Abstract
Neuronal release of noradrenaline is primarily responsible for the contraction of prostatic smooth muscle in all species, and this forms the basis for the use of α1-adrenoceptor antagonists as pharmacotherapies for benign prostatic hyperplasia. Previous studies in mice have demonstrated that a residual nonadrenergic component to nerve stimulation remains after α1-adrenoceptor antagonism. In the guinea pig and rat prostate and the vas deferens of guinea pigs, rats, and mice, ATP is the mediator of this residual contraction. This study investigates the mediator of residual contraction in the mouse prostate. Whole prostates from wild-type, α1A-adrenoceptor, and P2X1-purinoceptor knockout mice were mounted in organ baths, and the isometric force that tissues developed in response to electrical field stimulation or exogenously applied agonists was recorded. Deletion of the P2X1 purinoceptor did not affect nerve-mediated contraction. Furthermore, the P2-purinoceptor antagonist suramin (30 μM) failed to attenuate nerve-mediated contractions in wild-type, α1A-adrenoceptor, or P2X1-purinoceptor knockout mice. Atropine (1 μM) attenuated contraction in prostates taken from wild-type mice. In the presence of prazosin (0.3 μM) or guanethidine (10 μM), or in prostates taken from α1A-adrenoceptor knockout mice, residual nerve-mediated contraction was abolished by atropine (1 μM), but not suramin (30 μM). Exogenously administered acetylcholine elicited reproducible concentration-dependent contractions of the mouse prostate that were atropine-sensitive (1 μM), but not prazosin-sensitive (0.3 μM). Acetylcholine, but not ATP, mediates the nonadrenergic component of contraction in the mouse prostate. This cholinergic component of prostatic contraction is mediated by activation of muscarinic receptors.
Introduction
Benign prostatic hyperplasia (BPH) is a disease of the human prostate resulting from an age- and androgen-dependent noncancerous proliferation of both the prostatic epithelium and stromal tissue (Wilson, 1980). The enlarged hyperplasic prostate places pressure on the urethra and the base of the bladder and leads to the manifestation of lower urinary tract symptoms. These symptoms arise from a dynamic component, caused by an increase in the prostatic smooth muscle tone, and a static component, caused by the proliferation of the prostatic tissue. Currently, drugs that target the dynamic component by relaxing prostatic smooth muscle, such as the α1A-adrenoceptor antagonist tamsulosin, are the most effective treatments for relieving the symptoms associated with BPH (Lepor, 2007; Miano et al., 2008).
The smooth muscle tone of the prostate gland is controlled predominantly by neuronally released noradrenaline acting at α1A adrenoceptors in humans, guinea pigs, and rats (Haynes and Ventura, 2005) and mice (Gray and Ventura, 2006). This therefore forms the basis for the use of selective α1A-adrenoceptor antagonists for the treatment of BPH (Cooper et al., 1999; Lepor, 2007). Although α1A adrenoceptors are seen as the principal mediator of nerve-mediated prostatic smooth muscle contraction, a residual nerve-mediated nonadrenergic contraction is observed after pharmacological α1-adrenoceptor blockade and more prominently after genetic α1A-adrenoceptor deletion in mice (Gray et al., 2008). It has been shown previously that ATP, released as a cotransmitter with noradrenaline, acting at P2X1 purinoceptors, mediates the residual nonadrenergic nerve-mediated contraction in the prostates of guinea pigs (Buljubasich and Ventura, 2004) and rats (Ventura et al., 2003). However, this is not the case in the mouse prostate (Gray and Ventura, 2005).
A large muscarinic receptor population has been identified in the prostates of humans, rats, and guinea pigs (Ventura et al., 2002). These receptors are confined mostly to the epithelium and are therefore thought to be responsible for the production and secretion of prostatic fluid. Little is known about the contribution of muscarinic receptor and cholinergic innervation to contraction in the mouse prostate. Binding studies of whole prostates indicate the presence of muscarinic receptors (Oki et al., 2006) of the M1 and M3 subtypes (Ito et al., 2009); however, the location or function of these receptors has not been described. Nevertheless, muscarinic receptor expression is not confined entirely to the glandular epithelium and has been observed in the stromal tissue of prostate glands taken from humans (Lepor and Kuhar, 1984), dogs (Fernández et al., 1998), rats (Lau and Pennefather, 1998), and guinea pigs (Lau et al., 2000). Furthermore, cholinesterase-positive nerves have been shown histochemically in the mouse prostatic smooth muscle (Gray and Ventura, 2005), and functional studies have indicated that muscarinic receptors play a role in nerve-mediated prostatic contraction of other species (Haynes and Hill, 1997; Najbar-Kaszkiel et al., 1997; Lau et al., 2000). The aim of this study was to investigate whether the mediator of the nonadrenergic nonpurinergic residual component of nerve-mediated contraction in the mouse prostate is cholinergic in nature.
Materials and Methods
Animals.
Adult α1A-adrenoceptor knockout mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and P2X1-purinoceptor knockout mice were generated in the laboratory of Professor R. J. Evans (University of Leicester, Leicester, UK). Colonies of knockout mice were maintained on a C57BL/6 background by heterozygous breeding pairs and routinely genotyped by polymerase chain reaction using genomic DNA from tail samples obtained at weaning (21 days) as described previously (Mulryan et al., 2000; Rokosh and Simpson, 2002). All mice were bred and housed at the Monash Animal Services facility, were exposed to a 12-h light/dark photoperiod, and had free access to food and water. Adult male mice (≥ 8 weeks) for experimentation were killed by cervical dislocation. Prior approval for animal experimentation was granted by the Monash University Standing Committee on Animal Ethics, ethics numbers VCPA 2006/08 RSV.4 2006 and VCPA 2006/09 RSV.5 2006 for the use of genetically modified and wild-type mice, respectively. All studies conformed to the National Health and Medical Research Council, Australian code of practice for the care and use of animals for scientific purposes.
Tissue Dissection.
An incision along the midline of the lower abdomen was made, and the urogenitial tract was exposed. The penile muscles, excess fat, and connective tissue were cut away to reveal the prostate and vas deferens. The whole prostate and one vas deferens from alternating sides were carefully dissected out and placed in a specimen jar containing Krebs-Henseleit solution (118.1 mM NaCl, 25.0 mM NaHCO3, 11.7 mM glucose, 4.69 mM KCl, 1.2 mM KH2PO4, 0.5 mM MgSO4, 2.5 mM CaCl2, pH 7.4).
Isolated Organ Bath Studies.
The dissected prostate and, as a comparator, the vas deferens were mounted in 10-ml water-jacketed organ baths containing Krebs-Henseleit solution maintained at 37°C and bubbled with 95% O2/5% CO2. One end of the tissue was attached to a perspex tissue holder and the other to a Grass FT03 force displacement transducer (Grass Instruments, Quincy, MA) for the measurement of isometric contractions. The force of contraction was recorded by using a PowerLab 4/SP data acquisition system (ADInstruments Pty Ltd., Castle Hill, Australia) and LabChart software version 5 (ADInstruments Pty Ltd.) run on a personal computer. Tissues were maintained under a resting force of approximately 0.7 g. Before experimentation tissue preparations were equilibrated for 1 h, during which time tissues were stimulated with electrical pulses of 0.5-ms duration, 60 V at 0.01 Hz. Electrical stimulation occurred via two parallel platinum electrodes incorporated into the tissue holder, connected to a Grass S88 stimulator (Grass Instruments).
Frequency Response Curves.
Frequency response curves to electrical field stimulation were constructed by using frequencies of 1, 2, 5, 10, and 20 Hz (0.5-ms duration, 60 V), and electrical stimulation was delivered at 10-min intervals in trains of pulses lasting 10 s.
An initial control frequency response curve was constructed to determine the contractile response of the tissue at each frequency. A second frequency response curve was then constructed after the tissue had been washed with three to four times the volume of the organ bath and had been exposed to the test drug for 1 h. When a third frequency response curve was constructed, the same washing and dosing protocol as described previously was used. Appropriate time control curves were constructed concurrently when no test drug was administered. The volume of test drug added to the organ bath was ≤200 μl.
In a subset of electrical field stimulation experiments, prazosin (0.3 μM) or guanethidine (10 μM) was present for the equilibration period and construction of the initial frequency response curve. The second frequency response curve was then constructed after the tissues had been washed with three to four times the volume of the organ bath and had been exposed for 1 h to the readded initial test drug and atropine (1 μM). An appropriate time control curve was also constructed in the presence of prazosin (0.3 μM), whereby the tissues were exposed to prazosin (0.3 μM) alone for both the first and second frequency response curves.
Agonist Concentration-Response Studies.
To assess sites of action, contractions of the isolated tissues were elicited by the application of exogenous acetylcholine on unstimulated tissues. After a 1-h equilibration period when tissues were stimulated with electrical pulses of 0.5-ms duration, 60 V at 0.01 Hz, an initial discrete concentration response curve to acetylcholine (10 nM to 1 mM) was constructed without electrical stimulation by using a log unit concentration-progression ratio.
For each agonist concentration, the contractile response of the tissue was allowed to reach a maximum and plateau before the tissue was washed with three to four times the volume of the organ bath. The tissue was then allowed to recover for 15 min before the addition of a subsequent concentration. After the initial concentration response curve, a second concentration response curve was constructed in the same manner; however in this instance, the tissue was exposed to an antagonist for 1 h before the addition of the first agonist concentration and after each washout the antagonist was replaced. Antagonists used were prazosin (0.3 μM) or atropine (1 μM). An appropriate time control curve was constructed in a parallel experiment, without the addition of antagonists.
Data Analysis.
The peak force (g) (prostate) or integral force (g × s; where s = 30 s) (vas deferens) of electrical field stimulation or agonist-mediated contraction was measured at each frequency or concentration. Baseline variance was removed from the peak contractile response by subtraction of the baseline height from the contractile response peak height. Mean frequency and concentration response curves were constructed by using Prism version 5.00 for Windows (GraphPad Software Inc., San Diego, CA). The mean curves were formed from the average of data from n experiments, where n is equal to the number of mice used. Results are expressed as mean ± S.E.M.
In mean frequency and concentration response curves, differences between the initial and subsequent drug-exposed curves were analyzed by GraphPad Prism version 5.00 using a two-way repeated-measures analysis of variance (ANOVA), followed by a Bonferroni post-test where required. The P values stated were used to evaluate the statistical significance of any difference between the two curves and represent the probability of the drug treatment causing a significant change. P < 0.05 was considered significant. GraphPad Prism version 5.00 was also used to calculate EC50 and pKB values from concentration response curves. All receptor nomenclature conforms to the International Union of Basic and Clinical Pharmacology nomenclature guidelines (Harmar et al., 2009)
Reagents.
Acetylcholine chloride, atropine sulfate, prazosin hydrochloride, suramin sodium salt, and tetrodotoxin were obtained from Sigma-Aldrich (St. Louis, MO). Guanethidine sulfate was attained from Ciba-Geigy (Sydney, Australia). All drugs were dissolved and diluted in distilled water.
Results
Contractile Response to Electrical Field Stimulation.
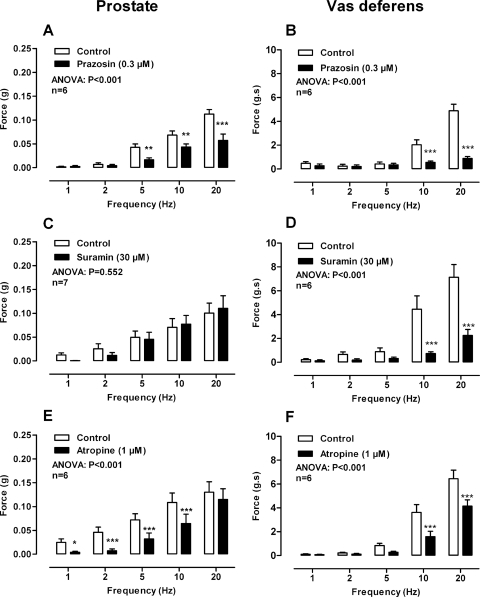
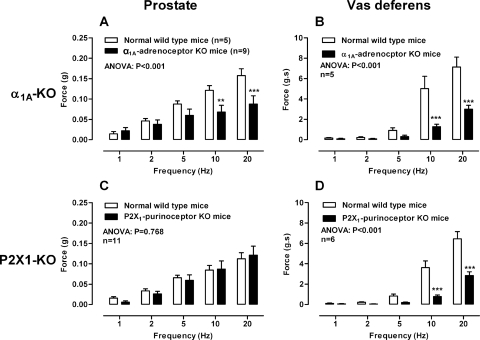
Electrical field stimulation (1–20 Hz) of the mouse prostate produced frequency-dependent contractions that were abolished by tetrodotoxin (1 μM; P < 0.001, data not shown). The noradrenergic component of contraction was attenuated to a similar degree by pharmacological α1-adrenoceptor blockade with prazosin (0.3 μM; Fig. 1A; P < 0.001), the genetic deletion of the α1A adrenoceptor (Fig. 2A; P < 0.001), or noradrenergic neuron blockade with guanethidine (10 μM; P < 0.001; data not shown). Maximum attenuation of contraction by prazosin (0.3 μM) was observed at 5 and 10 Hz for α1A-adrenoceptor deletion with a reduction in contraction of 60 and 44%, respectively.
Fig. 1.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in the prostate (A, C, and E) and vas deferens (B, D, and F) in the absence (open bars) and presence of (solid bars) prazosin (0.3 μM) (A and B), suramin (30 μM) (C and D), and atropine (1 μM) (E and F). Bars represent mean force ± S.E.M. (n = 6–7). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. *, P < 0.05; **, P < 0.01; ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests.
Fig. 2.
Comparison of mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in prostates (A and C) and vas deferens (B and D) taken from wild-type mice (open bars) and α1A-adrenoceptor knockout mice (solid bars) (A and B) or P2X1-purinoceptor knockout mice (solid bars) (C and D). Bars represent mean force ± S.E.M. (n = 5–11). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. **, P < 0.01; ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests.
In the isolated vas deferens taken from wild-type mice, tetrodotoxin (1 μM; P < 0.001, data not shown) and the noradrenergic neuron blocker guanethidine (10 μM; Fig. 3D) abolished contraction. Contraction was attenuated by the α1-adrenoceptor antagonist prazosin (0.3 μM; Fig. 1B; P < 0.001) and to the same degree by genetic deletion of the α1A adrenoceptor (Fig. 2B; P < 0.001). A reduction in contraction of 74% at 10 Hz was observed in both cases. Furthermore, in vasa deferentia taken from P2X1-purinoceptor knockout mice prazosin (0.3 μM; Fig. 4D; P < 0.001) abolished contraction.
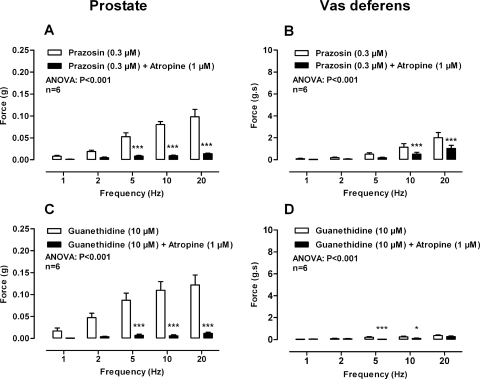
Fig. 3.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in the prostate (A and C) and vas deferens (B and D) after the administration of prazosin (0.3 μM) (open bars) and prazosin (0.3 μM) plus atropine (1 μM) (solid bars) (A and B) and guanethidine (10 μM) (open bars) and guanethidine (10 μM) plus atropine (1 μM) (solid bars) (C and D). Bars represent mean force ± S.E.M. (n = 5–6). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. **, P < 0.01; ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests.
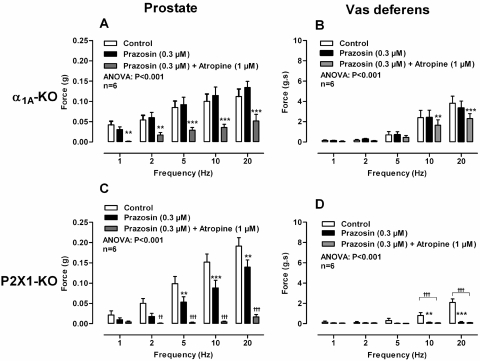
Fig. 4.
A and B, mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in prostates (A) and vas deferens (B) taken from α1A-adrenoceptor knockout mice after the administration of no drug (open bars), prazosin (0.3 μM) (solid bars), and prazosin (0.3 μM) plus atropine (1 μM) (gray bars). C and D, mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in prostates (C) and vas deferens (D) taken from P2X1-purinoceptor knockout mice after the administration of no drug (open bars), prazosin (0.3 μM) (solid bars), and prazosin (0.3 μM) plus atropine (1 μM) (gray bars). Bars represent mean force ± S.E.M. (n = 6). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. **, P < 0.01; ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests. ††, P < 0.01; †††, P < 0.001; gray bars versus control calculated by Bonferroni post-tests.
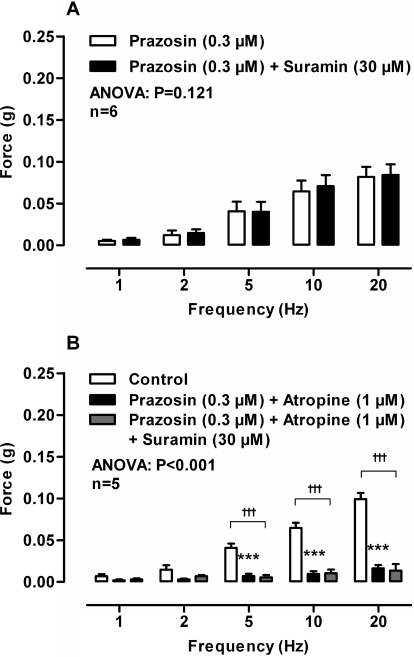
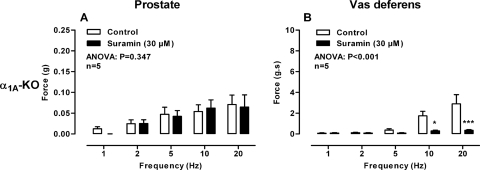
In isolated prostates taken from wild-type mice, suramin (30 μM) had no effect on the magnitude of contraction in the absence (Fig. 1C; P = 0.552) or presence (Fig. 4A; P = 0.121) of prazosin (0.3 μM). Moreover, in the presence of prazosin (0.3 μM) and atropine (1 μM) suramin (30 μM) did not affect the magnitude of the small residual contraction (Fig. 5B). Nor did suramin (30 μM) affect the residual nonadrenergic contraction in prostates taken from α1A-adrenoceptor knockout mice (Fig. 6A; P = 0.347). Furthermore, genetic deletion of the P2X1 purinoceptor did not result in reduction of electrical field stimulation-induced contractions of the isolated mouse prostate (Fig. 2C; P = 0.768).
Fig. 5.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in the prostate after the administration of prazosin (0.3 μM) (open bars) and prazosin (0.3 μM) plus suramin (30 μM) (solid bars) (A) and no drug (open bars), prazosin (0.3 μM) plus atropine (1 μM) (solid bars) and prazosin (0.3 μM) plus atropine (1 μM) plus suramin (30 μM) (gray bars) (B). Bars represent mean force ± S.E.M. (n = 5–6). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests. †††, P < 0.001; gray bar versus control calculated by Bonferroni post-tests.
Fig. 6.
Mean contractile responses to electrical field stimulation (0.5 ms, 60 V, 1–20 Hz, 10-s pulses) in prostates (A) and vas deferens (B) taken from α1A-adrenoceptor knockout mice in the absence (open bars) and presence of suramin (30 μM) (solid bars). Bars represent mean force ± S.E.M. (n = 6). P values determined by a two-way repeated-measures of ANOVA represent the probability of the drug treatment causing a significant change. *, P < 0.05; ***, P < 0.001; solid bar versus control calculated by Bonferroni post-tests.
In the isolated vas deferens pharmacological blockade by the P2-purinoceptor antagonist suramin (30 μM; Fig. 1D; P < 0.001) or genetic deletion of the P2X1-purinoceptor attenuated contraction (Fig. 2D; P < 0.001). In the vas deferens taken from α1A-adrenoceptor knockout mice suramin (30 μM) abolished contraction (Fig. 6B; P < 0.001).
In isolated prostates taken from wild-type mice atropine (1 μM) attenuated electrical field stimulation-induced contractions (Fig. 1E; P < 0.001). Atropine (1 μM) produced a maximum inhibition of contraction of 84% at 1 and 2 Hz. Furthermore, atropine (1 μM) abolished the residual nonadrenergic contractile response to electrical field stimulation observed in the presence of prazosin (0.3 μM; Fig. 3A; P < 0.001) or guanethidine (10 μM; Fig. 3C; P < 0.001). The contractile response to electrical field stimulation was also attenuated by atropine in prostates taken from α1A-adrenoceptor knockout mice (Fig. 4A; P < 0.001). Likewise, in isolated prostates taken from P2X1-purinoceptor knockout mice, electrical field stimulation-induced contractions were inhibited by prazosin (0.3 μM), and the residual nonadrenergic contraction was then abolished by atropine (1 μM; Fig. 4C; P < 0.001).
In the isolated vas deferens the muscarinic receptor antagonist atropine (1 μM; Fig. 1F; P < 0.001) attenuated contraction. Likewise, in the presence of prazosin (0.3 μM; Fig. 3B; P < 0.001) or in vas deferens taken from α1A-adrenoceptor knockout mice (Fig. 4B; P < 0.001) contraction was further attenuated by atropine (1 μM). However, in the presence of prazosin (0.3 μM) in vas deferens taken from P2X1-purinoceptor knockout mice atropine (1 μM; Fig. 6D) had no effect.
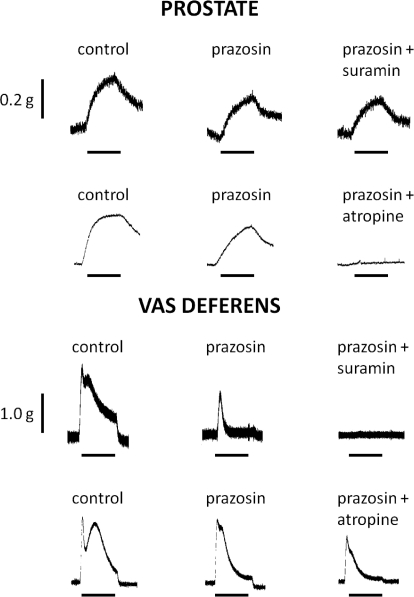
Representative traces illustrating the different effects of suramin (30 μM) and atropine (1 μM) on the residual responses of the mouse prostate and vas deferens to trains of electrical field stimulation (10 Hz) in the presence of prazosin (0.3 μM) are shown in Fig. 7.
Fig. 7.
Representative traces of the contractile responses to electrical field stimulation (0.5 ms, 60 V, 10 Hz, 10-s trains) in isolated preparations of prostate and vas deferens from wild-type mice in the absence and presence of prazosin (0.3 μM), prazosin (0.3 μM), and suramin (30 μM) or prazosin (0.3 μM) and atropine (1 μM). Bars indicate period of electrical field stimulation (10 s). Traces are representative of six experiments.
Agonist-Induced Contractile Response.
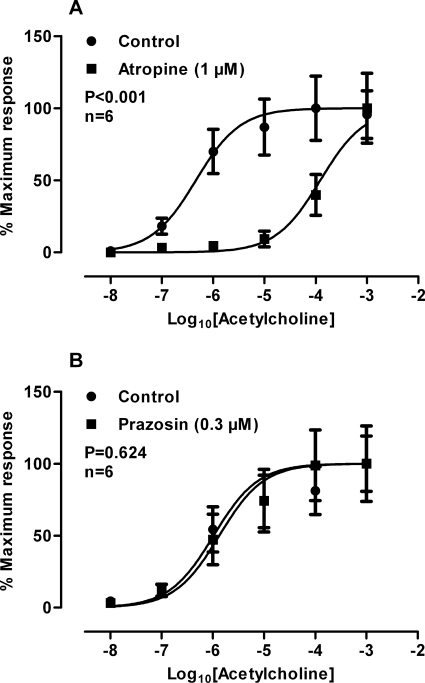
Administration of exogenously applied acetylcholine produced a reproducible concentration-dependent contractile response (−log10[EC50] = 6.33, 95% confidence limits: 5.88–6.71). Second discrete concentration response curves constructed without the application of a test drug were not different from the original curve (P = 0.794).
Atropine (1 μM), shifted the acetylcholine concentration response curve to the right approximately 250-fold (Fig. 8A; P < 0.001; −log10[EC50] = 3.91; 95% confidence limits: 3.55–4.27) with a pKB of 8.42 (95% confidence limits: 7.84–9.01), whereas prazosin (0.3 μM) had no effect (Fig. 8B; P = 0.624).
Fig. 8.
Normalized mean log concentration response curves to acetylcholine of prostates before and after the administration of atropine (1 μM) (A) and prazosin (0.3 μM) (B). Bars represent mean force ± S.E.M. (n = 6). P values determined by a two-way repeated-measures of ANOVA represent the probability of a significant interaction between concentration and treatment.
Discussion
Previous studies of the mouse prostate have identified that after pharmacological α1-adrenoceptor blockade or genetic deletion of the α1A adrenoceptor a residual nerve-mediated contraction remains (Gray and Ventura, 2005; Gray et al., 2008). The mediator of this component is not purinergic (Gray and Ventura, 2005), as has been shown in the guinea pig and rat prostate (Ventura et al., 2003; Buljubasich and Ventura, 2004) and the vas deferens of the guinea pig, rat, and mouse (Burnstock and Verkhratsky, 2010). The objective of the present study was to characterize the residual nonadrenergic component, using both pharmacological antagonists and genetically modified receptor knockout mice.
In the present study we confirmed earlier observations that genetic deletion of the α1A adrenoceptor attenuated but did not abolish nerve-mediated contraction compared with wild-type mice from the same breeding colony (Gray et al., 2008). The possibility exists that compensatory up-regulation of the remaining α1B,α1D-adrenoceptor subtypes occurs, as has been reported in the hepatocytes of α1B-adrenoceptor knockout mice (Deighan et al., 2004). However, this seems unlikely in the mouse prostate because the nonsubtype-selective α1-adrenoceptor antagonist prazosin failed to attenuate nerve-mediated contraction in prostates taken from α1A-adrenoceptor knockout mice. Likewise, prazosin and guanethidine attenuated but did not abolish nerve-mediated contractions of prostates taken from wild-type mice. This is in agreement with previous studies, and it is therefore reasonable to conclude that contraction of the mouse prostate is not mediated solely by noradrenergic mechanisms.
The guanethidine-insensitive residual nerve-mediated contraction indicates that although the mouse prostate is innervated by noradrenergic nerves, nonadrenergic, possibly cholinergic, nerves capable of eliciting contraction also exist. Compared with adrenergic blockade, we observed that inhibition of cholinergic mechanisms consistently reduced nerve-mediated contraction to a larger extent at lower frequencies (1, 2, and 5 Hz), whereas adrenergic blockade produced a marked inhibition at 10 and 20 Hz. This suggests that cholinergic nerves contribute to a greater proportion of contraction at lower frequencies and are more sensitive than adrenergic nerves in the mouse prostate.
Immunohistochemical studies of the mouse prostate have identified P2X1 purinoceptors in the smooth muscle (Gray and Ventura, 2005). However, activation of P2X1 purinoceptors by neuronally released ATP does not seem to play a physiological role in contraction of the mouse prostate. The mouse prostate is different in this regard to the guinea pig and rat prostate (Ventura et al., 2003; Buljubasich and Ventura, 2004) where ATP acts as a cotransmitter with noradrenaline at P2X1 purinoceptors in the prostatic smooth muscle to elicit contraction. In contrast, ATP mediates contraction in the mouse vas deferens via P2X1 purinoceptors (Mulryan et al., 2000) as it does in the guinea pig and rat vas deferens (Burnstock and Verkhratsky, 2010) and human vas deferens (Banks et al., 2006). The P2-purinoceptor antagonist suramin inhibited contraction in the vas deferens of wild-type and α1A-adrenoceptor knockout mice but did not inhibit nerve-mediated contraction in prostates taken from wild-type or α1A-adrenoceptor knockout mice. Despite the presence of other P2 purinoceptors in the mouse prostate (Gray and Ventura, 2005) it would seem unlikely that we have targeted the wrong purinoceptor with the P2X1-purinoceptor knockout mouse because the nonspecific P2-purinoceptor antagonist suramin is without effect on contraction in prostates taken from wild-type mice. Therefore, we have demonstrated that ATP is not a neuronally released mediator of contraction in the mouse prostate.
Histochemical observation of cholinesterase-containing nerves in the prostatic smooth muscle and glandular epithelium has been observed in human (Higgins and Gosling, 1989; Dixon et al., 2000), guinea pig and rat (Lau et al., 1998), and mouse prostates (Gray and Ventura, 2005). In contrast to a previous study of the mouse prostate (Gray and Ventura, 2005), the current study shows inhibitory effects of atropine on nerve-mediated contractions. On closer examination the noted difference between the two studies seems to be caused by statistical analysis rather than experimental origin. However, in agreement with this study, atropine has previously been observed to inhibit nerve-mediated cholinergic contractile responses in rabbit, guinea pig, and rat prostates (Haynes and Hill, 1997; Najbar-Kaszkiel et al., 1997; Lau et al., 1998).
In the mouse prostate when the adrenergic component of the nerve-mediated contractile response was blocked by guanethidine, prazosin, or α1A-adrenoceptor deletion muscarinic receptor antagonism abolished the residual response. Previously, this residual nonadrenergic response has been observed in the mouse prostate (Gray et al., 2008), but its mediator was not identified, except to state that it is also nonpurinergic. In the guinea pig and rat it has also been reported that contraction can be abolished by a combination of guanethidine and atropine (Najbar-Kaszkiel et al., 1997). The use of guanethidine may be misleading because ATP is the major nonadrenergic transmitter but is costored with noradrenaline, and the contractile effects of both are abolished by guanethidine (see Fig. 3D). Our results imply that acetylcholine, rather than ATP, is the major nonadrenergic transmitter and is released from non-noradrenergic, cholinergic nerves that innervate the prostatic smooth muscle and mediate contraction via muscarinic receptors in addition to noradrenaline released from noradrenergic nerves.
Exogenous muscarinic agonists have previously been shown to mediate contraction in prostates of the dog, rabbit, guinea pig, and rat (Normandin and Lodge, 1996; Najbar-Kaszkiel et al., 1997; Fernández et al., 1998; Lau and Pennefather, 1998), albeit at a decreased magnitude compared with the adrenergic response. Some studies have also reported that muscarinic receptor agonists mediate contraction in the human prostate (Caine et al., 1975; Gup et al., 1989; Kester et al., 2003), whereas in other human studies no contraction was observed (Hedlund et al., 1985). When observed, cholinergic receptor agonist-mediated contraction seems to be localized to the prostatic capsule. Acetylcholine is reported to potentiate the adrenergic component of nerve-mediated prostatic contraction in the guinea pig (Lau et al., 1998, 2000), while inhibiting noradrenaline-mediated contraction in the prostates of dogs (Arver and Sjöstrand, 1982). Acetylcholine has also been shown to enhance noradrenaline-mediated contraction in the human prostate (Roosen et al., 2009), indicating a possible synergism.
It has been shown that there are functional muscarinic receptors mediating contraction in vasa deferentia taken from the mouse (Cuprian et al., 2005) and guinea pig (Solanki et al., 2007), and we also observed that atropine attenuated contraction in vasa deferentia taken from wild-type mice. However, after purinergic and adrenergic blockade we did not observe a cholinergic residual response. This is in contrast to the mouse prostate where adrenergic and purinergic blockade still left a substantial residual response that could be abolished by atropine.
Traditionally, theoretical concerns over acute urinary retention have limited the use of anticholinergic drugs for the treatment of patients with BPH. Nevertheless, a recent review of clinical trial data indicated that anticholinergics are a safe treatment option for the lower urinary tract symptoms associated with BPH and patients may benefit from a combination therapy of α1-adrenoceptor and muscarinic receptor antagonists (Gallegos and Frazee, 2008). Although Gallegos and Frazee (2008) suggested that the mechanism of action was localized in the bladder, evidence of cholinergically mediated contraction of the prostatic smooth muscle suggests that the mechanism of action of solifenacin for relief of the lower urinary tract symptoms associated with BPH may be achieved by relaxing prostatic smooth muscle as suggested by Roosen et al. (2009). This prostatic relaxation would alleviate the urethral blockade caused by the hyperplastic prostate allowing for an easier flow of urine. In conclusion, the present study indicates that cholinergic rather than purinergic innervation is predominantly responsible for the nonadrenergic component of nerve-mediated contraction in the mouse prostate. Therefore, if the same were true for humans, anticholinergic drugs in combination with existing α1-adrenoceptor antagonists may provide a more effective treatment for lower urinary tract symptoms associated with BPH.
This work was supported by the ANZ Trustees [Grant 09/3164] (to S.V.), the National Health and Medical Research Council (Australia) [Grant 334136] (to S.V.), and the Wellcome Trust [Grant 080487/Z/06/Z] (to R.J.E.) for the generation of the P2X1 purinoceptor knockout mouse.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.172130.
- BPH
- benign prostatic hyperplasia
- ANOVA
- analysis of variance
- KO
- knockout.
References
- Arver S, Sjöstrand NO. ( 1982) Functions of adrenergic and cholinergic nerves in canine effectors of seminal emission. Acta Physiol Scand 115: 67– 77 [DOI] [PubMed] [Google Scholar]
- Banks FC, Knight GE, Calvert RC, Thompson CS, Morgan RJ, Burnstock G. ( 2006) The purinergic component of human vas deferens contraction. Fertil Steril 85: 932– 939 [DOI] [PubMed] [Google Scholar]
- Buljubasich R, Ventura S. ( 2004) Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol 499: 335– 344 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. ( 2010) Vas deferens–a model used to establish sympathetic cotransmission. Trends Pharmacol Sci 31: 131– 139 [DOI] [PubMed] [Google Scholar]
- Caine M, Raz S, Zeigler M. ( 1975) Adrenergic and cholinergic receptors in human prostate, prostatic capsule and bladder neck. Br J Urol 47: 193– 202 [DOI] [PubMed] [Google Scholar]
- Cooper KL, McKiernan JM, Kaplan SA. ( 1999) α-Adrenoceptor antagosnists in the treatment of benign prostatic hyperplasia. Drugs 57: 9– 17 [DOI] [PubMed] [Google Scholar]
- Cuprian AM, Solanki P, Jackson MV, Cunnane TC. ( 2005) Cholinergic innervation of the mouse isolated vas deferens. Br J Pharmacol 146: 927– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighan C, Woollhead AM, Colston JF, McGrath JC. ( 2004) Hepatocytes from α1B-adrenoceptor knockout mice reveal compensatory adrenoceptor subtype substitution. Br J Pharmacol 142: 1031– 1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JS, Jen PY, Gosling JA. ( 2000) The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its co-localization with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn 19: 185– 194 [DOI] [PubMed] [Google Scholar]
- Fernández JL, Rivera L, López PG, Recio P, Vela-Navarrete R, García-Sacristán A. ( 1998) Characterization of the muscarinic receptor mediating contraction of the dog prostate. J Auton Pharmacol 18: 205– 211 [DOI] [PubMed] [Google Scholar]
- Gallegos PJ, Frazee LA. ( 2008) Anticholinergic therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Pharmacotherapy 28: 356– 365 [DOI] [PubMed] [Google Scholar]
- Gray KT, Ventura S. ( 2005) Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods 51: 41– 50 [DOI] [PubMed] [Google Scholar]
- Gray KT, Ventura S. ( 2006) α1L-Adrenoceptors mediate contractions of the isolated mouse prostate. Eur J Pharmacol 540: 155– 161 [DOI] [PubMed] [Google Scholar]
- Gray K, Short J, Ventura S. ( 2008) The α1A-adrenoceptor gene is required for the α1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate. Br J Pharmacol 155: 103– 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gup DI, Shapiro E, Baumann M, Lepor H. ( 1989) Contractile properties of human prostate adenomas and the development of infravesical obstruction. Prostate 15: 105– 114 [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, et al. ( 2009) IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucl Acids Res 37: D680– D685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JM, Hill SJ. ( 1997) β-Adrenoceptor-mediated inhibition of α1-adrenoceptor-mediated and field stimulation-induced contractile responses in the prostate of the guinea pig. Br J Pharmacol 122: 1067– 1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JM, Ventura S. ( 2005) Current models of human prostate contractility. Clin Exp Pharmacol Physiol 32: 797– 804 [DOI] [PubMed] [Google Scholar]
- Hedlund H, Andersson KE, Larsson B. ( 1985) α-Adrenoceptors and muscarinic receptors in the isolated human prostate. J Urol 134: 1291– 1298 [DOI] [PubMed] [Google Scholar]
- Higgins JRA, Gosling JA. ( 1989) Studies on the structure and intrinsic innervation of the normal human prostate. Prostate 2 ( Suppl) 5– 16 [DOI] [PubMed] [Google Scholar]
- Ito Y, Oyunzul L, Seki M, Fujino , Oki T, Matsui M, Yamada S. ( 2009) Quantitative analysis of the loss of muscarinic receptors in various peripheral tissues in M1–M5 receptor single knockout mice. Br J Pharmacol 156: 1147– 1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester RR, Mooppan UM, Gousse AE, Alver JE, Gintautas J, Gulmi FA, Abadir AR, Kim H. ( 2003) Pharmacological characterization of isolated human prostate. J Urol 170: 1032– 1038 [DOI] [PubMed] [Google Scholar]
- Lau WA, Pennefather JN. ( 1998) Muscarinic receptor subtypes in the rat prostate gland. Eur J Pharmacol 343: 151– 156 [DOI] [PubMed] [Google Scholar]
- Lau WA, Pennefather JN, Mitchelson FJ. ( 2000) Cholinergic facilitation of neurotransmission to the smooth muscle of the guinea-pig prostate gland. Br J Pharmacol 130: 1013– 1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WA, Ventura S, Pennefather JN. ( 1998) Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J Auton Pharmacol 18: 349– 356 [DOI] [PubMed] [Google Scholar]
- Lepor H. ( 2007) Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol 9: 181– 190 [PMC free article] [PubMed] [Google Scholar]
- Lepor H, Kuhar MJ. ( 1984) Characterization and localization of the muscarinic cholinergic receptor in human prostatic tissue. J Urol 132: 397– 402 [DOI] [PubMed] [Google Scholar]
- Miano R, De Nunzio C, Asimakopoulos AD, Germani S, Tubaro A. ( 2008) Treatment options for benign prostatic hyperplasia in older men. Med Sci Monit 14: RA94– RA102 [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, et al. ( 2000) Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403: 86– 89 [DOI] [PubMed] [Google Scholar]
- Najbar-Kaszkiel AT, Di Iulio JL, Li CG, Rand MJ. ( 1997) Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea pigs, rabbits and pigs. Eur J Pharmacol 337: 251– 258 [DOI] [PubMed] [Google Scholar]
- Normandin DE, Lodge NJ. ( 1996) Pharmacological characterization of the isolated canine prostate. J Urol 155: 1758– 1761 [PubMed] [Google Scholar]
- Oki T, Maruyama S, Takagi Y, Yamamura HI, Yamada S. ( 2006) Characterization of muscarinic receptor binding and inhibition of salivation after oral administration of tolterodine in mice. Eur J Pharmacol 529: 157– 163 [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. ( 2002) Knockout of the α 1A/C-adrenergic receptor subtype: The α1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA 99: 9474– 9479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosen A, Blake-James BT, Wood D, Fry CH. ( 2009) Clinical and experimental aspects of adreno-muscarinic synergy in the bladder base and prostate. Neurourol Urodyn 28: 938– 943 [DOI] [PubMed] [Google Scholar]
- Solanki P, Cuprian-Beltechi AM, Cunnane TC. ( 2007) Cholinergic innervation of the guinea-pig isolated vas deferens. Naunyn Schmiedebergs Arch Pharmacol 376: 265– 274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Dewalagama RK, Lau LC. ( 2003) Adenosine 5′-triphosphate (ATP) is an excitatory cotransmitter with noradrenaline to the smooth muscle of the rat prostate gland. Br J Pharmacol 138: 1277– 1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Pennefather J, Mitchelson F. ( 2002) Cholinergic innervation and function in the prostate gland. Pharmacol Ther 94: 93– 112 [DOI] [PubMed] [Google Scholar]
- Wilson JD. ( 1980) The pathogenesis of benign prostatic hyperplasia. Am J Med 68: 745– 756 [DOI] [PubMed] [Google Scholar]