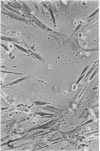
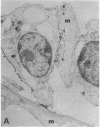
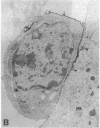
Abstract
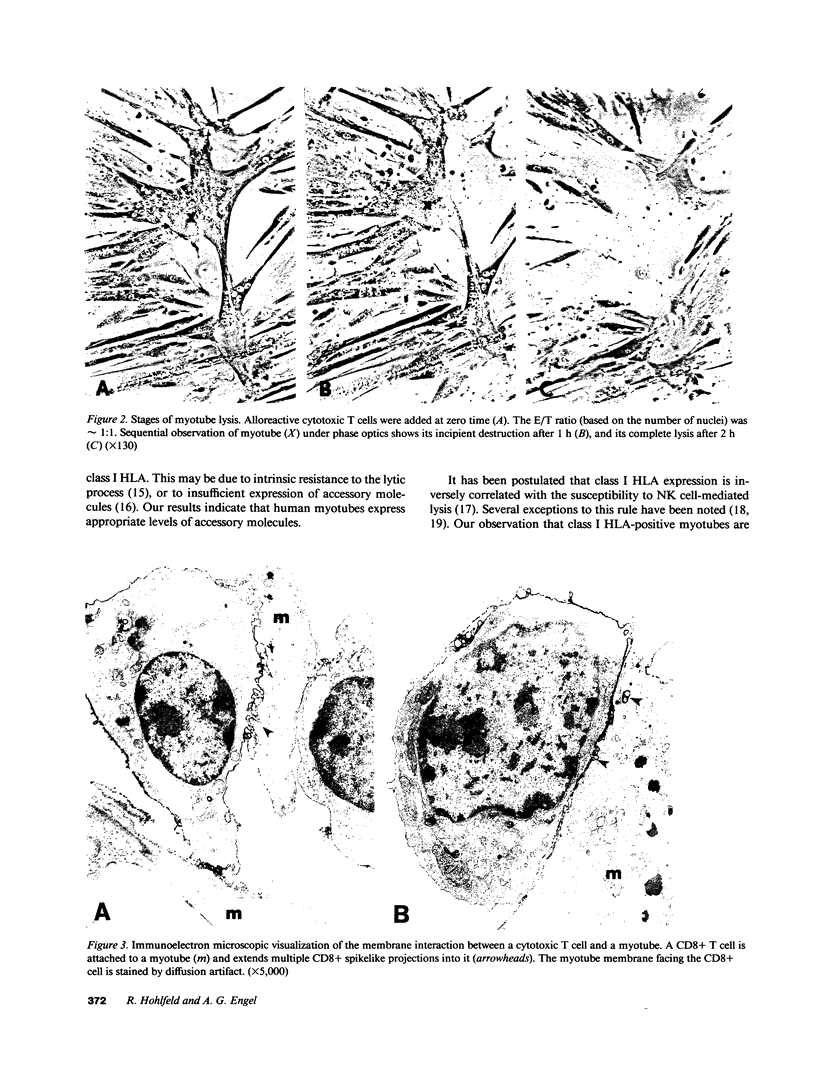
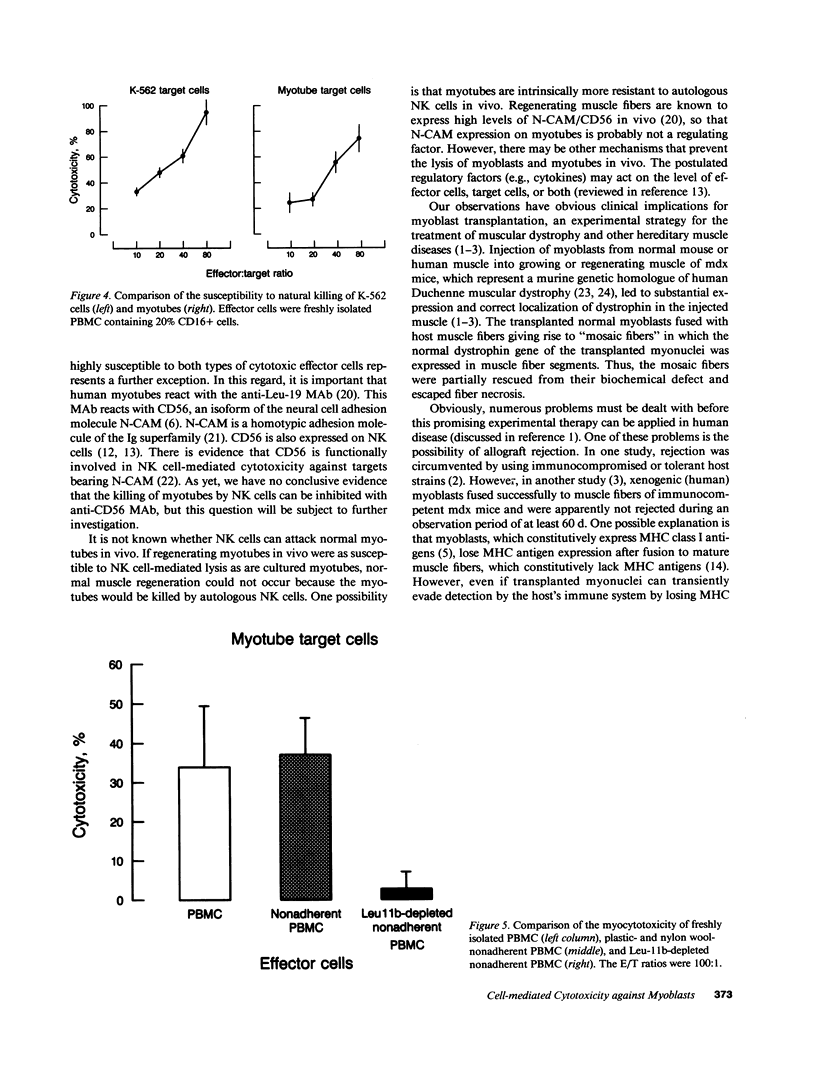
The aim of this study was to investigate the susceptibility of human myotubes to lysis by the two major types of cytotoxic effector cells, CD3+CD8+ cytotoxic T cells (CTL) and CD16+CD56+ natural killer (NK) cells. The myoblasts preparations used as target cells were greater than 90% pure as assessed by immunostaining with the Leu19 monoclonal antibody (MAb) that cross-reacts with the neural cell adhesion molecule N-CAM. Allospecific CTL lines were generated from mixed lymphocyte cultures, and freshly isolated allogeneic and autologous peripheral blood cells were used as a source of NK cells. The cytotoxicity was observed under phase optics and by immunoelectron microscopy, and was quantitated with a chromium release assay. Myotubes were efficiently killed by allospecific CTL and by autologous and allogeneic NK cells. The killing by CTL was inhibited with an anti-class I HLA MAb, and the killing by NK cells was inhibited by depleting peripheral blood cells of CD16+ cells with anti-CD16 MAb and complement. The results have important implications for myoblast transplantation, an experimental therapy of muscular dystrophy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Engel A. G. Monoclonal antibody analysis of mononuclear cells in myopathies. III: Immunoelectron microscopy aspects of cell-mediated muscle fiber injury. Ann Neurol. 1986 Feb;19(2):112–125. doi: 10.1002/ana.410190203. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenak R., Wolcott R. M. Target cell expression of MHC antigens is not (always) a turn-off signal to natural killer cells. J Immunol. 1988 Jun 1;140(11):3712–3716. [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Dougherty E. P. Improved method for transmission electron microscopy of ciliated cell monolayers maintained on gas-permeable membranes. J Microsc. 1983 Nov;132(Pt 2):165–169. doi: 10.1111/j.1365-2818.1983.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Gutmann D. H., Fischbeck K. H. Molecular biology of Duchenne and Becker's muscular dystrophy: clinical applications. Ann Neurol. 1989 Aug;26(2):189–194. doi: 10.1002/ana.410260202. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Engel A. G. Induction of HLA-DR expression on human myoblasts with interferon-gamma. Am J Pathol. 1990 Mar;136(3):503–508. [PMC free article] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988 Jan;23(1):64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Zubrzycka-Gaarn E., Carpenter S., Ray P. N., Worton R. G., Holland P. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989 Jul;135(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lanier L. L., Testi R., Bindl J., Phillips J. H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989 Jun 1;169(6):2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Karpinski B. A., Gottschalk L., Kornbluth J. Susceptibility to natural killer cell-mediated cytolysis is independent of the level of target cell class I HLA expression. J Immunol. 1989 Mar 15;142(6):2140–2147. [PubMed] [Google Scholar]
- Main E. K., Monos D. S., Lampson L. A. IFN-treated neuroblastoma cell lines remain resistant to T cell-mediated allo-killing, and susceptible to non-MHC-restricted cytotoxicity. J Immunol. 1988 Nov 1;141(9):2943–2950. [PubMed] [Google Scholar]
- Mandel J. L. Dystrophin. The gene and its product. Nature. 1989 Jun 22;339(6226):584–586. doi: 10.1038/339584a0. [DOI] [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Moretta L., Cerottini J. C., Mingari M. C. Direct demonstration of the clonogenic potential of every human peripheral blood T cell. Clonal analysis of HLA-DR expression and cytolytic activity. J Exp Med. 1983 Feb 1;157(2):743–754. doi: 10.1084/jem.157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T., Yagita H., Sato K., Okumura K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer-target cell interaction. J Exp Med. 1989 Nov 1;170(5):1757–1761. doi: 10.1084/jem.170.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Reinherz E. L. T lymphocytes: ontogeny, function, and relevance to clinical disorders. N Engl J Med. 1987 Oct 29;317(18):1136–1142. doi: 10.1056/NEJM198710293171807. [DOI] [PubMed] [Google Scholar]
- Schubert W., Zimmermann K., Cramer M., Starzinski-Powitz A. Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci U S A. 1989 Jan;86(1):307–311. doi: 10.1073/pnas.86.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F. A., Head J. R. Murine trophoblast resists cell-mediated lysis. I. Resistance to allospecific cytotoxic T lymphocytes. J Immunol. 1987 Nov 1;139(9):2856–2864. [PubMed] [Google Scholar]