Abstract
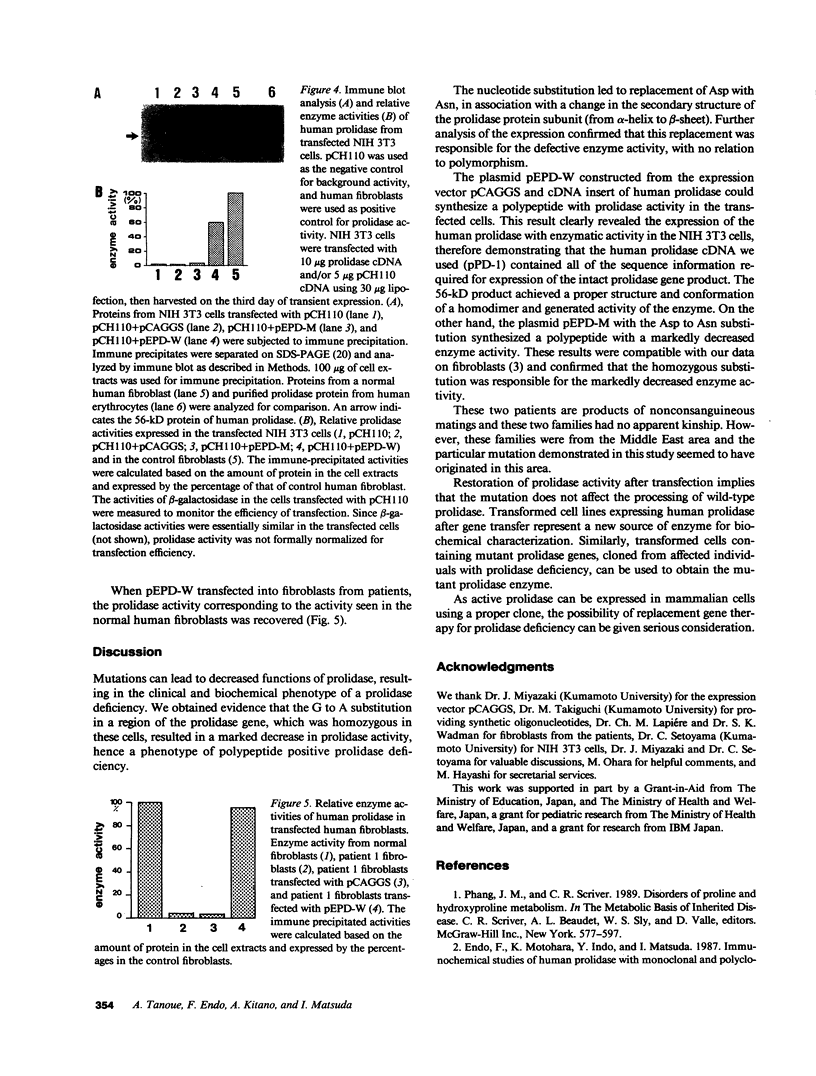
Prolidase deficiency is an autosomal recessive disorder characterized by mental retardation and various skin lesions. Cultured skin fibroblasts were obtained from two independent patients with abnormal prolidase. Using the polymerase chain reaction, we amplified the entire coding region of human prolidase mRNA derived from patients' fibroblasts. Nucleotide sequence analysis of amplified cDNA products revealed a G to A substitution at position 826 in exon 12, where aspartic acid was replaced by asparagine at the amino acid residue 276, in cells from both patients. An analysis of the DNA showed that the substitution was homozygous. An expression plasmid clone containing a normal human prolidase cDNA (pEPD-W) or mutant prolidase cDNA (pEPD-M) was prepared, transfected, and tested for expression in NIH 3T3 cells. Incorporation of pEPD-W and pEPD-M resulted in the synthesis of an immunological polypeptide that corresponded to human prolidase. Active human enzyme was detected in cells transfected with pEPD-W, but not in those transfected with pEPD-M. These results were compatible with our observation of fibroblasts and confirmed that the substitution was responsible for the enzyme deficiency. As active prolidase was recovered in prolidase-deficient fibroblasts transfected with pEPD-W, this restoration of prolidase activity after transfection means that gene replacement therapy for individuals with this human disorder can be given due consideration.
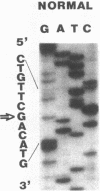
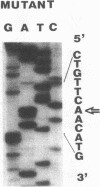
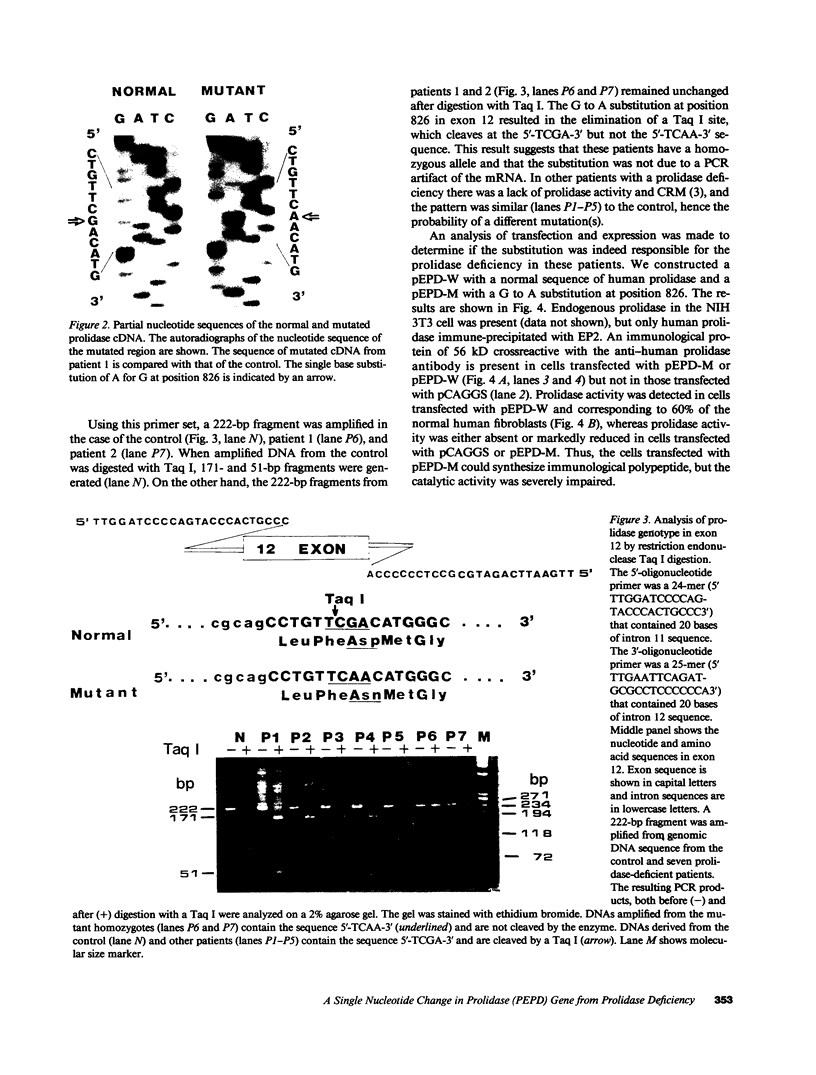
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boright A. P., Scriver C. R., Lancaster G. A., Choy F. Prolidase deficiency: biochemical classification of alleles. Am J Hum Genet. 1989 May;44(5):731–740. [PMC free article] [PubMed] [Google Scholar]
- Endo F., Hata A., Indo Y., Motohara K., Matsuda I. Immunochemical analysis of prolidase deficiency and molecular cloning of cDNA for prolidase of human liver. J Inherit Metab Dis. 1987;10(3):305–307. doi: 10.1007/BF01800088. [DOI] [PubMed] [Google Scholar]
- Endo F., Matsuda I. Screening method for prolidase deficiency. Hum Genet. 1981;56(3):349–351. doi: 10.1007/BF00274691. [DOI] [PubMed] [Google Scholar]
- Endo F., Motohara K., Indo Y., Matsuda I. Immunochemical studies of human prolidase with monoclonal and polyclonal antibodies: absence of the subunit of prolidase in erythrocytes from a patient with prolidase deficiency. Pediatr Res. 1987 Dec;22(6):627–633. doi: 10.1203/00006450-198712000-00002. [DOI] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Kitano A., Arata J., Danks D. M., Lapière C. M., Sei Y., Wadman S. K., Matsuda I. Biochemical basis of prolidase deficiency. Polypeptide and RNA phenotypes and the relation to clinical phenotypes. J Clin Invest. 1990 Jan;85(1):162–169. doi: 10.1172/JCI114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo F., Tanoue A., Nakai H., Hata A., Indo Y., Titani K., Matsuda I. Primary structure and gene localization of human prolidase. J Biol Chem. 1989 Mar 15;264(8):4476–4481. [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B. Plaies cutanees torpides et trouble du metabolisme du collagene. Arch Belg Dermatol Syphiligr. 1969;25(3):353–356. [PubMed] [Google Scholar]
- Lombeck I., Wendel U., Versieck J., van Ballenberghe L., Bremer H. J., Duran R., Wadman S. Increased manganese content and reduced arginase activity in erythrocytes of a patient with prolidase deficiency (iminodipeptiduria). Eur J Pediatr. 1986 Apr;144(6):571–573. doi: 10.1007/BF00496038. [DOI] [PubMed] [Google Scholar]
- Miyazaki J., Takaki S., Araki K., Tashiro F., Tominaga A., Takatsu K., Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989 Jul 15;79(2):269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]