) | 0.70 (0.60–0.83) |
| Recipient age (years) | ||
| 0–17 vs. 18–39 | 1.41 (1.04–1.91) | 1.17 (1.02–1.34) |
| 40–54 vs. 18–39 | 1.06 (0.91–1.24) | 0.95 (0.89–1.00) |
| 55+ vs. 18–39 | 1.25 (1.05–1.49) | 1.12 (1.05–1.19) |
| Recipient gender | ||
| Male vs. female | 1.17 (1.02–1.35) | 1.04 (0.98–1.09) |
| Recipient race | ||
| African-American vs. Caucasian | 1.35 (1.13–1.61) | 1.28 (1.21–1.35) |
| Other vs. Caucasian | 0.57 (0.42–0.78) | 0.86 (0.79–0.92) |
| Recipient BMI (kg/m2) | ||
| 21–30 vs. 0–20 | 1.03 (0.80–1.32) | 1.05 (0.98–1.12) |
| 31+ vs. 0–20 | 1.33 (0.94–1.89) | 1.17 (1.07–1.27) |
| Recipient diabetes status | ||
| Yes vs. no | 1.23 (1.06–1.44) | 1.19 (1.12–1.26) |
| Recipient peak PRA (%) | ||
| 1–10 vs. 0 | 0.93 (0.78–1.11) | 1.00 (0.95–1.06) |
| 11–50 vs. 0 | 1.03 (0.85–1.25) | 1.09 (1.02–1.16) |
| 51+ vs. 0 | 1.43 (1.14–1.79) | 1.31 (1.20–1.43) |
| Donor age (years) | ||
| 0–17 vs. 18–39 | 1.08 (0.93–1.27) | 1.21 (1.13–1.29) |
| 40–54 vs. 18–39 | 1.34 (1.10–1.63) | 1.41 (1.33–1.50) |
| 55+ vs. 18–39 | 1.75 (1.37–2.24) | 1.91 (1.78–2.04) |
| Donor gender | ||
| Male vs. female | 1.03 (0.90–1.19) | 0.92 (0.88–0.97) |
| Donor race | ||
| African-American vs. Caucasian | 1.24 (0.95–1.63) | 1.18 (1.10–1.27) |
| Other vs. Caucasian | 1.50 (0.97–2.32) | 1.06 (0.98–1.15) |
| Cold ischemia time (hr) | ||
| 24–35 vs. 0–23 | 0.96 (0.78–1.17) | 1.08 (1.02–1.13) |
| 36–47 vs. 0–23 | 1.10 (0.86–1.41) | 1.17 (1.08–1.28) |
| 48+ vs. 0–23 | 1.23 (0.92–1.65) | 1.15 (0.97–1.36) |
| ABO compatibility | ||
| Identical vs. nonidentical | 1.32 (0.87–2.01) | 0.96 (0.86–1.08) |
| Transplant era | ||
| 1988 onward vs. before 1988 | 0.77 (0.63–0.95) | Not applicable |
Abbreviations are as defined in Table 1.
Although other factors influenced graft survival significantly (e.g., type of immune suppression, donor and recipient age, recipient race, insulin-dependent diabetes, and peak panel-reactive antibody), none of the corresponding HRs reached the magnitudes attained for the full range of HLA matching. Inasmuch as the regimen of immune suppression i i.e., cyclosporine vs. tacrolimus) was identified, on the basis of the change-in-estimate criterion, to be the only confounder for measuring the HLA effect, this factor alone was retained in the final Pittsburgh regression model. The HRs in this final model (Table 3) were within 10% of the HR adjusted for all covariates (Table 2).
Table 3.
Adjusted HRs from final Cox proportional hazards regression analyses
| Baseline factors | Pittsburgh 1981–1995 (n= 1,780) |
UNOS 1991–1995 (n=31,291) |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| HLA-A,B,DR mismatches | ||||
| 1 vs. 0 | 1.08 (0.52–2.24) | 0.83 | 1.22 (1.04–1.43) | 0.013 |
| 2 vs. 0 | 1.92 (1.05–3.51) | 0.035 | 1.26 (1.12–1.43) | <0.001 |
| 3 vs. 0 | 2.17 (1.21–3.89) | 0.009 | 1.33 (1.19–1.49) | <0.001 |
| 4 vs. 0 | 2.13 (1.19–3.80) | 0.011 | 1.50 (1.34–1.67) | <0.001 |
| 5 vs. 0 | 2.16 (1.20–3.88) | 0.010 | 1.54 (1.37–1.73) | <0.001 |
| 6 vs. 0 | 2.44 (1.32–4.51) | 0.004 | 1.64 (1.43–1.89) | <0.001 |
| Immunosuppressive therapy | ||||
| Tacrolimus vs. cyclosporine | 0.66 (0.55–0.78) | <0.001 | 0.75 (0.64–0.88) | 0.001 |
Adjusted graft survival curves and HRs are shown in Figure 1. Graft survival was inversely related to the degree of HLA mismatching (P<0.001), with a 3-year survival difference of 83% and 64% in recipients of zero- and six-HLA-mismatched kidneys, respectively. Between these extremes, however, there was only a 3% difference in survival in the recipients of two- to five-HLA mismatches, who made up 86.4% of all Pittsburgh cases. Within this spectrum, HRs rose from 1.92 to only 2.16 and half-lives were 9–10 years. The HRs measuring the risk of mismatch at the A and B loci (class I) were identical and exceeded the risk of mismatch at the DR locus (class II) (Table 4). Of particular interest, a second incompatibility at either the A, B, or DR locus did not increase the survival penalty imposed by a first one in two of these three loci.
Table 4.
Percentage of individual HLA-A, -B, and -DR loci mismatches at baseline, and adjusted HRs from Cox regression
| UNOS (n=31,291) | Pittsburgh (n=1,780) | UNOS 1991–1995 HR(95% CI) | Pittsburgh 1981– 1995 HR (95% CI) | |
|---|---|---|---|---|
| HLA-A | ||||
| 0 | 17.2 | 15.5 | 1.00 | 1.00 |
| 1 vs. 0 | 41.2 | 44.9 | 1.19 (1.10–1.27) | 1.51 (1.23–1.85) |
| 2 vs. 0 | 41.6 | 39.6 | 1.26 (1.17–1.35) | 1.39 (1.13–1.71) |
| HLA-B | ||||
| 0 | 17.0 | 10.4 | 1.00 | 1.00 |
| 1 vs. 0 | 43.8 | 43.0 | 1.17 (1.09–1.26) | 1.51 (1.14–1.99) |
| 2 vs. 0 | 39.2 | 46.5 | 1.29 (1.20–1.39) | 1.61 (1.22–2.13) |
| HLA-DR | ||||
| 0 | 32.6 | 22.7 | 1.00 | 1.00 |
| 1 vs. 0 | 49.7 | 52.6 | 1.16 (1.09–1.22) | 1.26 (1.06–1.50) |
| 2 vs. 0 | 17.7 | 24.7 | 1.27 (1.19–1.36) | 1.18 (0.97–1.45) |
Because HLA typing methods and reagents had improved with time and because immune suppression was different in the later period of this study, the cyclosporine- and tacrolimus-defined cohorts were examined in two further analyses. First, a test for interaction between the level of HLA mismatch and the type of immunosuppression on graft survival was performed using a regression analysis. The test statistic yielded a nonsignificant result (P=0.81), indicating that the relationship between HLA matching and graft survival could not be considered different under the two types of therapy.
For completeness, maximum likelihood estimates of A, B, and DR loci antigen and gene frequencies were computed for all 1780 Pittsburgh patients. These estimates also were computed for the 896 and 508 Caucasian recipients treated with cyclosporine and tacrolimus, respectively, and separately for the 1060 and 545 Caucasian donors in the two treatment cohorts. The analyses indicated that, for both recipients and donors, the Pittsburgh HLA-A, -B, and -DR antigen and gene frequencies were indistinguishable (within 1 standard error) from those reported in the UNOS Scientific Renal Registry, and between drug cohorts. These additional analyses indicated that the HLA types observed in the Pittsburgh patients were expressed consistently and are generally representative of the transplant population.
UNOS database
Based on complete follow-up data for 31,291 UNOS transplantations between 1991 and 1995, 6,960 (22.2%) grafts failed after a median time to failure of 0.37 years. Among all patients, the median time to failure or end of study (December 1996) was 1.60 years. Differences in median times between Pittsburgh and UNOS transplantations reflect differences in follow-up periods and not differences in overall graft survival (see comparative rates in Fig. 1 The results of the adjusted analysis for the full model are shown in Table 2, and the final model in Table 3. Although immune suppression was not identified as a confounder for the UNOS data, the final model nevertheless included this factor to ensure comparability between the two study cohorts. Note that the 95% confidence intervals about the HRs for the UNOS data are almost always enclosed within the corresponding interval estimates for the Pittsburgh data.
As in the Pittsburgh series, graft survival was related inversely to the extent of HLA mismatch (P<0.001) but with a more graded effect from the predicted 80% 3-year survival with zero mismatches to 69% with six-antigen mismatches (Fig. 1). Within the two- to five-HLA-mismatch spectrum that encompassed 82.5% of the recipients, HR rose modestly from 1.26 to 1.54 and 3-year survival was clustered within a range of 5%. Post-1-year half-lives were bracketed at 9–11 years with Cox regression analysis and 9–10 years with conventional half-life calculations, respectively (Fig. 1)
Separate analysis showed that the negative effects of mismatching at the A, B, and DR loci were essentially the same at each locus (Table 4). Unlike the analysis of Pittsburgh data, a double incompatibility added to the HR imposed by the first one.
Secondary Analysis
In the UNOS collection, the percentage of CREG matches dwindled rapidly with each further HLA mismatch, from tiers 1 (35.3%) to 4 (0.7%). No CREG matches were identified in tiers 5 and 6. The same pattern was seen in the Pittsburgh series (Table 5).
Table 5.
Percentage of 0 CREGs at each level of 1–4 ABDR mismatch, and adjusted hazard ratios from Cox regression
| UNOS 1991–95 |
|||||
|---|---|---|---|---|---|
| 1 (n=1.334) | 2 (n=4.008) | 3 (n=8.071) | 4 (n=8.542) | 2–4 (n=20.621) | |
| Percentage of 0 CREG (n) | 35.3 (471) | 10.4 (416) | 2.5 (206) | 0.7 (57) | 3.3 (679) |
| HR (95% CI) | |||||
| 0 CREG vs. 0 ABDR | 1.02 (0.80–1.30) | 1.34 (1.07–1.69) | 1.36 (0.99–1.85) | 1.15 (0.61–2.16) | 1.33 (1.11–1.62) |
| 1 + CREG vs. 0 ABDR | 1.33 (1.11–1.58) | 1.26 (1.11–1.42) | 1.33 (1.19–1.49) | 1.50 (1.34–1.68) | 1.39 (1.25–1.54) |
| Pittsburgh 1981–95 |
|||||
|---|---|---|---|---|---|
| 1 (n=65) | 2 (n=196) | 3 (n=456) | 4 (n=522) | 2–4 (n=1.174) | |
| Percentage of 0 CREG (n) | 27.7 (18) | 6.6 (13) | 0.9 (4) | 0.6 (3) | 1.7 (20) |
| HR (95% CI) | |||||
| 0 CREG vs. 0 ABDR | 1.30 (0.49–3.46) | Not estimated | Not estimated | Not estimated | 1.96 (0.80–4.80) |
| 1 − CREG vs. 0 ABDR | 1.01 (0.46–2.21) | Not estimated | Not estimated | Not estimated | 2.11 (1.19–3.75) |
Using the zero-HLA mismatch results as the reference category (HR 1.0), only the CREG-matched allografts extracted from the UNOS one-HLA-mismatch collection had comparable graft survival (HR= 1.02, 95% confidence interval [CI]: 0.80–1.30) (Table 5). The companion CREG-mismatched kidneys at this one-HLA-mismatch level had an HR of 1.33 (95% CI: 1.11–1.58). Paradoxically, in the small Pittsburgh series, the CREG-mismatched kidneys had better survival (HR 1.01) than those that were matched (HR 1.30) (Fig. 2). There was no benefit in either the UNOS or Pittsburgh analysis of a CREG match at the two- to four-HLA mismatch level (Table 5). These findings also are shown in Figure 2, expressed in terms of graft survival. For clarity in presenting the survival curves, HLA mismatch categories 2–4 shown individually in Table 5 were combined for Figure 2 inasmuch as there were no discernible differences among the HRs nor among the graft survival rates. The aggregation of categories also allowed the Pittsburgh CREG data, which consists of fewer numbers, to be analyzed alongside the UNOS data.
Figure 2.
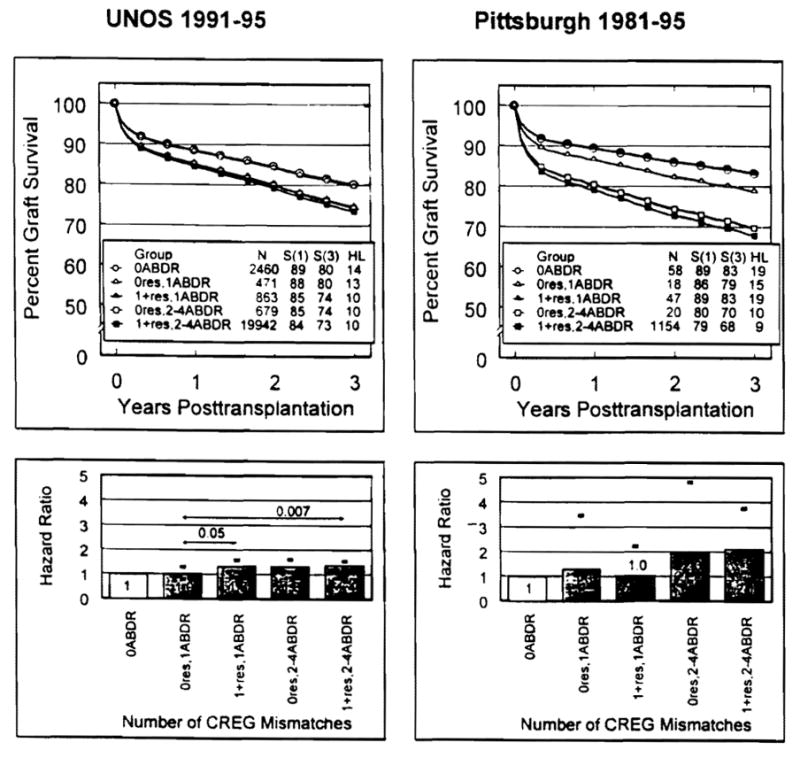
Adjusted graft survival curves (top panels) and HRs (bottom panels) from a secondary analysis of the UNOS (left panels) and Pittsburgh (right panels) databases that bifurcated each degree (1–4) of HLA-A, -B, -DR-mismatched transplants by the presence (1 +res) or absence (0res) of a CREG mismatch. The differences in HRs (95% confidence limits given by darkened error bars shown in the bottom panels) between CREG-HLA groups and the 0res-one-HLA antigen-mismatched group were significant when indicated by P-value (listed above arrow line depicting comparison).
Exploratory Analysis
The problems of organ allocation by tissue matching in an ethnically pluralistic society were evident in our HLA analysis of the 31,291 UNOS cases. Of the 2,460 zero-HLA-mismatched kidneys used during 1991–1995, only 174 were transplanted into the 8111 African-American recipients, for a rate of 2.1%. The other 2,286 went to the 23,180 “all others,” for a rate of 9.9%.
Allocation of the 1334 one-HLA-mismatched kidneys was also unequal: 206 went to the 8,111 African-Americans (2.5% rate) versus 1,128 to the 23,180 all others (4.9%). Because this was the only HLA mismatch tier within which there was any evidence of a CREG match benefit (see previous section), we determined the ethnic allocation rates of the CREG-matched and -mismatched organs within the one-HLA category. Of the 206 given to African-Americans, 81 (39.3%) were CREG-matched compared with 390/1,128 (34.6%) transplanted to the “all other” population.
Although under-represented overall in the one-HLA mismatch tier, the 81 African-Americans in this category with a CREG match were estimated to have better graft survival than the 125 with a CREG mismatch, far exceeding the advantage of a CREG match in the “all other” population (Fig. 3), Incongruously, survival of the CREG-matched one-HLA-mismatched kidneys in African-Americans exceeded that of zero-HLA-mismatched kidneys (Fig. 3), which by definition also were CREG-matched.
Figure 3.
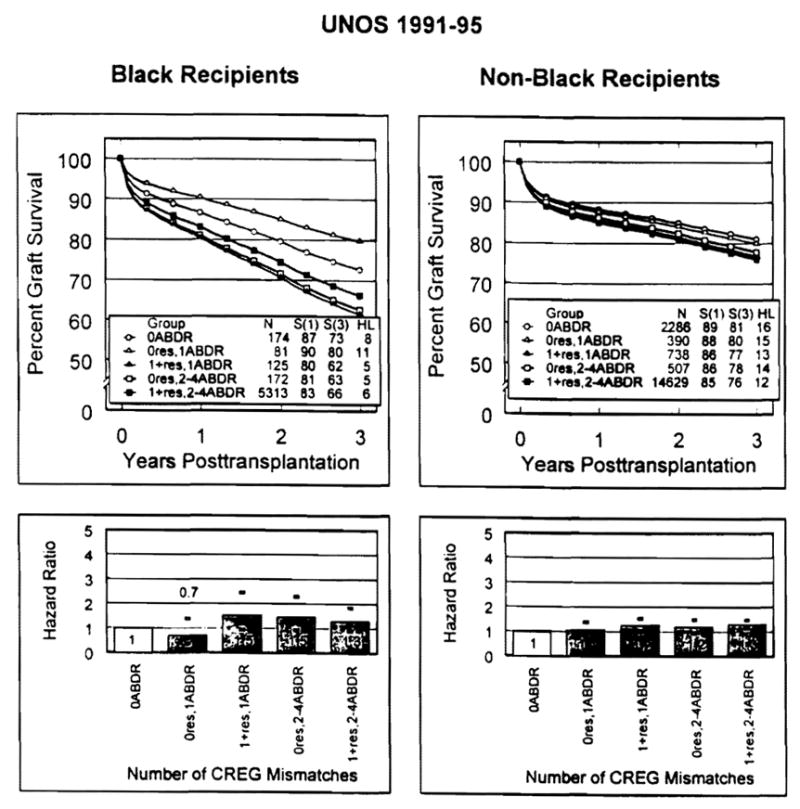
Adjusted graft survival curves (top panels) and HRs (bottom panels) from an exploratory analysis of the UNOS database stratifying CREG histocompatibility by recipient race (black vs. non-black). Darkened error bars (shown in the bottom panels) represent 95% confidence limits for HRs, using zero mismatch as the reference. Note that no formal tests of significance were conducted due to the exploratory nature of the analysis.
For easier comparison of graft survival in the African-American and non-African-American populations of the one-HLA mismatch category, the HRs in Figure 3 were converted to relative risks using the CREG match results as the reference (i.e., 1.5/0.7=2.14 for African-Americans; 1.2/1.1=1.09 for non-African-Americans). With this calculation, the 125 African-American patients with a one-HLA mismatch who were CREG-mismatched were 2.14 times (95% CI: 1.11–4.10) more likely to fail than the 81 with a CREG match. In contrast, the concurrent non-African-American relative risk was only 1.09 (95% CI: 0.88–1.58).
It is important to emphasize, first, that the number of cases in the foregoing exploratory analysis of CREG matching in the one-HLA match tier was small and, second, that a CREG match appeared to have no advantage for either race in the two- to four-HLA mismatch groups (Table 5 and Fig. 3).
DISCUSSION
Except for the prevention of hyperacute rejection by observance of ABO compatibility guidelines (26) and the routine use of the cytotoxic cross-match (27), immunologic screening has had a limited impact on survival after cadaver kidney transplantation. The failure of prospective HLA matching to upgrade overall graft survival documented in the report by Held et al. (28) from the United States Renal Data System was in stark contrast to the historically rooted conviction that matching would be the most important means of up grading organ transplant outcome (see Brent [29]). It also was inconsistent with experience in clinical bone marrow transplantation, where incompatibility of even one or two HLA alleles was associated with nearly universal failure (30–32) until the 1990s. Instead, the benefit of graded HLA matching for kidney transplantation has been more subtle, frequently not obvious in single-center studies, and most convincingly displayed in registry compilations (29, 33).
Ultimately, a generic explanation for the disparity between bone marrow and organ transplantation was suggested by the discovery that passenger leukocytes including pluripotent stem cells migrate after kidney and other organ transplantation and result in persistent multilineage chimerism (34, 35). Variable induction of mutual nonreactivity by the genetically disparate leukocyte populations, each obeying its own genetic program, would obscure the expected histocompatibility influence unless the recipient is immunologically defenseless: i.e., in the neonatal tolerance model, recipient cytoablation in all species, or as the consequence of breeding (e.g., the F1 hybrid preparations) (36).
In addition, experimental (37) and epidemiologic evidence (38, 39) suggests that much of the late allograft attrition is due to the non-HLA factors that were evident in the present study (see Table 2) and elsewhere (40). These factors help explain why the average time to loss of 50% of cadaver kidneys still functioning at 1 year (the “half-life”) has been fixed until recently at 8–10 years (41) except when there is a zero-HLA mismatch, as confirmed in our current analyses.
HLA matching in organ transplantation was first tested retrospectively (42) and prospectively (43) in live donor kidney recipients treated with azathioprine and prednisone, with or without adjunct antilymphocyte globulin. As had been predicted, HLA-matched allografts had the best survival and function, least dependence on maintenance prednisone, and fewest histopathologic abnormalities in routine 2-year postoperative biopsies (44). The results of these studies were generally consistent with those from the classical skin graft investigations in nonimmunosuppressed healthy volunteers by Rapaport and Dausset (45–47) upon which the “laws of HLA” are based. Unexpectedly, however, a cumulative adverse effect of mismatching in the kidney recipients could not be identified. When similar observations were made in live donor recipients during the cyclosporine era (48, 49), it was concluded that the lower survival of contemporaneously transplanted cadaveric kidneys commonly attributed to generally inferior HLA compatibility was due largely to premortem and preservation damage.
The equally imprecise prognostic discrimination of HLA matching in cadaver kidney transplant cases, first recognized by Mickey and Terasaki et al. (50), was evident in the current analyses. Given the large sample sizes in both the single-center and UNOS databases, virtually every comparison of the different levels of mismatching showed statistical significance. These observations confirm the scientific validity of the matching technology and the continuing need for first-class histocompatibility laboratories. However, as Held et al. (28) have demonstrated, the important clinical issues are the magnitude of the observed differences and the numbers of patients in the HLA-defined groups. The absence of a large or consistent matching effect except at the top was noteworthy. In both the Pittsburgh and UNOS series, approximately 85% of the cases were in the two- to five-HLA mismatch spectrum where 1-year survival was clustered within 3%, with half-life projections thereafter in the narrow spread of 9–11 years.
In Pittsburgh, access to the national donor pool beginning in January 1988 resulted in a reduction of average mismatches from 4.0 to 3.3, but only 2.0% of recipients were moved out of the two- to five-HLA incompatibility range within which matching has little benefit. A second management change, the preferential use of tacrolimus-based immune suppression after 1989 (23,24), was associated with an increased early and late graft survival. If the 15-year experience had been simply pooled as in many registry reports (33), the better survival in the later period would have been attributed erroneously to the lower average number of mismatches under tacrolimus therapy, whereas the poorer earlier results would have been ascribed to the more frequent use of mismatched allografts under cyclosporine. To control for the potential confounding effect of drug therapy, graft survival was adjusted for the treatment factor in the regression analyses, which showed that the relationship between survival and match was similar with both immunosuppressive regimens.
The findings reported herein should be considered in reforming cadaver kidney allocation. It is an established fact that the absence of HLA mismatches improves short- and long-term cadaver kidney allograft survival (21,28,39) and that there is no weak locus (i.e., A locus) that can be minimized in importance as is current UNOS policy (14). Such highly compatible organs were found until recently for less than 5% of cadaver renal transplant recipients in the United States (28). The incidence rose to 7.9% in the 1991–1995 UNOS experience, but with the ethnic bias that was also seen with one-HLA-mismatched kidneys.
Presumably, the prospects for the African-American candidates could be improved by efforts, such as those that Callender et al. (51) have described, to recruit donor participation by minorities. The combination of increased minority donation and national sharing theoretically could result in zero-mismatched allografts for approximately 20% of candidates (52). Despite the inherent inequity of obligatory national sharing of zero-mismatched kidneys under present circumstances, this group of privileged recipients is needed as a baseline for investigations of alternative strategies such as CREG matching.
Although CREG matching is currently being promoted by the histocompatibility committee of UNOS, its future role in organ transplantation can only be considered speculative. No CREG matches were found in recipients of five- to six-HLA-mismatched kidneys, and a CREG match in a small number of cases with two to four HLA mismatches did not confer a graft survival advantage. The only encouraging notation was that a subset (n=471) of the 1334 one-HLA-mismatched UNOS recipients had significantly better survival than their 863 CREG-mismatched counterparts. It must be emphasized, however, that this observation was not confirmed in the Pittsburgh analysis, in which exactly the opposite association was seen. In addition, the survival rate of the 81 Mrican-Americans in the UNOS experience who received CREG-matched grafts in the one-HLA mismatch tier was inexplicably higher than in the 174 African-American patients given zero-HLA-mismatched organs, which by definition (9) were also CREG-matched.
Whether the approximately one third of the one-HLA-mismatched kidneys which are CREG-matched actually have an improved prognosis can only be answered by a rigorously controlled prospective trial(s). It has been estimated that if obligatory sharing were extended to the one-HLA match, 50% of the national pool of cadaver kidneys exclusive of zero-HLA-mismatched cases could be consumed (53). If such a policy were enacted, either to enlarge the pool from which CREG matches could be extracted or for the statistically significant but clinically inconsequential increase in one-HLA mismatch graft survival seen in the 1991–1995 UNOS analysis, only 30% of the organs would be left for allocation without an HLA-related ethnic bias.
Even if a CREG match in one-HLA-mismatched recipients were associated with improved graft survival, there is no reason to expect correction of the distribution inequity. Our UNOS analysis suggests that the rate of distribution of the CREG-matched kidneys to African-American recipients would be only about half that to the “all other” population. Consequently, a CREG matching trial of one-HLA-mismatched kidneys, if it is done at all, should be restricted to demographically defined local or regional populations in which there is a substantial donor as well as recipient African-American constituency. Furthermore, such a trial should be viewed as a Helsinki Declaration Type I “innovative attempt to treat” or “acquisition of human knowledge” human experiment (54), subject to informed consent, frequent specified analyses, and conditions of trial stoppage.
Finally, it should be recognized that the polymorphism of the human HLA system, as well as the complexities of organ transplantation as practiced currently, may preclude improvement of matching performance beyond what already has been delineated with conventional HLA typing techniques. In bone marrow transplantation, the assumption that HLA-A, -B, and -DR were of overriding importance was not challenged until recently, in part because HLA-C and -DR were not analyzable in related donors because of linkage disequilibrium. However, Petersdorf et al. (55,56) have been able to show that C and DQ loci are just as important as A, B, and DR in determining the outcome after unrelated bone marrow transplantation. Whether the effect of these alleles would override CREGs in the current organ transplant setting is uncertain. Our preliminary studies (57, 58) have suggested that C and DQ matching would have a more limited role than that of the other loci in kidney transplantation.
Acknowledgments
The authors acknowledge the contributions of the following individuals: Mark L. Jordan, M.D., Carlos Vivas, M.D., H. Albin Gritsch, M.D., Thomas R. Hakala, M.D., Richard L. Simmons, M.D., J. Thomas Rosenthal, M.D., Rodney Taylor, M.D., Christopher Jensen, M.D., William Lopatin, M.D., Andreas Tzakis, M.D., Robert Gordon, M.D., Shelia Fedorek, RN, Lynn Ostrowski, RN, BSN, Regina Fenton, RN, BSN, Loraine Oczypok, RN, BSN. Deborah Good, RN, BSN, Holly Woods, RN, Jareen Flohr, RN, BSN, Jennifer Ovesney, RN, Sharon Orlofske, RN, Mark Paynter RN, BSN, and Gerri James, RN. All of these individuals have been involved, at one time or another, in the care of renal transplant recipients over the past 16 years at the University of Pittsburgh.
Footnotes
This work was supported by project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
Abbreviations: CI, confidence interval; CREG, cross-reactive antigen group; HLA, human leukocyte antigen; HR, hazard ratio; UNOS, United Network for Organ Sharing.
References
- 1.Starzl TE, Shapiro R, Teperman L. The point system for organ distribution. Transplant Proc. 1989;21:3432. [PMC free article] [PubMed] [Google Scholar]
- 2.Van Buren CT, Kerman RH, Lewis RM, Kahan BD. Exchanging donor kidneys. N Engl J Med. 1988;319:1092. doi: 10.1056/NEJM198810203191613. [DOI] [PubMed] [Google Scholar]
- 3.Guttman RD. The graft survival curve: ideology and rhetoric. Transplant Proc. 1992;24:2407. [PubMed] [Google Scholar]
- 4.Gaston RS, Ayres I, Dooley LG, Diethelm AG. Racial equity in renal transplantation: the disparate impact of HLA-based allocation. JAMA. 1983;270:1352. [PubMed] [Google Scholar]
- 5.Starzl TE, Fung JJ. The politics of grafting cadaver kidneys. Lancet. 1996;348:454. doi: 10.1016/S0140-6736(96)03435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanfilippo F, Vaughn WK, Spees EK, Heise ER, LeFor WM. The effect of HLA-A, -B matching on cadaver renal allograft rejection comparing public and private specificities. Transplantation. 1984;38:483. doi: 10.1097/00007890-198411000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Duquesnoy RJ, White LT, Fierst JW, et al. Multiscreen serum analysis of highly sensitized renal dialysis patients for antibodies toward public and private class I HLA determinants: implications for computer-predicted acceptable and unacceptable donor mismatches in kidney transplantation. Transplantation. 1990;50:427. doi: 10.1097/00007890-199009000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodey GE, Fuller TC. Public epitopes and the antigenic structure of the HLA molecules. Crit Rev Immunol. 1987;7(3):229. [PubMed] [Google Scholar]
- 9.Takemoto S, Terasaki PI, Gjertson DW, Cecka JM. Equitable allocation of HLA-compatible kidneys for local pools and for minorities. N Engl J Med. 1994;331:760. doi: 10.1056/NEJM199409223311202. [DOI] [PubMed] [Google Scholar]
- 10.Thompson JS, Thacker LR. CREG matching for first cadaveric kidney transplants (TNX) performed by SEOPF centers between October 1987 and September 1995. Clin Transplant. 1996;10:586. [PubMed] [Google Scholar]
- 11.Bodmer JG, Marsh SGE, Albert ED, et al. Nomenclature for factors of the HLA system. Hum Immunol. 1997;53:98. doi: 10.1016/S0198-8859(97)00031-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhou YC, Cecka JM. Effect of HLA matching on renal transplant survival. In: Cecka JM, Terasaki PI, editors. Clinical transplants 1993. Los Angeles: UCLA Tissue Typing Laboratory; 1994. p. 499. [PubMed] [Google Scholar]
- 13.Starzl TE, Hakala T, Tzakis A, et al. A multifactorial system for equitable selection of cadaveric kidney recipients. JAMA. 1987;257:3073. [PMC free article] [PubMed] [Google Scholar]
- 14.Norman DJ, Ellison MD, Breen TJ, Davies DB, Daily OP. Cadaveric kidney allocation in the US: a critical analysis of the point system. Transplant Proc. 1995;27:800. [PubMed] [Google Scholar]
- 15.Takemoto S, Gjertson DW, Terasaki PI. HLA matching: a comparison of conventional and molecular approaches. In: Terasaki PI, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1992. p. 413. [PubMed] [Google Scholar]
- 16.Rodey GE, Nalan JF, Whelchel JD, Revels KW, Bray RA. Epitope specificity of HLA class I alloantibodies. I. Frequency analysis of antibodies to private versus public specificities in potential transplant recipients. Hum Immunol. 1994;39:272. doi: 10.1016/0198-8859(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 17.Dixon WJ, Brown MB, Engelman L, Jennrich RI. BMDP statistical software. Berkeley, CA: University of California Press; 1990. p. 769. [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 19.Kleinbaum DG. Survival analysis: a self-learning text. New York: Springer-Verlag; 1996. p. 104. [Google Scholar]
- 20.Opelz G, Mickey MR, Terasaki PI. Calculations of long-term graft and patient survival in human kidney transplantation. Transplant Proc. 1987;9:27. [PubMed] [Google Scholar]
- 21.Takemoto BS, Terasaki PI, Cecka JM, Cho YW, Gjertson DW. Survival of nationally shared, HLA-matched kidney transplants from cadaveric donors. N Engl J Med. 1992;327:834. doi: 10.1056/NEJM199209173271202. [DOI] [PubMed] [Google Scholar]
- 22.Gjertson DW. Multifactorial analysis of renal transplants reported to the United Network of Organ Sharing registry: a 1994 update. In: Terasaki PI, Cecka JM, editors. Clinical transplants. Los Angeles: UCLA Tissue Typing Laboratory; 1994. p. 519. [PubMed] [Google Scholar]
- 23.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro R, Jordan ML, Scantlebury VP, et al. Randomized trial of FK506/prednisone versus FK506/azathioprine/prednisone after renal transplantation: preliminary report. Transplant Proc. 1993;25:669. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Donner A, Eliasziw M, et al. Randomized trialomania? The multicenter liver transplant trials. Lancet. 1995;346:1346. doi: 10.1016/s0140-6736(95)92349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE. Experience in renal transplantation. Philadelphia: WE Saunders; 1964. Patterns of permissible donor-recipient tissue transfer in relation to ABO blood groups; p. 37. [Google Scholar]
- 27.Terasaki PI, Marchioro TL, Starzl TE. Histocompatibility testing. Washington, DC: National Academy of Sciences-National Research Council; 1965. Sero-typing of human lymphocyte antigens: preliminary trials on long-term kidney homograft survivors; p. 83. [Google Scholar]
- 28.Held PJ, Kahan BD, Hunsicker LG, et al. The impact of HLA mismatches on the survival of first cadaveric kidney transplants. N Engl J Med. 1994;331:765. doi: 10.1056/NEJM199409223311203. [DOI] [PubMed] [Google Scholar]
- 29.Brent L. A history of transplantation immunology. London: Academic Press; 1997. Immunogenetics; histocompatibility antigens: structure and function; p. 131. [Google Scholar]
- 30.Meuwissen HJ, Gatti RA, Terasaki PI, Hong R, Good RA. Treatment of lymphopenic hypogammaglobulinemia and bone-marrow aplasia by transplantation of allogeneic marrow: crucial role of histocompatibility matching. N Engl J Med. 1969;281:691. doi: 10.1056/NEJM196909252811302. [DOI] [PubMed] [Google Scholar]
- 31.Thomas ED. Allogeneic marrow grafting: a story of man and dog. In: Terasaki PI, editor. History of transplantation: thirty-five recollections. Los Angeles: UCLA Tissue Typing Laboratory; 1991. p. 379. [Google Scholar]
- 32.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 33.Opelz G. HLA matching should be utilized for improving kidney transplant success rates. Transplant Proc. 1991;23:46. [PubMed] [Google Scholar]
- 34.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Demetris AJ, Murase N, Trucco M, Thomson AW, Rao AS. The lost chord: microchimerism. Immunol Today. 1996;17:577. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azuma H, Nadeau K, Mackenzie HS, Brenner BM, Tilney NL. Nephron mass modulates the hemodynamic, cellular, and molecular response of the rat renal allograft. Transplantation. 1997;63:519. doi: 10.1097/00007890-199702270-00006. [DOI] [PubMed] [Google Scholar]
- 38.Brenner BM, Milford EL. Nephron underdosing: a programmed cause of chronic renal allograft failure. Am J Kidney Dis. 1993;21:66. doi: 10.1016/0272-6386(93)70097-i. [DOI] [PubMed] [Google Scholar]
- 39.Chertow GM, Milford EL, Mackenzie HS, Brenner BM. Antigen-independent determinants of cadaveric kidney transplant failure. JAMA. 1996;276:1732. [PubMed] [Google Scholar]
- 40.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S, Cho YW, Yuge J. Risk rate and long-term kidney transplant survival. In: Cecka JM, Terasaki PI, editors. Clinical transplants 1996. Los Angeles: UCLA Tissue Typing Laboratory; 1996. p. 443. [PubMed] [Google Scholar]
- 41.Gjertson DW, Cecka JM, Terasaki PI. The relative effects of FK 506 and cyclosporine on short- and long-term kidney graft survival. Transplantation. 1995;60:1384. doi: 10.1097/00007890-199560120-00002. [DOI] [PubMed] [Google Scholar]
- 42.Starzl TE, Marchioro TL, Terasaki PI, et al. Chronic survival after human renal homotransplantations: lymphocyte-antigen matching, pathology and influence of thymectomy. Ann Surg. 1965;162:749. doi: 10.1097/00000658-196510000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terasaki PI, Vredevoe DL, Mickey MR, et al. Serotyping for homotransplantation: VI. Selection of kidney donors for thirty-two recipients. Ann NY Acad Sci. 1966;129:500. doi: 10.1111/j.1749-6632.1966.tb12873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl TE, Porter KA, Andres G, et al. Long-term survival after renal transplantation in humans: with special reference to histocompatibility matching, thymectomy, homograft glomerulonephritis, heterologous ALG, and recipient malignancy. Ann Surg. 1970;172:437. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapaport FT, Lawrence HS, Thomas L, Converse JM, Tillett WS, Mulholland JH. Cross-reactions to skin homografts in man. J Clin Invest. 1962;41:2166. doi: 10.1172/JCI104675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dausset J, Rapaport FT. The Hu-1 system of human histocompatibility. In: Rapaport FT, Dausset J, editors. Human transplantation. New York: Grune & Stratton; 1968. p. 369. [Google Scholar]
- 47.Rapaport FT, Dausset J. Behavior of HLA-compatible and incompatible skin allografts in human recipients preimmunized with pooled leukocyte extracts obtained from randomly selected donors. Transplantation. 1983;36:592. [PubMed] [Google Scholar]
- 48.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 49.D’Alessandro AM, Sollinger HW, Knechtle SJ, et al. Living related and unrelated donors for kidney transplantation: a 28-year experience. Ann Surg. 1995;222:353. doi: 10.1097/00000658-199509000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mickey MR, Kreisler M, Albert ED, Tanaka N, Terasaki PI. Analysis of HLA incompatibility in human renal transplants. Tissue Antigens. 1971;1:57. doi: 10.1111/j.1399-0039.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Callender CO, Hall LE, Yeager CL, Barber JB, Dunston GM, Pinn-Wiggins VW. Organ donation and blacks: a critical frontier. N Engl J Med. 1991;325:442. doi: 10.1056/NEJM199108083250631. [DOI] [PubMed] [Google Scholar]
- 52.Port FK, Held PJ, Wolfe RA, Garcia JR, Rocher LL. The impact of non-identical ABO cadaveric renal transplantation on waiting times and graft survival. Am J Kidney Dis. 1991;17:519. doi: 10.1016/s0272-6386(12)80492-6. [DOI] [PubMed] [Google Scholar]
- 53.Mickey MR, Cook DJ, Terasaki PI. Recipient pool sizes for prioritized HLA matching. Transplantation. 1989;47:401. [PubMed] [Google Scholar]
- 54.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925. [PubMed] [Google Scholar]
- 55.Petersdorf EW, Longton GM, Anasetti C, et al. Definition of HLA-DQ as a transplantation antigen. Proc Natl Acad Sci USA. 1996;93:15358. doi: 10.1073/pnas.93.26.15358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersdorf EW, Longton GM, Anasetti C, et al. Association of HLA-C disparity with graft failure after marrow transplantation from unrelated donors. Blood. 1997;89:1818. [PubMed] [Google Scholar]
- 57.Cho YW, Cecka JM, Terasaki PI. HLA matching effect: better survival rates and graft quality. In: Terasaki PI, Cecka JM, editors. Clinical transplants 1994. Los Angeles: UCLA Tissue Typing Laboratory; 1994. p. 435. [PubMed] [Google Scholar]
- 58.Terasaki PI, Cecka JM, Gjertson DW, Cho Y, Takemoto S, Cohn M. A ten-year prediction for kidney transplant survival. In: Terasaki PI, Cecka JM, editors. Clinical transplants 1992. Los Angeles: UCLA Tissue Typing Laboratory; 1992. p. 501. [PubMed] [Google Scholar]


