Abstract
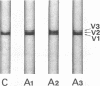
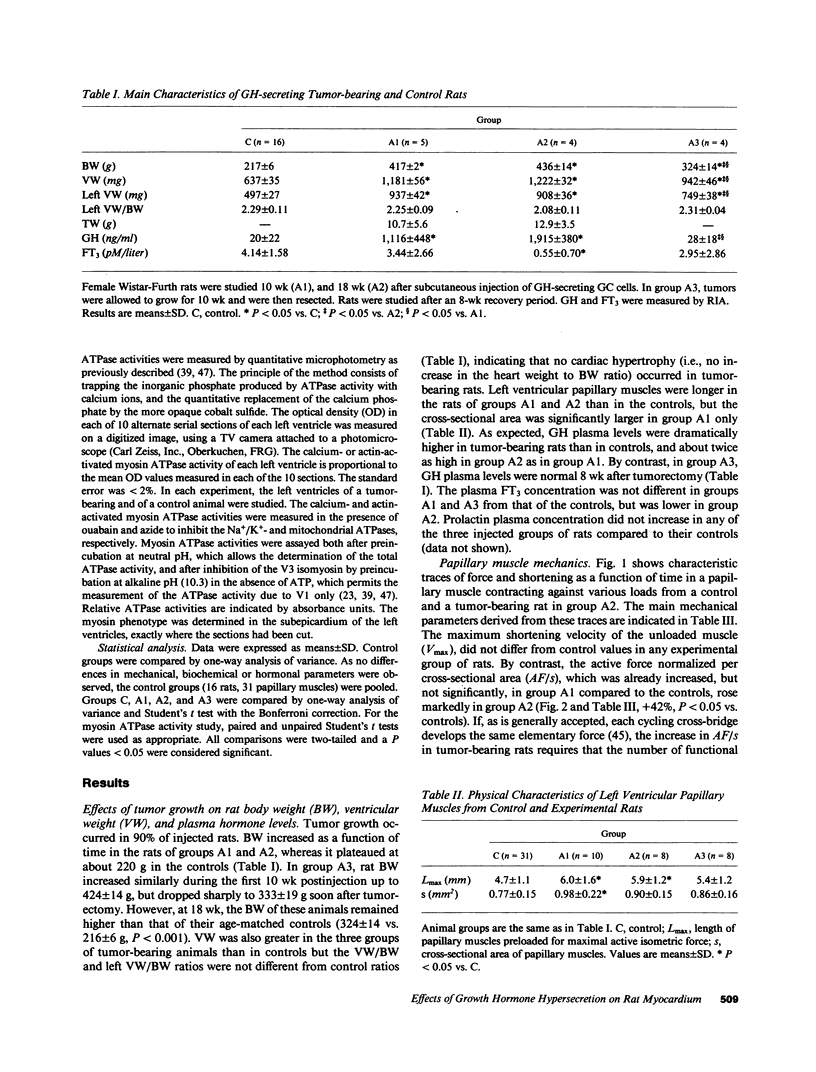
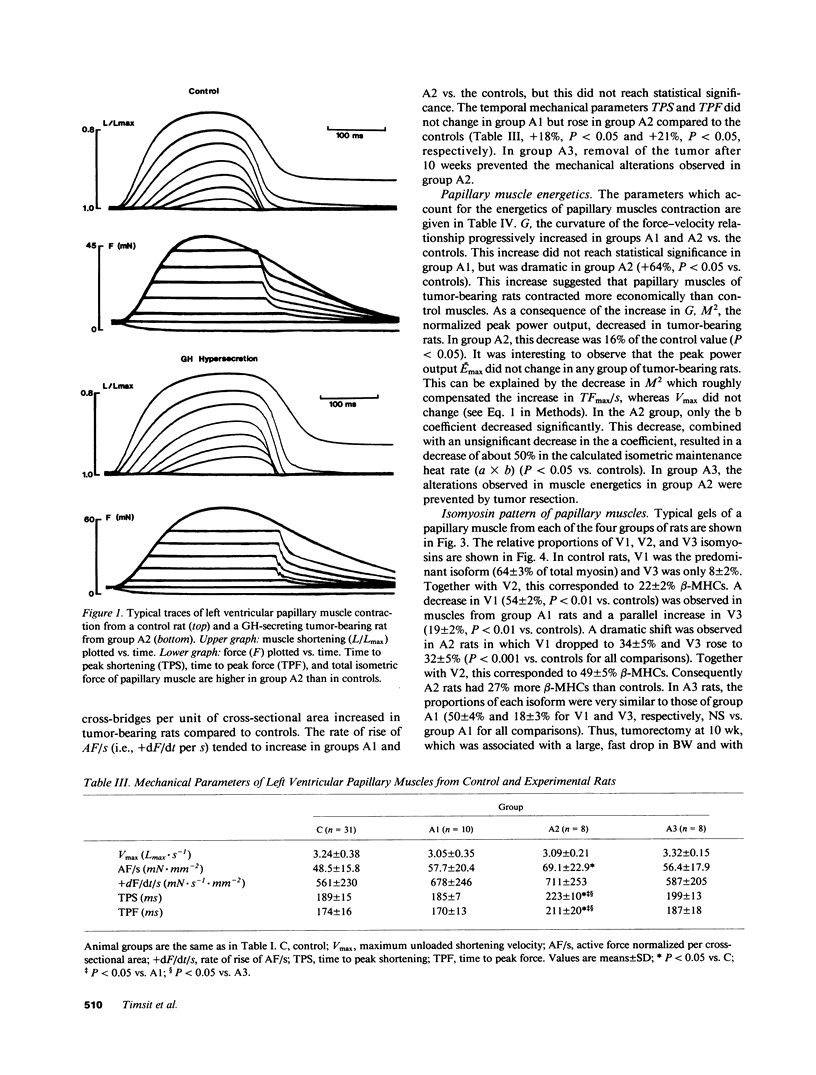
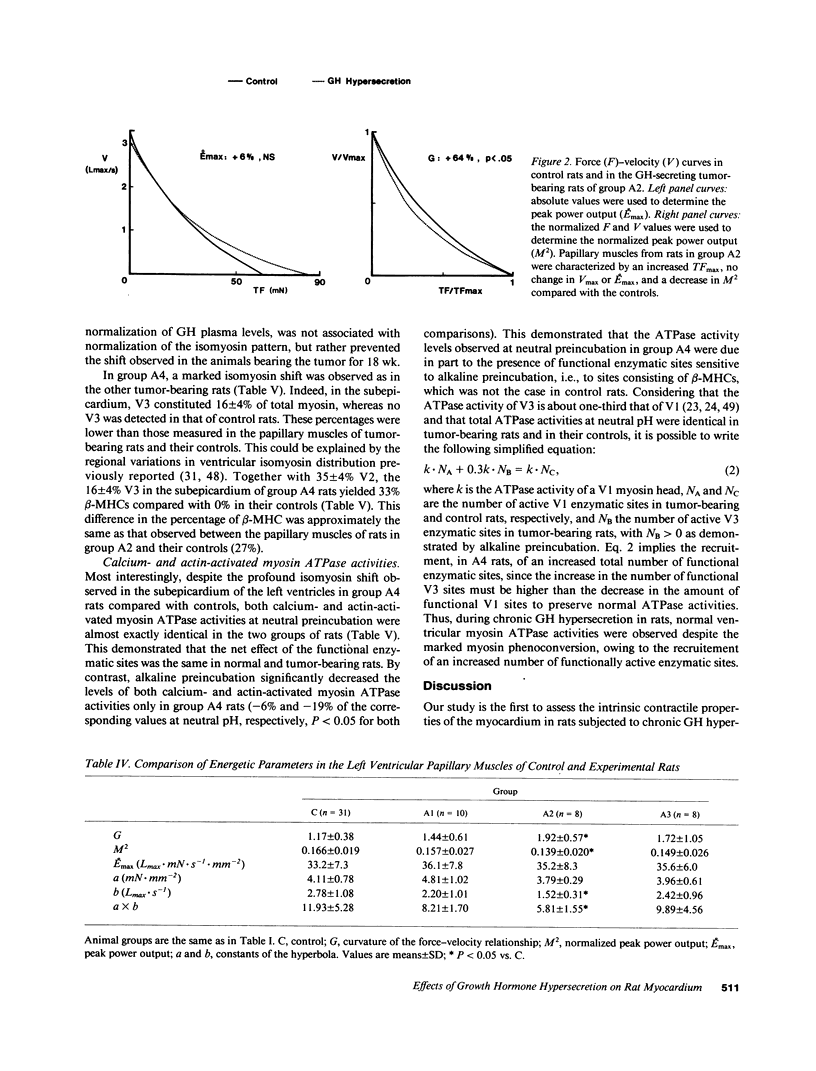
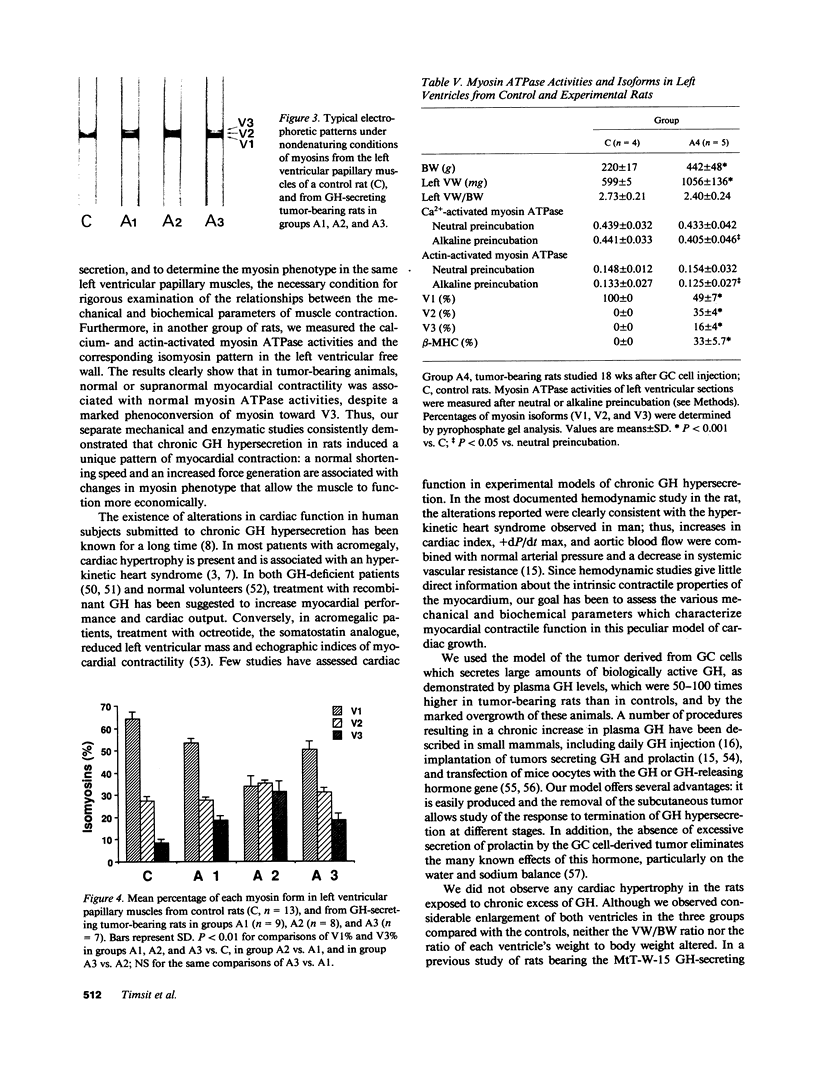
We studied papillary muscle mechanics and energetics, myosin phenotype, and ATPase activities in left ventricles from rats bearing a growth hormone (GH)--secreting tumor. 18 wk after tumor induction, animals exhibited a dramatic increase in body weight (+101% vs. controls) but no change in the ventricular weight/body weight ratio. The maximum isometric force of papillary muscles normalized per cross-sectional area rose markedly (+42%, P less than 0.05 vs. controls), whereas the maximum unloaded shortening velocity did not change. This was observed despite a marked isomyosin shift towards V3 (32 +/- 5% vs. 8 +/- 2% in controls, P less than 0.001). Increased curvature of the force-velocity relationship (+64%, P less than 0.05 vs. controls) indicated that the muscles contracted more economically, suggesting the involvement of V3 myosin. Total calcium- and actin-activated myosin ATPase activities assayed on quickly frozen left ventricular sections were similar in tumor-bearing rats and in controls. After alkaline preincubation, these activities only decreased in tumor-bearing rats, demonstrating that V3 enzymatic sites were involved in total ATPase activity. These data demonstrate that chronic GH hypersecretion in the rat leads to a unique pattern of myocardial adaptation which allows the muscle to improve its contractile performance and economy simultaneously, thanks to myosin phenoconversion and an increase in the number of active enzymatic sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Claes V. A. Onset of mechanical activation of mammalian heart muscle in calcium- and strontium-containing solutions. Circ Res. 1974 Sep;35(3):345–357. doi: 10.1161/01.res.35.3.345. [DOI] [PubMed] [Google Scholar]
- Chemla D., Lecarpentier Y., Martin J. L., Clergue M., Antonetti A., Hatt P. Y. Relationship between inotropy and relaxation in rat myocardium. Am J Physiol. 1986 Jun;250(6 Pt 2):H1008–H1016. doi: 10.1152/ajpheart.1986.250.6.H1008. [DOI] [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984 Oct 25;259(20):12628–12632. [PubMed] [Google Scholar]
- Cuneo R. C., Wilmshurst P., Lowy C., McGauley G., Sönksen P. H. Cardiac failure responding to growth hormone. Lancet. 1989 Apr 15;1(8642):838–839. doi: 10.1016/s0140-6736(89)92290-3. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcayre C., Swynghedauw B. A comparative study of heart myosin ATPase and light subunits from different species. Pflugers Arch. 1975 Mar 22;355(1):39–47. doi: 10.1007/BF00584798. [DOI] [PubMed] [Google Scholar]
- Ebrecht G., Rupp H., Jacob R. Alterations of mechanical parameters in chemically skinned preparations of rat myocardium as a function of isoenzyme pattern of myosin. Basic Res Cardiol. 1982 Mar-Apr;77(2):220–234. doi: 10.1007/BF01908175. [DOI] [PubMed] [Google Scholar]
- Engelmann G. L., Boehm K. D., Haskell J. F., Khairallah P. A., Ilan J. Insulin-like growth factors and neonatal cardiomyocyte development: ventricular gene expression and membrane receptor variations in normotensive and hypertensive rats. Mol Cell Endocrinol. 1989 May;63(1-2):1–14. doi: 10.1016/0303-7207(89)90076-2. [DOI] [PubMed] [Google Scholar]
- Fong Y., Rosenbaum M., Tracey K. J., Raman G., Hesse D. G., Matthews D. E., Leibel R. L., Gertner J. M., Fischman D. A., Lowry S. F. Recombinant growth hormone enhances muscle myosin heavy-chain mRNA accumulation and amino acid accrual in humans. Proc Natl Acad Sci U S A. 1989 May;86(9):3371–3374. doi: 10.1073/pnas.86.9.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. L., Siegel R. J., Melmed S., Sherman C. T., Fishbein M. C. Cardiac morphology in rats with growth hormone-producing tumours. J Mol Cell Cardiol. 1985 Aug;17(8):805–811. doi: 10.1016/s0022-2828(85)80042-0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hammer R. E., Brinster R. L., Rosenfeld M. G., Evans R. M., Mayo K. E. Expression of human growth hormone-releasing factor in transgenic mice results in increased somatic growth. 1985 May 30-Jun 5Nature. 315(6018):413–416. doi: 10.1038/315413a0. [DOI] [PubMed] [Google Scholar]
- Hirsch E. Z., Sloman J. G., Martin F. I. Cardiac function in acromegaly. Am J Med Sci. 1969 Jan;257(1):1–8. doi: 10.1097/00000441-196901000-00001. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Rossmanith G. H., Kwan L. J., Hamilton A. M. Adrenaline increases the rate of cycling of crossbridges in rat cardiac muscle as measured by pseudo-random binary noise-modulated perturbation analysis. Circ Res. 1988 Mar;62(3):452–461. doi: 10.1161/01.res.62.3.452. [DOI] [PubMed] [Google Scholar]
- Holubarsch C., Goulette R. P., Litten R. Z., Martin B. J., Mulieri L. A., Alpert N. R. The economy of isometric force development, myosin isoenzyme pattern and myofibrillar ATPase activity in normal and hypothyroid rat myocardium. Circ Res. 1985 Jan;56(1):78–86. doi: 10.1161/01.res.56.1.78. [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 1987 Mar;79(3):970–977. doi: 10.1172/JCI112908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas E. A., Aloia J. F., Lane F. J. Evidence of subclinical heart muscle dysfunction in acromegaly. Chest. 1975 Feb;67(2):190–194. doi: 10.1378/chest.67.2.190. [DOI] [PubMed] [Google Scholar]
- Jørgensen J. O., Pedersen S. A., Thuesen L., Jørgensen J., Ingemann-Hansen T., Skakkebaek N. E., Christiansen J. S. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989 Jun 3;1(8649):1221–1225. doi: 10.1016/s0140-6736(89)92328-3. [DOI] [PubMed] [Google Scholar]
- Lecarpentier Y., Bugaisky L. B., Chemla D., Mercadier J. J., Schwartz K., Whalen R. G., Martin J. L. Coordinated changes in contractility, energetics, and isomyosins after aortic stenosis. Am J Physiol. 1987 Feb;252(2 Pt 2):H275–H282. doi: 10.1152/ajpheart.1987.252.2.H275. [DOI] [PubMed] [Google Scholar]
- Lei L. Q., Rubin S. A., Fishbein M. C. Cardiac architectural changes with hypertrophy induced by excess growth hormone in rats. Lab Invest. 1988 Sep;59(3):357–362. [PubMed] [Google Scholar]
- Lie J. T. Pathology of the heart in acromegaly: anatomic findings in 27 autopsied patients. Am Heart J. 1980 Jul;100(1):41–52. doi: 10.1016/0002-8703(80)90277-x. [DOI] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982 Jun;50(6):856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Luboshitzky R., Barzilai D. Bromocriptine for an acromegalic patient. Improvement in cardiac function and carpal tunnel syndrome. JAMA. 1980 Oct 17;244(16):1825–1827. doi: 10.1001/jama.244.16.1825. [DOI] [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Martins J. B., Kerber R. E., Sherman B. M., Marcus M. L., Ehrhardt J. C. Cardiac size and function in acromegaly. Circulation. 1977 Nov;56(5):863–869. doi: 10.1161/01.cir.56.5.863. [DOI] [PubMed] [Google Scholar]
- Mather H. M., Boyd M. J., Jenkins J. S. Heart size and function in acromegaly. Br Heart J. 1979 Jun;41(6):697–701. doi: 10.1136/hrt.41.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin W. L., Jr, Sherman B. M., Roth F., Gorden P., Kahn C. R., Roberts W. C., Frommer P. L. Acromegaly and cardiovascular disorders. A prospective study. Ann Intern Med. 1974 Jul;81(1):11–18. doi: 10.7326/0003-4819-81-1-11. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Lecarpentier Y., Delcayre C., Lompre A. M., Swynghedauw B., Schwartz K. Mécanismes biochimiques de l'adaptation myocardique dans l'hypertrophie cardiaque. Arch Mal Coeur Vaiss. 1982 Oct;75(10):1179–1186. [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Wisnewsky C., Samuel J. L., Bercovici J., Swynghedauw B., Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 1981 Aug;49(2):525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Bell G. I., Duckworth M. L., Friesen H. G. Identification, characterization, and regulation of a rat complementary deoxyribonucleic acid which encodes insulin-like growth factor-I. Endocrinology. 1987 Aug;121(2):684–691. doi: 10.1210/endo-121-2-684. [DOI] [PubMed] [Google Scholar]
- Nabarro J. D. Acromegaly. Clin Endocrinol (Oxf) 1987 Apr;26(4):481–512. doi: 10.1111/j.1365-2265.1987.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Pagani E. D., Julian F. J. Rabbit papillary muscle myosin isozymes and the velocity of muscle shortening. Circ Res. 1984 May;54(5):586–594. doi: 10.1161/01.res.54.5.586. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passa P., Masquet C., Cophignon J., Gourgon R., Bouvrain Y. Le coeur dans l'acromégalie. Etude hémodynamique. Arch Mal Coeur Vaiss. 1973 Dec;66(12):1517–1523. [PubMed] [Google Scholar]
- Penney D. G., Dunbar J. C., Jr, Baylerian M. S. Cardiomegaly and haemodynamics in rats with a transplantable growth hormone-secreting tumour. Cardiovasc Res. 1985 May;19(5):270–277. doi: 10.1093/cvr/19.5.270. [DOI] [PubMed] [Google Scholar]
- Pepine C. J., Aloia J. Heart muscle disease in acromegaly. Am J Med. 1970 Apr;48(4):530–534. doi: 10.1016/0002-9343(70)90055-0. [DOI] [PubMed] [Google Scholar]
- Pope B., Hoh J. F., Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980 Sep 8;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Prysor-Jones R. A., Jenkins J. S. Effect of excessive secretion of growth hormone on tissues of the rat, with particular reference to the heart and skeletal muscle. J Endocrinol. 1980 Apr;85(1):75–82. doi: 10.1677/joe.0.0850075. [DOI] [PubMed] [Google Scholar]
- Savage D. D., Henry W. L., Eastman R. C., Borer J. S., Gorden P. Echocardiographic assessment of cardiac anatomy and function in acromegalic patients. Am J Med. 1979 Nov;67(5):823–829. doi: 10.1016/0002-9343(79)90742-3. [DOI] [PubMed] [Google Scholar]
- Schwartz K., Lecarpentier Y., Martin J. L., Lompré A. M., Mercadier J. J., Swynghedauw B. Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol. 1981 Dec;13(12):1071–1075. doi: 10.1016/0022-2828(81)90297-2. [DOI] [PubMed] [Google Scholar]
- Smallridge R. C., Rajfer S., Davia J., Schaaf M. Acromegaly and the heart. An echocardiographic study. Am J Med. 1979 Jan;66(1):22–27. doi: 10.1016/0002-9343(79)90477-7. [DOI] [PubMed] [Google Scholar]
- Stier C. T., Jr, Cowden E. A., Friesen H. G., Allison M. E. Prolactin and the rat kidney: a clearance and micropuncture study. Endocrinology. 1984 Jul;115(1):362–367. doi: 10.1210/endo-115-1-362. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Thuesen L., Christensen S. E., Weeke J., Orskov H., Henningsen P. A hyperkinetic heart in uncomplicated active acromegaly. Explanation of hypertension in acromegalic patients? Acta Med Scand. 1988;223(4):337–343. doi: 10.1111/j.0954-6820.1988.tb15882.x. [DOI] [PubMed] [Google Scholar]
- Thuesen L., Christensen S. E., Weeke J., Orskov H., Henningsen P. The cardiovascular effects of octreotide treatment in acromegaly: an echocardiographic study. Clin Endocrinol (Oxf) 1989 Jun;30(6):619–625. doi: 10.1111/j.1365-2265.1989.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Thuesen L., Christiansen J. S., Sørensen K. E., Jørgensen J. O., Orskov H., Henningsen P. Increased myocardial contractility following growth hormone administration in normal man. An echocardiographic study. Dan Med Bull. 1988 Apr;35(2):193–196. [PubMed] [Google Scholar]
- Winegrad S., McClellan G., Horowits R., Tucker M., Lin L. E., Weisberg A. Regulation of cardiac contractile proteins by phosphorylation. Fed Proc. 1983 Jan;42(1):39–44. [PubMed] [Google Scholar]
- Winegrad S., Weisberg A. Isozyme specific modification of myosin ATPase by cAMP in rat heart. Circ Res. 1987 Mar;60(3):384–392. doi: 10.1161/01.res.60.3.384. [DOI] [PubMed] [Google Scholar]
- Winegrad S., Weisberg A., Lin L. E., McClellan G. Adrenergic regulation of myosin adenosine triphosphatase activity. Circ Res. 1986 Jan;58(1):83–95. doi: 10.1161/01.res.58.1.83. [DOI] [PubMed] [Google Scholar]
- d'Albis A., Pantaloni C., Bechet J. J. An electrophoretic study of native myosin isozymes and of their subunit content. Eur J Biochem. 1979 Sep;99(2):261–272. doi: 10.1111/j.1432-1033.1979.tb13253.x. [DOI] [PubMed] [Google Scholar]