Abstract
Background & Aims
Epidemiological studies have shown that obesity is a risk factor for hepatocellular carcinoma (HCC). Lower adiponectin levels are associated with poor prognosis in obese HCC patients hence it is plausible that adiponectin acts as a negative regulator of HCC. Here, we investigated the effects of adiponectin on HCC development and elucidated the underlying molecular mechanisms.
Methods
We utilized various in vitro assays using Huh7 and HepG2 HCC cells to examine the signal transduction pathways involved in protective function of adiponectin in HCC. These studies were followed by in vivo approaches using HCC xenografts and tumor analysis. Results from in vitro and in vivo findings were corroborated using human HCC tissue micro-array (TMA) and analysis of clinicopathological characteristics.
Results
Adiponectin treatment resulted in increased apoptosis of HCC cells via activation of caspase-3. Adiponectin increased phosphorylation of c-Jun-N-terminal kinase (JNK) and inhibition of JNK-phosphorylation inhibited adiponectin-induced apoptosis and caspase-3 activation. Adiponectin increased phosphorylation of AMP-activated protein kinase (AMPK) and tumor suppressor TSC2 and inhibited mammalian target of rapamycin (mTOR) phosphorylation. Inhibition of AMPK phosphorylation not only inhibited adiponectin-induced JNK phosphorylation but also effectively blocked biological effects of adiponectin. In vivo study showed that adiponectin treatment substantially reduced liver tumorigenesis in nude mice. Importantly, analysis of adiponectin expression levels in TMA of human HCC patients revealed an inverse correlation of adiponectin expression with tumor size.
Conclusions
These novel findings show protective role of adiponectin in liver tumorigenesis and could help explain poor prognosis of obese HCC patients who typically have low adiponectin levels.
Keywords: Hepatocellular carcinoma, Adiponectin, TSC2, mTOR
Introduction
Obesity is an important risk factor for numerous health problems including carcinogenesis1 and its biological effects are mediated through adipocytokines2. Adiponectin is an adipocytokine3–6 whose expression is reduced in obese/diabetic murine model db/db mice7. Plasma adiponectin levels have also been reported to be significantly reduced in obese humans8 and in various disease states9. Adiponectin reduces tissue triglyceride content, upregulates insulin signaling10 and has direct antiatherosclerotic effects11. More recently, low levels of plasma adiponectin have been associated with many common forms of cancer2. Adiponectin receptors exist in two isoforms, AdipoR1 and AdipoR2; AdipoR2 has 67% homology (amino acids) with AdiopR112. AdipoR2 is expressed most abundantly in liver13. These receptors mediate cellular functions of adiponectin via activation of various intracellular signaling pathways14. Several signaling molecules and pathways, such as 5'-AMP-activated protein kinase (AMPK), nuclear factor (NF)-κB, peroxisome proliferators-activated receptor (PPAR)-α, and p38 mitogen–activated protein (MAP) kinase, are known to mediate adiponectin induced signaling15–17.
Large population prevalence studies have shown that HCC is clearly associated with obesity18. A recent US study reported a great impact of obesity on HCC even after multivariate analysis. This study included 404,576 men and 495,477 women aged 30 years with a BMI of 18.5 kg/m2 at enrollment who were observed for 16 years. Stratification of overall and site-specific cancer-related deaths according to BMI showed that HCC was 1.68 times higher among women with high BMI and was 4.52 times higher for men with high BMI. Most notably, among the male group, HCC had the highest relative-risk increase as a consequence of obesity compared to all the cancers studied including prostate, kidney, gallbladder, colon, rectum, esophagus, stomach and pancreas19. At present a biological explanation for risk associated between obesity and HCC is not known. Therefore, the effects of obesity on human HCC represent a critical intersection between these two important health problems. Considering the fundamental role of adipocytokines in cancer progression, the growth regulation of HCC cells by adiponectin might effect their malignant progression. Therefore, in the present study, we investigated the effects of adiponectin on apoptosis and proliferation of HCC. We also elucidated the signal transduction pathways regulating adiponectin induced changes in the cancerous properties of HCC.
Materials and Methods
Antibodies
Antibodies for AdipoR1, AdipoR2, Bcl-2, Bax, cleaved-caspase-3, caspase-3, cyclin D1, PCNA, phospho-JNK, JNK, phospho-AMPK, AMPK, phospho-mTOR, mTOR, phospho-TSC-2, TSC-2 were purchased from Cell Signaling (Danvers, MA).
Cell culture and reagents
HepG2 cells (ATCC, Manassas, VA) derived from a human hepatoblastoma20 and Huh7 cells derived from a well differentiated HCC21 were cultured in MEM (ATCC) and DMEM (Invitrogen, Carlsbad, CA) respectively, supplemented with 10% fetal bovine serum (FBS) (Fisher Scientific (Hyclone) Pittsburgh, PA). THLE-2 cells (ATCC) derived from primary normal liver cells and human hepatocytes (Lonza, Walkersville, MD) were used as controls. THLE-2 cells were cultured in ATCC complete growth medium (ATCC) containing 5 ng/ml EGF, 70 ng/ml Phosphoethanolamine and 10% FBS. Human hepatocytes were cultured in HCM hepatocyte culture medium (Lonza). Full length and globular Adiponectin human-HEK293 was purchased from Biovendor, Candler, NC. For adiponectin treatment, cells were serum starved for 16h followed by adiponectin treatment in fresh media as indicated.
RNA isolation and RT-PCR
Total cellular RNA was extracted and RT-PCR was carried out using specific sense and antisense PCR primers. Please see supplementary section for primer details.
Western Blot
Whole cell lysate was prepared following previously described method22,23. Please see supplementary section for details.
Quantification of DNA/cell proliferation assay by bromodeoxyuridine (BrdU) incorporation
BrdU incorporation assays were performed as described previously22,23. For details of BrdU incorporation assay see supplementary section.
Caspase Activity Assay
Caspase-3 like activity was detected in cell lysates after various treatments using an Ac-DEVD-AFC substrate (Calbiochem, San Diego, CA). Measurements were made using a fluorescence microplate reader (Beckman Coulter, Fullerton, CA). Control groups treated with inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone, BD PharMingen) Alexis Biochemicals, San Diego, CA), were included to ensure specificity.
Cell viability/apoptosis assay
Cell viability assay was performed22 following our published protocol. For details of XTT reduction assay, please see supplementary section.
Inoculation of HepG2 cells into nude mice and treatment with adiponectin
HepG2-xenografts in nude mice were developed and treated with recombinant adenovirus-adiponectin. All the experimental protocols were approved by the Institutional Animal Care and Use Committee at Emory University. For details, please see supplementary section.
Immunohistochemistry of human HCC TMA
Adiponectin expression was examined in paraffin-embedded sections of human HCC samples using tissue micro-array. In total 140 cases of HCC diagnosed between 1985 and 2009 were studied. Immunohistochemistry was performed using monoclonal adiponectin (1:80) (Chemicon International, Temecula, CA), Bcl-2 (1:100), Bax (1:100), cleaved-caspase-3 (1:100) and caspase-3 (1:100). For TMA details, please see supplementary section. These studies were approved by the Institutional Review Board at Emory University.
Statistical Analysis
All experiments were independently performed three times in triplicates. The data were analyzed using a combination of Fisher's exact test, t-tests, two-tailed distribution, analysis of variance (ANOVA) and Pearson's correlation. TMA data were analyzed using the non-parametric Mann-Whitney test, Kruskal-Wallis test, and Spearman correlation coefficient. Specifically, when comparing the expression level of adiponectin between two categorical factors such as node status, Mann-Whitney tests were used. When comparing these expressions between more than two categories, Kruskal-Wallis tests were carried out. Correlation of the expression level of adiponectin with a continuous variable such as size was assessed using the Spearman correlation coefficient.
Results
Adiponectin inhibits proliferation of hepatocellular carcinoma cells
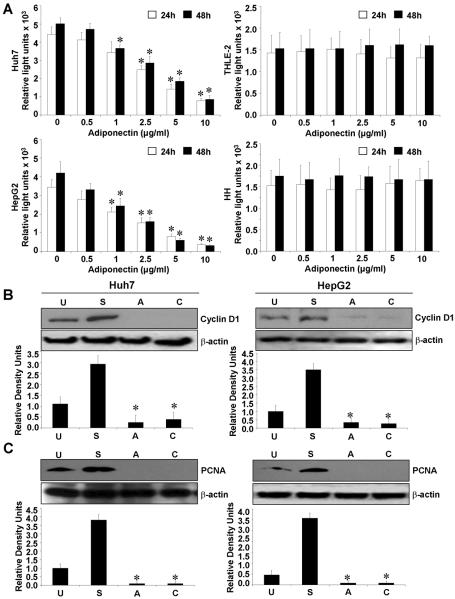
Adiponectin exerts its biological functions through binding to its receptors which mediate a downstream signal by activating multiple signaling pathways12,14,17. We examined mRNA and protein expression of adiponectin receptors, AdipoR1 and AdipoR2 in HepG2, Huh7 (hepatocellular carcinoma cells), THLE-2 (normal human hepatocyte cell line) and human hepatocytes (HH) using RT-PCR and western blot analysis. We found that HCC cells have higher expression levels of AdipoR1 and AdipoR2 as compared to normal human hepatocytes and THLE-2 cells (Supplementary Figure 1). We next examined the effect of full-length adiponectin (adiponectin) on HCC cell proliferation. Adiponectin treatment inhibited the growth of HepG2 and Huh7 cells in a dose-dependent manner (Figure 1A). Interestingly, adiponectin treatment did not effect proliferation of THLE-2 and human hepatocytes. Cell cycle analysis revealed that the proportion of both HepG2 and Huh7 cells was decreased in S-phase by adiponectin treatment (Supplementary Figure 2). We also examined the effect of globular-adiponectin on HCC cell proliferation and observed globular-adiponectin-mediated inhibition of proliferation in HepG2 and Huh7 cells while no effect was observed on THLE-2 and human hepatocytes (Supplementary Figure 3A). Hence, our studies showed similar effects of both full-length and globular adiponectin on HCC proliferation. D-type cyclins are active in G1 phase of the cell cycle. They complex with cyclin-dependent kinases (cdks) to catalyze the transition from G1 to S phase of the cell cycle23. Adiponectin inhibits proliferation of HCC cells and one of the targets for adiponectin action may be cyclin D1. Indeed, adiponectin decreased cyclin D1 expression (Figure 1B) and marker for proliferation, PCNA expression (Figure 1C). Collectively, these results showed that the anti-proliferative effect of adiponectin on HepG2 and Huh7 cells also involves down-regulation of cyclin D1 and PCNA.
Figure 1. Adiponectin inhibits proliferation of hepatocellular carcinoma cells.
A, Cells were serum starved for 16h followed by adiponectin treatment (0.5–10 μg/ml) for 24h and 48h and BrdU incorporation assays were performed. Adiponectin treatment decreased proliferation of HepG2 and Huh7 cells in a dose dependent manner whereas normal hepatocytes (THLE-2 and HH) remained unchanged. *p< 0.01, for different dose compared with untreated cells. The data represents mean values ± SEM and are the results of three independent experiments performed in triplicates. B and C, Cells were serum starved for 16h and treated with adiponectin (10μg/ml) (A) for 12h. Untreated cells (U), cells grown in complete medium containing 10% FBS (S) and cells treated with cycloheximide (C) were included as controls. Cycloheximide was employed as an inhibitor of proliferation. Total protein lysates were analyzed by immunoblot analysis with antibodies for cyclinD1 (B) and PCNA (C). Adiponectin treatment decreased expression of cyclinD1 and PCNA in HCC cells. The western blot signals were quantified by densitometry and normalized to β-actin. These data are representative of multiple independent experiments, *p< 0.05, compared with untreated controls.
Adiponectin-mediated apoptosis of HCC cells involves activation of caspase-3 via increased activation of c-Jun N-terminal kinase
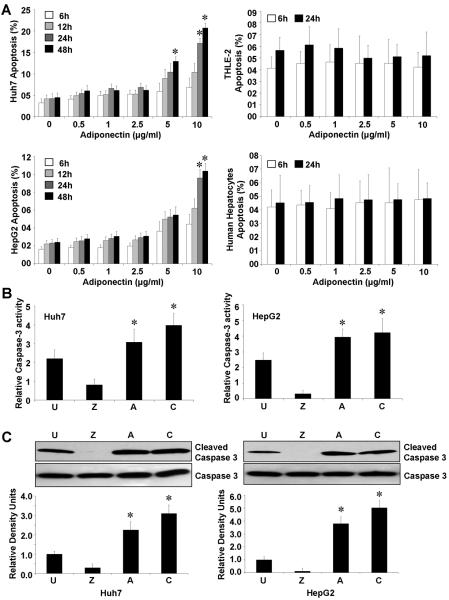
We also observed that adiponectin increases apoptosis in HCC cells in a dose- and time-dependent manner while adiponectin was unable to induce apoptosis in THLE-2 cells or primary human hepatocytes even at high doses (Figure 2A). We also examined the effect of globular-adiponectin on HCC apoptosis and found that globular-adiponectin increases apoptosis in HCC cells while no effect was observed on THLE-2 and human hepatocytes hence showing similar effects as full-length adiponectin (Supplementary Figure 3B). To examine if lack of adiponectin's effect on hepatocytes is simply due to non-proliferative nature of normal hepatocytes, we stimulated human hepatocytes with HGF24 followed by adiponectin treatment. In agreement with earlier studies, human hepatocytes showed very faint (non significant with p> 0.102) increased level of proliferation. Adiponectin treatment of these cells resulted in decreased (not significant) levels of proliferation but failed to induce apoptosis (Supplementary Figure 5A and 5B). Caspases have been characterized as the effectors and executioners of cell death and caspase activation is an important step towards the induction of apoptosis. We investigated caspase-3 like activity in HCC cells upon treatment with adiponectin and found that HCC cells treated with adiponectin displayed higher levels of caspase-3-like activity as compared to untreated cells (Figure 2B). We detected significantly increased levels of active caspase-3 in adiponectin treated HCC cells (Figure 2C). HCC cells treated with caspase-3 inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) denoted as “Z” were used as control.
Figure 2. Adiponectin is pro-apoptotic for HCC cells.
A, Cells were serum-starved for 16h followed by adiponectin treatment (0.5–10 μg/ml) for indicated time-intervals and apoptosis was analyzed by XTT method. The percentage of apoptotic cells represents the means ± SEM of viable cells present in treatment calculated with respect to cells grown in complete medium. *p< 0.01 as compared to respective untreated cells. Adiponectin induced apoptosis in HepG2 and Huh7 cells in a dose and time dependent manner, while no apoptosis was observed in THLE-2 and human hepatocytes. B, HepG2 and Huh7 cells were serum-starved for 16h followed by adiponectin (10μg/ml) treatment (A) for 12 hours and caspase-3 like activity was analyzed. Untreated cells (U), cells treated with cycloheximide (C) and cells treated with caspase-3 inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone) (Z) were included as controls. Adiponectin treatment increased caspase-3 like activity in HCC cells. The numbers in the figure represent the mean value of three repeat samples. C, Cells were treated as described in B. Cycloheximide was employed as an inhibitor of proliferation. Total protein lysates were analyzed by immunoblot analysis using caspase-3 and cleaved caspase-3 antibodies. The western blot signals were quantified by densitometry and normalized to β-actin. These data are representative of multiple independent experiments, *p < 0.05, compared with untreated controls.
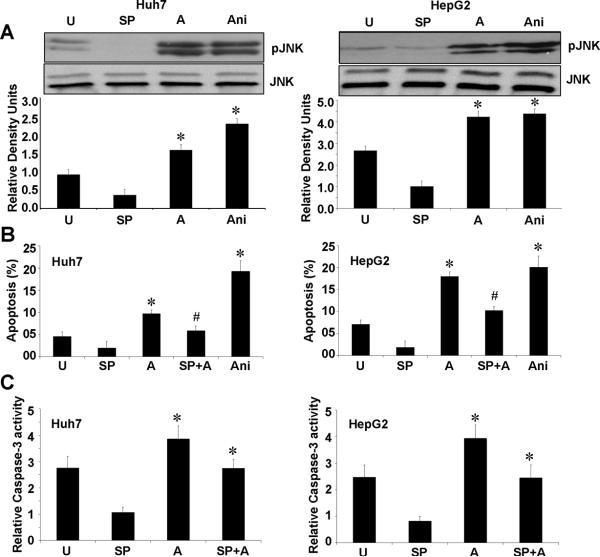
The stress-activated kinase, JNK have been implicated in apoptotic response of cells stimulated with UV irradiation, chemotherapy, heat shock and proinflammatory cytokines25. To better understand the signal transduction pathways underlying adiponectin-induced apoptosis, we examined the involvement of JNK in HCC cells treated with adiponectin. Adiponectin treatment induced significant phosphorylation of JNK in HepG2 and Huh7 cells (Figure 3A). These results are in agreement with an earlier study showing adiponectin-mediated activation of JNK in prostate cancer as well as HepG2 cells26. To determine the importance of JNK activation in adiponectin-induced apoptosis in HCC cells, cells were treated with JNK inhibitor (SP600125) 1h before adding adiponectin. Inhibition of the JNK pathway significantly blocked adiponectin-induced apoptosis in both HepG2 and Huh7 cells (Figure 3B). JNK activity has been found to be required for activation of the mitochondria-mediated apoptosis pathway27 demonstrating cytochrome c release and caspase-3 activation. We further examined the hierarchy of these events by treating HepG2 and Huh7 cells with JNK inhibitor prior to adiponectin treatment and examined caspase-3 activation. We found that inhibition of JNK activation inhibits adiponectin induced activation of caspase-3 (Figure 3C) showing that JNK activation is upstream of caspase-3 activation.
Figure 3. Adiponectin mediated increased phosphorylation of c-Jun N-terminal kinase is important for adiponectin-induced apoptosis and caspase-3 activation.
A, Cells were serum starved for 16h and treated with 10μg/ml adiponectin (A) for 12h. Untreated cells (U), cells treated with JNK inhibitor SP600125 (SP) and Anisomycin (Ani) were included as controls. Total protein lysates were analyzed by immunoblot analysis with JNK, phosphorylated-JNK antibodies. These data are representative of multiple independent experiments with a representative immunoblot for pJNK, JNK. The histogram represents densitometric mean values ± SEM of band intensities. *p< 0.05, compared with untreated control cells. B, HepG2 and Huh7 cells were serum starved for 16h and treated with 10μg/ml adiponectin (A) alone or in combination with JNK inhibitor SP600125 (SP) for 12h. Untreated cells (U), cells treated with SP600125 (SP) and Anisomycin (Ani) were included as controls. Apoptosis was analyzed by XTT method. The percentage of apoptotic cells represents the means ± SEM of viable cells present in treatment calculated with respect to cells grown in complete medium. *p< 0.01 as compared to respective untreated cells. The values represent three independent experiments performed in triplicate. JNK inhibition significantly reduces adiponectin induced apoptosis. C, HepG2 and Huh7 cells were treated as above; caspase-3 activity was measured as described in materials and methods. Adiponectin fails to increase caspase-3 activity in the presence of JNK inhibitor, SP600125.
Adiponectin-induced AMPK phosphorylation is an important step in mediating biological functions of adiponectin in HCC cells
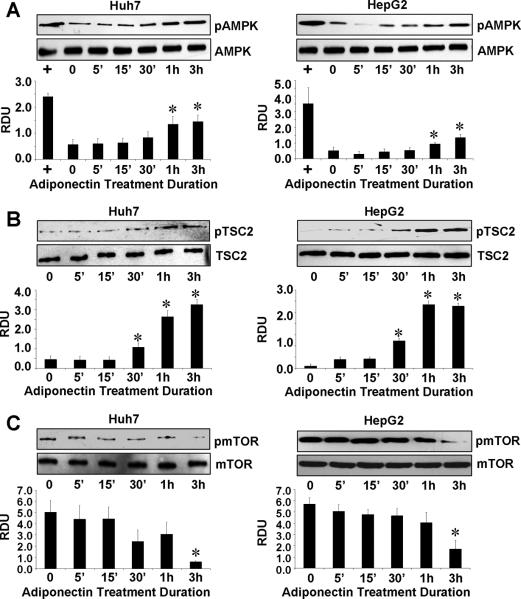
Previous studies investigating adiponectin signaling have reported the involvement of several signaling molecules and pathways15–17. Phosphorylation of AMPK has been shown in human hepatoma cells, Huh7 and Huh8 cells, and in hepatitis C virus-induced steatosis28. Adiponectin treatment significantly increased the phosphorylation of AMPK in both Huh7 and HepG2 cells (Figure 4A). Recent advances have linked AMPK with carcinogenesis as a suppressor of cell growth by controlling a variety of cellular events. Cell growth regulation by AMPK is mediated in part by regulation of the tumor suppressor gene TSC2 and mTOR (mammalian target of rapamycin) pathway. Intriguingly, investigating the downstream events of AMPK signaling, we found that adiponectin treatment led to increased phosphorylation of TSC2 (Figure 4B). Also we found decreased phosphorylation of mTOR in response to adiponectin treatment in both HCC cells (Figure 4C). These results indicate the involvement of AMPK-TSC2-mTOR axis in adiponectin action in HCC.
Figure 4. Adiponectin modulates AMPK-TSC2-mTOR axis.
A, B and C, Cells were serum starved for 16h and treated with 10μg/ml adiponectin for indicated time. Untreated cells are denoted by U. Cells treated with AICAR (+) were included as controls for pAMPK. Total protein lysates were analyzed by immunoblot analysis using AMPK, phosphorylated-AMPK (A), TSC2, phosphorylated-TSC2 (B) and mTOR, phosphorylated mTOR (C) antibodies. These data are representative of multiple independent experiments with a representative immunoblot for p-AMPK, AMPK, TSC2, p-TSC2, mTOR and p-mTOR. The histogram represents densitometric mean values ± SEM of band intensities of phosphorylated species with respect to total species of the target proteins. *p < 0.05, compared with untreated control cells.
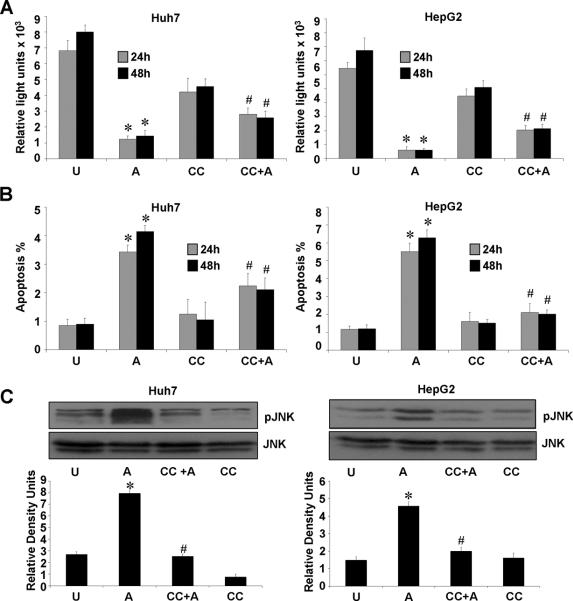
Next, to investigate if AMPK activation is directly involved in adiponectin-induced decreased proliferation and increased apoptosis of HCC cells and JNK activation, we examined the effect of compound C (a small-molecule-inhibitor of AMPK activity) on biological functions of adiponectin. Interestingly, we found that adiponectin decreased proliferation of HCC cells whereas pretreatment with compound C significantly blocked adiponectin-induced decrease in proliferation (Figure 5A). Also, treatment of HCC cells with compound C prior to adiponectin treatment significantly blocked adiponectin-induced increase in apoptosis (Figure 5B). Further, compound C pretreatment specifically inhibited adiponectin-induced activation of JNK in both HepG2 and Huh7 cells (Figure 5C). Revealing the hierarchy of these signaling events, our data show that AMPK activation is upstream of the activation of JNK and caspase-3. Collectively, our data suggests the involvement of AMPK-TSC2-mTOR and AMPK-JNK-caspase-3 pathways in anti-proliferative and pro-apoptotic effects of adiponectin in HCC.
Figure 5. Adiponectin-induced AMPK phosphorylation is essential for adiponectin-induced decreased proliferation, increased apoptosis and JNK activation.
A, Cells were serum starved for 16h, pretreated with compound C (CC) followed by treatment with 10μg/ml adiponectin (CC+A) or treated with 10μg/ml adiponectin alone (A). Untreated cells (U), cells treated with compound C alone (CC) were included as controls. BrdU incorporation assays were performed. *p< 0.01, for adiponectin (A) treated compared with untreated (U) cells; #p< 0.05, CC+A compared with adiponectin (A) treated cells. The data represents mean values ± SEM and are the results of three independent experiments performed in triplicates. B, Cells were treated as in A and apoptosis was analyzed by XTT method. The percentage of apoptotic cells represents the means ± SEM of viable cells present in treatment calculated with respect to cells grown in complete medium. *p< 0.01, for adiponectin (A) treated compared with untreated (U) cells; #p< 0.05, CC+A compared with adiponectin (A) treated cells. C, Cells were cultured and treated as in A. Total protein lysates were analyzed by immunoblot analysis using JNK, phosphorylated-JNK antibodies. These data are representative of multiple independent experiments with a representative immunoblot for pJNK and JNK. The histogram represents densitometric mean values ± SEM of band intensities. *p < 0.01, for adiponectin (A) treated compared with untreated (U) cells; #p< 0.05, CC+A compared with adiponectin (A) treated cells.
Adiponectin inhibits HCC tumor progression in athymic nude mice
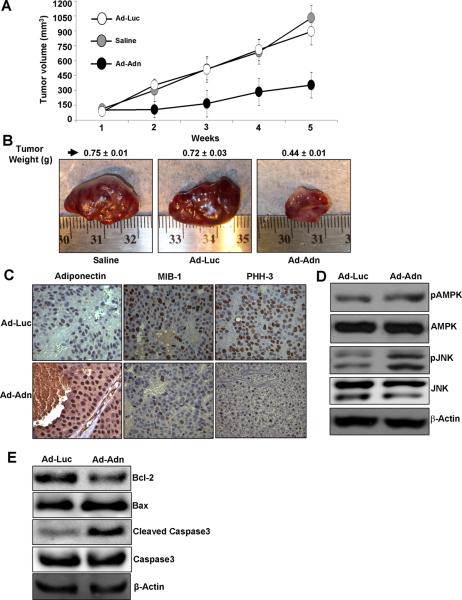
We investigated the physiological relevance of our in vitro findings by evaluating whether adiponectin has any suppressive effects on the development of HCC carcinoma in nude mouse models. We injected recombinant adenovirus that express either adiponectin or luciferase (as control) once a week into the tumor xenograft starting at 14 days after initial implantation. As shown in figure 6A, the visible tumors developed at ~one week after inoculation of HCC cells. In the experimental group treated with adiponectin adenovirus, the rate of tumor growth was significantly inhibited and the tumor size and weight were significantly reduced, compared to saline and adenovirus-luciferase control (Figure 6 A, B). Adiponectin adenovirus treated tumors showed elevated levels of adiponectin as compared to control group. The immunohistochemical assessment of tumor proliferation showed higher MIB-1 (Ki-67 receptor) and PHH3 expression in control group as compared to adiponectin treated group (Figure 6C). We also examined the plasma levels of adiponectin in tumor-bearing mice injected with recombinant adenovirus-adiponectin (Ad-Adn) or adenovirus-luciferase (Ad-Luc) and found that Ad-Adn injection led to a significant increase in serum adiponectin levels in tumor-bearing mice (Supplementary Figure 4). We further analyzed the adiponectin signaling molecules in tumors treated with adiponectin and control adenovirus. Importantly, we found that tumors treated with adiponectin adenovirus showed higher phosphorylation of AMPK and JNK (Figure 6D) showing the direct in vivo evidence of involvement of AMPK-JNK axis in adiponectin function. In addition, we also checked apoptotic markers in tumors from Ad-Adn and Ad-Luc treated mice. We found lower Bcl-2, higher Bax and cleaved caspase-3 levels in Ad-Adn treated mice tumors in comparison to Ad-luc treated mice which showed higher Bcl-2, lower Bax and low to none cleaved caspase-3 (Figure 6E). These data presented direct in vivo evidence of involvement of caspase-3 cleavage and Bcl-2, Bax ratio in adiponectin function as shown by our in vitro data.
Figure 6. The inhibitory effects of adiponectin on the growth of HepG2 cell- derived tumor in nude mice.
Saline or Ad-Luc or Ad-ADN (108 pfu) was locally injected into the HepG2-xenograft-tumor site once a week starting at day 14 after cell-implantation. A, Tumor growth was monitored by measuring the tumor volume for 5 weeks. B, At the end of 5 weeks, tumors were collected, measured, weighed and photographed. Ad-Adn treatment reduced tumor size as compared to Ad-Luc or Saline, * p < 0.05 Ad-Adn versus Ad-Luc. Average tumor weight and representative tumor images are shown here. C. Tumor samples were subjected to immunohistochemistry using adiponectin, MIB-1 and PHH-3 antibody. Ad-Adn treated tumors showed significant increase in adiponectin expression and reduction in MIB-1 and PHH-3 as compared to Ad-Luc treated tumors, *p < 0.05 Ad-Adn versus Ad-Luc. D. Tumor lysates were subjected to immunoblot analysis using AMPK, phospho-AMPK, phospho-JNK, JNK antibodies and cleaved-caspase-3, caspase-3, Bcl-2, Bax (E). Ad-Adn treated tumors show increased apoptotic markers and phosphorylation of AMPK and JNK as compared to Ad-Luc.
Expression of adiponectin in human hepatocellular carcinoma samples
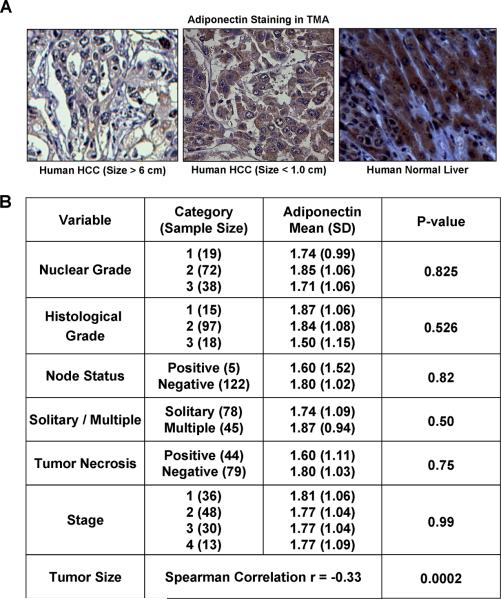
Our in vitro and in vivo data prompted us to investigate the pattern of adiponectin expression in a tissue microarray (TMA) of human HCC to understand its importance in tumor progression. Immunohistochemical studies showed that normal liver tissue expresses higher level of adiponectin as compare to HCC. Also, larger tumors show further decreased adiponectin expression as compared to smaller tumors. Representative photomicrographs are shown in Figure 7A. Analysis of clinicopathological characteristics showed that there is an inverse correlation between adiponectin expression and tumor size (Figure 7B). We also performed IHC analysis analyzing the expression levels of cleaved caspase-3, Bcl-2 and Bax in human HCC TMA. Results showed that human HCC samples showing higher adiponectin expression correlated positively with cleaved caspase-3 and Bax but the results were not statistically significant (data not shown). Our in vivo studies also showed that adiponectin reduces tumor size in athymic nude mice. Collectively, our studies show that adiponectin inhibits the progression of hepatocellular carcinoma.
Figure 7. Adiponectin expression in human HCC samples.
A. Representative photomicrograph of HCC samples from TMA showing decreased expression of adiponectin in larger tumors (6.0 ± 4.6) as compared to smaller tumors (1.0 ± 2.5), *p<0.001 low adiponectin versus high adiponectin. B. Adiponectin expression was analyzed using immunohistochemical analysis of tissue microarray (140 human HCC samples). Various clinicopathological characteristics were correlated with expression levels of adiponectin. Expression of adiponectin correlated significantly and inversely with tumor size (p<0.001).
Discussion
Modulations in the levels of adipocytokines represent a new molecular link explaining the complex relationship between obesity and related malignancies including cancer29–32. Of all the adipocytokines, adiponectin is of particular interest as it is inversely associated with the various consequences of obesity7. In recent epidemiological studies, HCC showed the highest relative-risk increase with increased obesity compared to all other cancers studied19. Adiponectin has received a great deal of attention due to its potent insulin-sensitizing, anti-inflammatory and antiatherogenic activities33, 34 and possible use for metabolic disorders35. We investigated the molecular mechanisms underlying the biological actions of adiponectin in HCC. The following novel findings are described in this study: (a) adiponectin treatment inhibits proliferation and increases apoptosis of HCC cells; (b) biological effects of adiponectin involve AMPK-JNK-caspase3 and AMPK-TSC2-mTOR axis in HCC cells; (c) adiponectin treatment inhibits HCC tumorigenesis in vivo; (d) adiponectin expression correlates significantly and inversely with tumor size in human HCC-tissue-microarray (TMA) (140 samples). Our studies also showed that HCC cells express significantly higher levels of adiponectin receptors adipoR1 and adipR2 in comparison to normal hepatocytes. Previous studies have shown that adipoR1 and adipoR2 serve as receptors for adiponectin and are positively correlated to adiponectin sensitivity. Depletion of adipoR1 and adipoR2 has been shown to inhibit the biological effects of adiponectin showing the importance of adiponectin receptors in adiponectin function36. Increase in adipoR1 and adipoR2 expression in hepatocellular carcinoma cells possibly represents a favored reaction balancing lower levels of adiponectin in liver cancer. As hypothetically, upregulation of adiponectin receptors expression increases adiponectin-binding capacity leading to increased levels of adiponectin action. Collectively, results from these in vitro, in vivo and human HCC-TMA studies show the negative regulatory role of adiponectin in HCC progression thus using adiponectin analogues may be a suitable therapeutic strategy for HCC.
Both clinical and epidemiological studies have linked adiponectin with carcinogenesis2. Our studies show the direct involvement of AMPK in adiponectin-mediated inhibition of HCC. AMPK has been shown to be involved in the regulation of diverse set of targets, including metabolic and endocrine functions37–39. AMPK inhibits cell growth via modulating regulation of the cell cycle, inhibition of protein synthesis, de novo fatty acid synthesis40, 41. Tumor suppressor TSC2 (Tuberous sclerosis complex 2), is a downstream effector of AMPK42, 43. Recent studies demonstrate that activation of AMPK phosphorylate and activates TSC2 that negatively regulates mTOR44. The mammalian target of rapamycin (mTOR)) is an evolutionary conserved serine/threonine kinase that occupies a central role in the regulation of cell growth and proliferation. Importantly, many cancers are characterized by increased expression and activity of mTOR44. The regulation of TSC2 and mTOR by adiponectin is important in the context of cancer because the PI3K-Akt signaling pathway is constitutively active in many cancers especially in those that have either inactivating mutations of the PTEN (Phosphatase and tensin homolog) gene45. Our studies support the concept that AMPK plays an important role in mediating biological effects of adiponectin on HCC via modulating JNK activation. JNK/SAPKs phosphorylate c-Jun in response to various extra-cellular stimuli and play an important role in the pathophysiology of several diseases46,47. Our studies show that adiponectin increases JNK activation in HCC which further regulates adiponectin-induced apoptosis and caspase-3 activation.
Most of the adipocytokines are casually linked to obesity-related diseases including carcinogenesis, whereas adiponectin has shown promising therapeutic role in various in vivo studies33–35. The clinical relevance of adiponectin has been suggested by studies showing that adiponectin can improve glucose/lipid homeostasis, increase insulin sensitivity and prevent atherosclerosis in animal models11,48,49. Here, we showed that a complex signaling module exists, involving AMPK-JNK-caspase-3 and AMPK-TSC2-mTOR, to mediate anti-cancerous biological actions of adiponectin in HCC. Importantly, our studies showed that adiponectin inhibits HCC tumorigenesis in vivo and adiponectin expression negatively correlates with tumor size in human HCC TMA thus providing important pre-clinical data for the development of adiponectin analogues. Considering the high prevalence of obesity in US and most of the developed world, our study has the potential to significantly impact the vast majority of obese HCC patients.
Supplementary Material
Acknowledgement
Grant Support: This work was supported by K01DK076742 and R03DK089130 from NIDDK, NIH to NKS, R01DK062092 and R01DK075397 from NIDDK, NIH to FAA, NCI NIH R01CA131294 to DS, CDMRP BCRP BC030963, The Susan G. Komen for the Cure BCTR0503526, and Mary K Ash Foundation to DS, DDRDC R24DK064399 to Division of Digestive Diseases. Data analysis was supported by NCI 1 P30 CA138292-01 to the Biostatics Shared Core Resource at Winship Cancer Institute of Emory University.
Footnotes
No conflicts of interest exist for all authors (NKS, AN, JH, PPF, CC, MT, DS, FAA)
References
- 1.Dizdar O, Alyamac E. Obesity: an endocrine tumor? Med Hypotheses. 2004;63:790–2. doi: 10.1016/j.mehy.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–44. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 5.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 6.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–12. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 7.Nawrocki AR, Scherer PE. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today. 2005;10:1219–30. doi: 10.1016/S1359-6446(05)03569-5. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 9.Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol. 2005;5:122–8. doi: 10.1016/j.coph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003;3:207–13. doi: 10.1007/s11892-003-0065-2. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–8. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 14.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–75. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–70. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 16.Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 18.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–50. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 20.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–9. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 21.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–63. [PubMed] [Google Scholar]
- 22.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–4. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–47. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 24.Efimova EA, Glanemann M, Liu L, Schumacher G, Settmacher U, Jonas S, Langrehr JM, Neuhaus P, Nussler AK. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res. 2004;36:300–7. doi: 10.1159/000079915. [DOI] [PubMed] [Google Scholar]
- 25.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem Biophys Res Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 27.Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–60. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 28.Rahman SM, Qadri I, Janssen RC, Friedman JE. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J Lipid Res. 2009;50:2193–202. doi: 10.1194/jlr.M800633-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 30.Amling CL, Kane CJ, Riffenburgh RH, Ward JF, Roberts JL, Lance RS, Friedrichs PA, Moul JW. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–8. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 31.Furuya Y, Akimoto S, Akakura K, Ito H. Smoking and obesity in relation to the etiology and disease progression of prostate cancer in Japan. Int J Urol. 1998;5:134–7. doi: 10.1111/j.1442-2042.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 32.Regimbeau JM, Colombat M, Mognol P, Durand F, Abdalla E, Degott C, Degos F, Farges O, Belghiti J. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transpl. 2004;10:S69–73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 33.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzawa Y. Adiponectin: Identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler Suppl. 2005;6:7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 37.Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205–15. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 40.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 42.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 43.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 44.Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–66. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–8. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun H, Kim HS, Lee S, Kang I, Kim SS, Choe W, Ha J. AMP Kinase Signaling Determines Whether c-Jun N-Terminal Kinase Promotes Survival or Apoptosis during Glucose Deprivation. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn259. [DOI] [PubMed] [Google Scholar]
- 48.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 49.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.