Abstract
The human and simian immunodeficiency viruses contain small open reading frames known as vpr and vpx. These genes encode proteins that are highly related both at the amino acid level and functionally, although key differences do exist. This review describes the main functions ascribed to Vpr and Vpx in the context of both viral replication and modulation of host cell biology.
1. Introduction
HIV-1 Vpr (short for viral protein, regulatory) is a small, 96-amino acid protein of about 14kDa. The name assigned to this protein originated from the observation that disruption of its open reading frame in HIV-1 resulted in a virus that replicated with a slower kinetics (Hattori et al., 1990; Ogawa et al., 1989; Wong-Staal, Chanda, and Ghrayeb, 1987). HIV-1 Vpr is a small, 96-amino acid (14 kDa) protein. Vpr is packaged in the virus particles via a direct interaction with the p6 subunit of the Gag precursor (reviewed in (Tungaturthi et al., 2003)). Vpr is also expressed de novo by the provirus, from a singly-spliced, late mRNA (Schwartz, Felber, and Pavlakis, 1991).
A multiplicity of effects and functions have been ascribed to Vpr. As a virion-bound protein, Vpr has been proposed to participate in the nuclear import of pre-integration complexes in macrophages and other non-dividing cells; and to enhance the fidelity of reverse transcription. As a late protein produced in the infected cell, Vpr induces cell cycle arrest in the G2 phase, transactivation of the viral promoter, and ultimately apoptosis (reviewed in (Le Rouzic and Benichou, 2005; Planelles and Benichou, 2010)).
2. Structure of Vpr
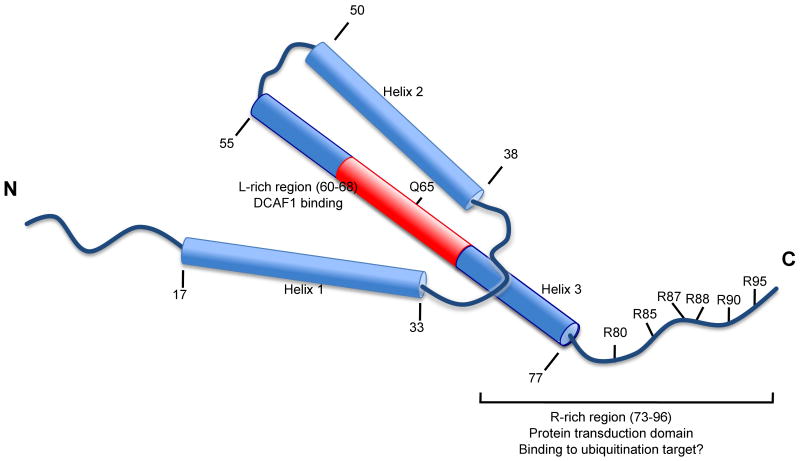
The structure of Vpr consists of three bundled α-helices spanning residues 17-33, 38-50 and 55-77, respectively. Flanking the triple helix bundle are flexible, unstructured n- and c-terminal domains that are negatively and positively charged, respectively (Figure 1) (Morellet et al., 2003). The carboxy-terminus of Vpr contains six arginine residues between positions 73 and 96 (Figure 1). This domain shows similarity with those of arginine-rich protein transduction domains, and may explain the transducing properties of Vpr, including its ability to cross the lipid bilayers (Coeytaux et al., 2003; Kichler et al., 2000; Sherman et al., 2002). The third helix of Vpr is rich in leucine residues (Schuler et al., 1999), and one side of the helix presents a stretch of hydrophobic side chains that can form a leucine zipper-like motif (Schuler et al., 1999). This region is thought to mediate the formation of Vpr oligomers (Fritz et al., 2008; Mahalingam et al., 1997; Schuler et al., 1999; Wang et al., 1996) and the interaction with a ubiquitin ligase complex (see below).
Figure 1.
Diagramatic structure of Vpr as determined by nuclear magnetic resonance (adapted from Morellet et al., 2003). Cilinders denote regions of alpha helix comprised between residues indicated by numbers. N, amino-terminus. C, carboxy-terminus.
3. Effects of Vpr on the cell cycle
The ability of Vpr to manipulate the cell cycle and, more specifically, to induce arrest at the G2-to-M transition was first reported in 1995 (He et al., 1995; Jowett et al., 1995; Re et al., 1995; Rogel, Wu, and Emerman, 1995). About one year prior to those reports, Zhao and collaborators described the first cellular protein found in association with Vpr in co-precipitation experiments (Zhao, Mukherjee, and Narayan, 1994). This was a novel cellular protein of unknown function, and was named Vpr-binding protein (VprBP) (Zhao, Mukherjee, and Narayan, 1994). Initial studies did not link VprBP to the cell cycle effects of Vpr and it was only recently that a direct link was found (see below).
4. Vpr induces genotoxic stress
The effects of Vpr on the cell cycle resemble those of DNA damage. More specifically, the presence of hyper-phosphorylated Cdk1, and the ability of methylxanthines, such as caffeine, to relieve the cell cycle block, suggested that the underlying stimulus was DNA damage (Poon et al., 1997; Shostak et al., 1999).
Roshal et al. showed that Vpr induced G2 arrest via activation of ataxia telangiectasia-mutated and Rad 3- related kinase, ATR (Roshal et al., 2003). ATR is a sensor for replication stress, a cellular condition that involves the stalling of replication forks. Replication stress be induced by deoxyribonucleotide depletion, topoisomerase inhibition or ultraviolet light-induced DNA damage (reviewed in (McGowan and Russell, 2004)). The ATR phosphorylation target that controls G2 checkpoint activation is Chk1 (Figure 2) (Cimprich and Cortez, 2008). In agreement with this idea, Roshal et al. also showed that depletion of Chk1 or ATR relieved Vpr-induced G2 arrest (Roshal et al., 2003).
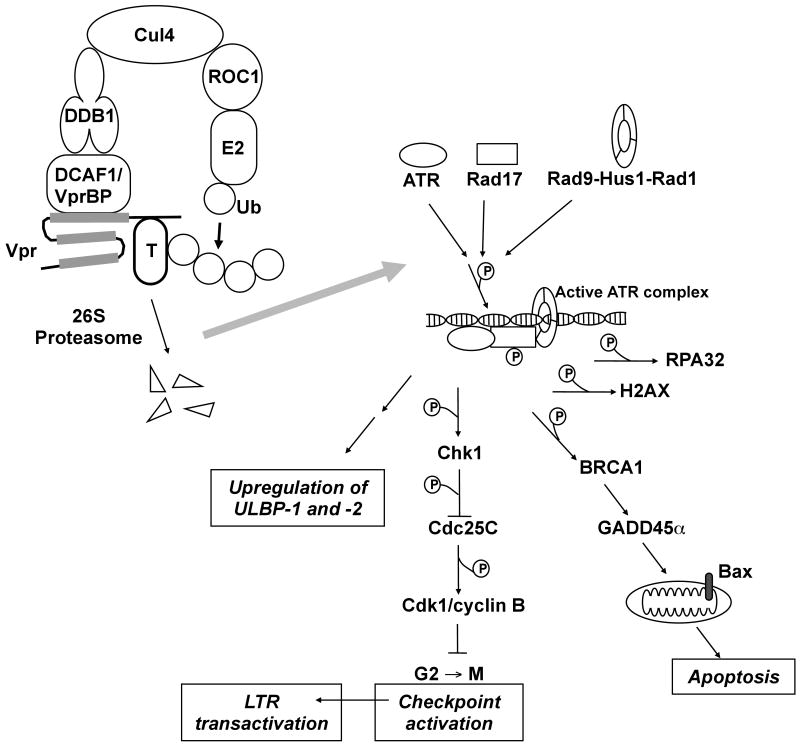
Figure 2.
Signaling pathways proposed to mediate induction of G2 arrest, ULBP-1 and -2 expression, apoptosis, and LTR transactivation by HIV-1 Vpr. Ub, ubiquitin.
It remains unclear whether the Vpr effect on the cell cycle is a primary function of Vpr or, alternatively, a consequence of an unknown function of Vpr. Nevertheless, recent observations with infected cells from HIV-1-infected individuals indicate that infected CD4+ lymphocytes, in vivo, display a DNA content that is consistent with that of cells in the G2/M transition (Zimmerman et al., 2006).
5. Vpr manipulates a ubiquitin ligase complex
In 2006, several groups identified a family of proteins that were associated with the damaged DNA-specific binding protein 1 (DDB1), a Cullin 4 adaptor (Angers et al., 2006; He et al., 2006; Higa et al., 2006a; Jin et al., 2006). This novel family of proteins, which include VprBP, act as the substrate receptors in a Cullin 4- and DDB1-based ubiquitin ligase complex or E3 (Angers et al., 2006; He et al., 2006; Higa et al., 2006a; Jin et al., 2006). VprBP was, accordingly, renamed DDB1- and Cullin 4-associated factor (DCAF) -1. Shortly thereafter, it was shown that, through its interaction with VprBP/DCAF1, Vpr is capable of binding to a larger complex that includes Cul4A, DDB1, Rbx1/Roc1 and a ubiquitin conjugating enzyme or E2 (Belzile et al., 2007; DeHart et al., 2007; Hrecka et al., 2007; Le Rouzic et al., 2007; Schrofelbauer, Hakata, and Landau, 2007; Tan, Ehrlich, and Yu, 2007; Wen et al., 2007).
DDB1 links Cul4A to a number of possible substrate receptors, collectively referred to as DCAFs. The ubiquitination targets for several DCAFs have been identified. For example, CDT2 (DCAF2) recruits the origin of replication licensing factor, CDT1 (Higa et al., 2003; Hu et al., 2004) to prevent re-replication of DNA. The damaged DNA-binding 2/xeroderma pigmentosum complementation group E protein (DDB2/XPE) is another DCAF that interacts with DDB1-Cul4A to promote degradation of XPC (Sugasawa et al., 2005), and the histones 3 and 4 (Wang et al., 2006), as part of the response to DNA damage. Cul4A- and Cul4B-containing E3 ligases are also responsible for destruction of the cyclin-dependent kinase inhibitor, p27, and cyclin E, respectively (Higa et al., 2006b). Thus, the general roles of Cul4ADDB1 E3 ligases involve genome stability, DNA replication and cell cycle checkpoint control. Merlin, a tumor suppressor protein, is the only target of DCAF1 found to date (Huang and Chen, 2008). In vitro, Merlin accumulates in serum-starved cells, and blocks proliferation. Merlin is then degraded in response to serum stimulation, and this relieves the block to cell proliferation (Huang and Chen, 2008).
Based on the above evidence, a model has emerged in which Vpr binds to a Cul4ADDB1/DCAF1 E3 ligase, to trigger polyubiquitination and, presumably, degradation of an unknown cellular protein (represented as “T” for “target” in Figure 2), resulting in activation of the G2 checkpoint (reviewed in (DeHart and Planelles, 2008)). Direct evidence for the polyubiquitination and degradation of the putative target is not available. However, Richard et al. recently reported that overexpression of the ubiquitin K48R mutant, which blocks formation of poly-ubiquitin chains with the K48 linkage, abrogated the induction of γH2A-X foci in Vpr-expressing cells (Belzile et al., 2010). Since K48-linked poly ubiquitin chains target proteins for proteasomal destruction, it is tempting to speculate that the ubiquitination target of Vpr is ultimately degraded.
This model predicts that Vpr would be using two different interfaces to bind to VprBP/DCAF1 and the putative target protein. The domain of Vpr that binds to DCAF1 was mapped to the leucine-rich (LR) motif 60-LIRILQQLL-68 within the third α-helix of HIV-189.6 Vpr (Zhao, Mukherjee, and Narayan, 1994). A Vpr mutant disrupting the DCAF1 interaction, Vpr(Q65R), was described by Le Rouzic et al. (Le Rouzic et al., 2007). Consistent with the idea that DCAF1-Vpr interaction is required for Vpr function, Vpr(Q65R) failed to induce G2 arrest (Le Rouzic et al., 2007).
In contrast, truncation of the c-terminal 18 residues of Vpr [Vpr(1-78)] or replacement of arginine at position 80 by alanine, Vpr(R80A), resulted in mutants with unaltered binding to DCAF1, but unable to induce G2 arrest (DeHart et al., 2007; Le Rouzic et al., 2007). In addition, co-expression of either Vpr(1-78) or Vpr(R80A) (Figure 1) with wild-type Vpr resulted in a dominant-negative effect by either of the previous mutants (DeHart et al., 2007; Le Rouzic et al., 2007).
Together, the above observations indicate that (a) binding of Vpr to DCAF1 is necessary, but not sufficient, for induction of G2 arrest; and (b) the carboxy-terminal domain of Vpr is likely required for the recruitment of a cellular protein, whose ubiquitination leads to G2 arrest (Figure 2).
Vpr-induced G2 arrest has two known downstream effects that likely contribute to the pathogenesis of HIV-1. First, the transcriptional activity of the viral promoter is increased by several fold during G2/M (Goh et al., 1998; Hrimech et al., 1999; Zhu et al., 2001), leading to enhanced production of viral particles (Goh et al., 1998). Secondly, G2 arrest leads to the commitment of infected cells to death by apoptosis (see below).
6. Vpr is a potent pro-apoptotic protein
Vpr was found to be a potent inducer of cell death both when expressed alone or in the context of HIV-1. Vpr-induced cell death has the hallmarks of apoptosis (Andersen et al., 2005; Muthumani et al., 2002; Shostak et al., 1999; Stewart et al., 2000).
Recombinant Vpr was found to associate with purified mitochondria (Jacotot et al., 2000; Vieira et al., 2000). This interaction was mediated via binding to the adenine nucleotide transporter (ANT), a component of the permeability transition pore (PTPC) that resides at the inner mitochondrial membrane (Jacotot et al., 2000; Vieira et al., 2000). The addition of recombinant Vpr to purified mitochondria triggered mitochondrial membrane permeabilization and release of pro-apoptotic proteins, such as cytochrome c (Jacotot et al., 2000; Vieira et al., 2000).
Recent studies of ANT and cyclophilin D (an essential regulatory element of ANT (Tsujimoto and Shimizu, 2007)) in knockout mice suggest that these mitochondrial pore components may promote necrotic, but not apoptotic cell death (Baines et al., 2005; Kokoszka et al., 2004; Nakagawa et al., 2005). In recent studies, siRNA-mediated depletion of ANT did not affect Vpr-induced apoptosis, whereas depletion of another mitochondrial pore-forming protein, Bax (Figure 2), effectively blocked apoptosis (Andersen et al., 2006).
An alternative explanation for Vpr-induced cell death is that apoptosis represents a downstream consequence of prolonged G2 arrest (Andersen et al., 2006; Jacquot et al., 2007; Yuan, Xie, and Chen, 2003). Specifically, the pro-apoptotic activity of Vpr was eliminated when cells were artificially synchronized at the G1/S boundary and therefore not allowed to progress into G2 (Andersen et al., 2006). Furthermore, G2 arrest and apoptosis induction by Vpr are dependent on the activation of ATR, and phosphorylation of its target, BRCA1 (Andersen et al., 2006). BRCA1 phosphorylation leads to GADD45α upregulation and induction of apoptosis (Andersen et al., 2006) (Figure 2). The signaling events that connect GADD45α and Bax are not known.
Tissue macrophages infected with HIV-1 are relatively resistant to the viral cytopathic effects (Gartner et al., 1986; Gorry et al., 2005; Kedzierska and Crowe, 2002). Thus, macrophages are considered one of the reservoirs for HIV-1 infection, and are capable of disseminating the virus to various tissues including the brain (Ghorpade et al., 1998; Orenstein, Fox, and Wahl, 1997). We have observed that macrophages are refractory to Vpr-induced apoptosis (Zimmerman et al., 2006). Western blot demonstrated the absence of three essential proteins in the ATR signaling axis: ATR itself, Chk1 and Rad17 (Zimmerman et al., 2006). In view of these results, we have speculated that the apparent lack of Vpr-induced cytopathicity in macrophages may due to the absence of ATR signaling (Zimmerman et al., 2006).
7. Vpr modulates the expression of natural killer cell ligands
Activation of the DNA damage pathway proteins ATR and ATM, after cells were exposed to genotoxic stress, resulted in the expression of ligands for the NK cell activation receptor, natural killer group 2, member D (NKG2D) (Gasser et al., 2005; Guerra et al., 2008). While the observations by Gasser et al. were obtained in the areas of cancer and genomic stability (Gasser et al., 2005), they opened the possibility that a virus that is capable of inflicting genotoxic stress may also induce NKG2D ligands. Specifically, two different teams tested whether Vpr-mediated activation of ATR may trigger upregulation of NKG2D ligands (Richard et al., 2009; Ward et al., 2009).
NKG2D is a single pass type II transmembrane protein consiting of 216 amino acid residues (Houchins et al., 1991). This receptor exists on the cell surface as a homodimer that contains a single C-lectin domain on each chain. NKG2D is part of an oligomeric complex with the transducing polypeptide DAP10 (Wu et al., 1999). DAP10 is a signaling adaptor protein that contains a cytoplasmic YINM motif that allows the recruitment and subsequent activation of phosphatidyl inositol 3-kinase (Wu et al., 1999). NKG2D is expressed on virtually all NK cells in the blood and on subsets of activated CD8+ T cells, γδ T cells and NKT cells (Bauer et al., 1999).
The ligands for NKG2D are are evolutionarily related to MHC class I molecules, although with clear functional differences. Unlike MHC I molecules, NKG2D ligands are devoid of CD8 binding, do not load peptides, and fail to associate with β2-microglobulin (Bahram et al., 1994; Groh et al., 1998). The human ligands for NKG2D include the transmembrane proteins, MHC class I polypeptide-related sequence-A (MICA) and –B (MICB) (Bahram et al., 1994) and the GPI-linked proteins, cytomegalovirus unique long 16 (UL16)-binding protein (ULBP) 1-4 (Cosman et al., 2001).
NKG2D ligands are expressed on certain tumor cell lines and in fetal tissues. Normal adult tissues may express these ligands but at much lower levels than those found on cell lines (Cosman et al., 2001). NKG2D ligands are induced on normal adult tissues after infection by certain viruses (Jonjic, Polic, and Krmpotic, 2008). Binding of NKG2D ligands to NKG2D on NK cells triggers a cytotoxic response as well as the release of multiple cytokines and chemokines (Kubin et al., 2001; Pende et al., 2002). Because NKG2D ligation elicits strong NK responses, viruses have developed strategies to down modulate NKG2D ligands on the infected cell surface. For example, human cytomegalovirus, which induces the expression of NKG2D ligands on infected cells, also encodes UL16 and UL142 that, in turn, cause intracellular sequestration of the same ligands, resulting in inhibition of their function (Cosman et al., 2001; Kubin et al., 2001).
HIV-1-infection of primary CD4+ T-cells leads to the induction of NKG2D ligand surface expression (Cerboni et al., 2007; Ward et al., 2007). These molecules were not only found on in vitro-infected primary CD4+ T-cell blasts but also on infected cells obtained from HIV-1-infected patients after amplification of the virus-infected cells ex vivo (Fogli et al., 2008). In addition, these studies, which used primary CD4+ T-cells infected with HIV-1 as targets for autologous NK cells in cytotoxicity assays, revealed that NK cells can respond to the HIV-1-infected cells in an NKG2D-dependent manner (Fogli et al., 2008; Ward et al., 2007; Ward et al., 2009).
Recent studies have shown that expression of the HIV-1 Vpr protein is sufficient for induction of expression of NKG2D ligands on the cell surface and that this action was mediated solely through ATR (Richard et al., 2009; Ward et al., 2009). HIV-1 Vpr, however, only upregulates the expression of ULBP-1 and -2 but not that of ULBP-3, MICA and MICB in primary CD4+ T-cells (Ward et al., 2009). The upregulation of ULBP-1 and -2 proteins on the cell surface was accompanied by increased mRNA levels for ULBP-1 and -2 (Ward et al., 2009). The presence of ULBP-1 and ULBP-2 on HIV-1 infected cells is dependent on the ability of Vpr to associate with the Cul4ADDB1/DCAF1 ubiquitin ligase (Richard et al., 2009; Ward et al., 2009). Thus, the Vpr mutation, Q65R, which disabled binding of Vpr to DCAF-1, or knockdown of DCAF-1, abolished the Vpr effect on ULBP-1 and -2 (Ward et al., 2009).
To determine whether the expression of ULBP-1 and -2 could trigger NK cell killing of infected T-cells, NK cells were co-cultured with target cells infected with wild-type or Vpr-deleted HIV-1. NK cells, when exposed to T-cells infected with a Vpr-deficient virus, were two-fold less as efficient at lysing infected cells than when exposed to T-cells infected with a wild-type virus (Ward et al., 2009). Moreover, blocking the NKG2D receptor on NK cells diminished the lysis of T-cells infected with virus containing Vpr (Ward et al., 2009).
In studies by Cerboni et al., HIV-1 Nef had been implicated in down-modulation of the NKG2D ligands, MICA, ULBP-1 and -2 on CD4+ Jurkat cell lines (Cerboni et al., 2007). However, in our recent studies we observed that deletion of Nef did not affect expression of these ligands on primary CD4+ T-cells (Ward et al., 2009). The reasons for this discrepancy are not known.
Efficient lysis by NK cells requires the recognition of NKG2D ligands in combination with co-activation receptors, such as 2B4 or NTB-A (Bryceson, Ljunggren, and Long, 2009; Bryceson et al., 2005; Bryceson et al., 2006a; Bryceson et al., 2006b). The previous idea is consistent with the observation that HIV-1 down modulates the ligands of 2B4 and NTB-A (CD48 and NTB-A, respectively) on infected cells (Ward et al., 2007). Therefore, we speculate that the modest killing effect that was observed in the presence of ULBP-1 and -2 downregulation (Ward et al., 2009) would be enhanced if simultaneous downregulation of 2B4 and/or NTB-A were abrogated. This remains to be tested.
8. Vpx as a paralog of Vpr
Viruses in the HIV-2/SIVsm/SIVmac lineage encode two proteins that are homologous to HIV-1 Vpr, namely Vpr and Vpx. While HIV-2 Vpr shares the ability to induce G2 arrest with HIV-1 Vpr (Fletcher et al., 1996; Planelles et al., 1996), HIV-2 Vpx has no effect on the cell cycle and, instead, is required for efficient infection of non-dividing cells such as macrophages and dendritic cells (Fletcher et al., 1996; Guyader et al., 1989; Pancio, Vander Heyden, and Ratner, 2000; Yu et al., 1991). Vpx is thought to have arisen via a duplication of vpr within the HIV-2/SIVsm/SIVmac group (Sharp et al., 1996; Tristem et al., 1992), which diverged from the other primate lentiviral groups (Tristem et al., 1992). Given the common evolutionary origin, the high degree of homology, and the divergent functions of Vpx with respect to HIV-1 Vpr, Vpx is considered a paralog of HIV-1 Vpr.
Vpx facilitates the nuclear import of pre-integration complexes and/or promotes the accumulation of full-length viral DNA in non-dividing cells (Fletcher et al., 1996; Fujita et al., 2008; Goujon et al., 2007; Sharova et al., 2008; Srivastava et al., 2008). To explain the ability of Vpx to enhance lentiviral infection of dentritic cells, it has been proposed that Vpx overcomes an unknown restriction factor (Goujon et al., 2007). It has also been proposed that the restriction mechanism involves the ubiquitin/proteasome system, since treatment with proteasome inhibitors has a similar effect to that of Vpx expression (Goujon et al., 2007). Restriction factors are typically genetically dominant. In agreement with that, when Sharova et al. fused permissive cells (infection of which does not require Vpx) with restricting ones, the resulting heterokaryons had the restricting phenotype (Sharova et al., 2008).
The finding that HIV-1 Vpr manipulates the Cul4ADDB1/DCAF1 ubiquitin ligase prompted studies to examine the interaction of Vpr and Vpx alleles from other primate lentiviruses with DCAF1. It was shown that SIVMAC and HIV-2 Vpr interacted with DCAF1 (Le Rouzic et al., 2007; Wen et al., 2007). Le Rouzic et al. found that the SIVmac Vpx, which has no apparent effect on the cell cycle, also binds to DCAF1 (Le Rouzic et al., 2007). Binding of lentiviral Vpr and Vpx proteins to DCAF1 is mediated by a highly conserved leucine-rich motif (Le Rouzic et al., 2007). It is tempting, then, to speculate that Vpr and Vpx have preserved through evolution the ability to recruit DCAF1, although for different purposes.
Two recent reports demonstrated that the interaction of Vpx with DCAF1 is required for the enhancement of infectivity of non-dividing cells (Sharova et al., 2008; Srivastava et al., 2008). These studies show that depletion of DCAF1 via RNA interference (Sharova et al., 2008; Srivastava et al., 2008) or expression of a Vpx mutant, Q76A, devoid of DCAF1 binding (Srivastava et al., 2008) ablated the enhancement of infectivity by Vpx.
Unlike lentiviruses, gammaretroviruses, such as the murine leukemia virus (MLV) are unable to infect non-dividing cells. Examples of physiologically relevant non-dividing cells include monocytes, monocyte-derived macrophages (MDM), microglial cells and dendritic cells. In the laboratory, non-dividing cells can artificially be generated by treatment of immortalized cell lines with certain chemicals, such as aphidicolin, an inhibitor of DNA polymerases. Kaushik et al. recently demonstrated that the restriction in primary cell types is of a different nature from that observed in artificially arrested cell lines (Kaushik et al., 2009). While the block to MLV replication in macrophages is at or prior to the step of reverse transcription, this is not the case in arrested HeLa cells, where reverse transcription proceeds similarly to that in dividing cells (Kaushik et al., 2009). Kaushik et al. also showed that the ability of Vpx to overcome a putative restriction to SIV and HIV-1 in non-dividing primary cells was also active in overcoming the restriction to MLV in such cell types. The ability of Vpx to overcome the block to MLV infection was shown by preinfection with viruses encoding Vpx, but also by constructing a chimeric MLV Gag protein encoding SIV p6. SIV p6 is binds to Vpx and mediates Vpx encapsidation in virus particles. This p6-chimeric MLV acquired the ability to infect macrophages, but only when it was produced in the presence of Vpx (Kaushik et al., 2009).
Acknowledgments
This work was supported by NIH grants AI49057 to V.P; and AI52809 and AI56923 to EB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JL, DeHart JL, Zimmerman ES, Ardon O, Kim B, Jacquot G, Benichou S, Planelles V. HIV-1 Vpr-induced apoptosis is cell cycle dependent and requires Bax but not ANT. PLoS Pathog. 2006;2(12):e127. doi: 10.1371/journal.ppat.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JL, Zimmerman ES, DeHart JL, Murala S, Ardon O, Blackett J, Chen J, Planelles V. ATR and GADD45alpha mediate HIV-1 Vpr-induced apoptosis. Cell Death Differ. 2005;12(4):326–34. doi: 10.1038/sj.cdd.4401565. [DOI] [PubMed] [Google Scholar]
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443(7111):590–3. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1994;91(14):6259–63. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- Belzile JP, Duisit G, Rougeau N, Mercier J, Finzi A, Cohen EA. HIV-1 Vpr-Mediated G2 Arrest Involves the DDB1-CUL4A(VPRBP) E3 Ubiquitin Ligase. PLoS Pathog. 2007;3(7):e85. doi: 10.1371/journal.ppat.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzile JP, Richard J, Rougeau N, Xiao Y, Cohen EA. HIV-1 Vpr Induces the K48-Linked Polyubiquitination and Proteasomal Degradation of Target Cellular Proteins to Activate ATR and Promote G2 Arrest. J Virol. 2010 doi: 10.1128/JVI.02590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114(13):2657–66. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202(7):1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006a;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006b;107(1):159–66. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88(Pt 1):242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeytaux E, Coulaud D, Le Cam E, Danos O, Kichler A. The cationic amphipathic alpha-helix of HIV-1 viral protein R (Vpr) binds to nucleic acids, permeabilizes membranes, and efficiently transfects cells. J Biol Chem. 2003;278(20):18110–6. doi: 10.1074/jbc.M300248200. [DOI] [PubMed] [Google Scholar]
- Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- DeHart JL, Planelles V. Human immunodeficiency virus type 1 vpr links proteasomal degradation and checkpoint activation. J Virol. 2008;82(3):1066–72. doi: 10.1128/JVI.01628-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHart JL, Zimmerman ES, Ardon O, Monteiro-Filho CM, Arganaraz ER, Planelles V. HIV-1 Vpr activates the G2 checkpoint through manipulation of the ubiquitin proteasome system. Virol J. 2007;4(1):57. doi: 10.1186/1743-422X-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher TM, 3rd, Brichacek B, Sharova N, Newman MA, Stivahtis G, Sharp PM, Emerman M, Hahn BH, Stevenson M. Nuclear import and cell cycle arrest functions of the HrV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) EMBO J. 1996;15(22):6155–65. [PMC free article] [PubMed] [Google Scholar]
- Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, Barker E, Marcenaro E, Moretta A, Fauci AS. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4(7):e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JV, Didier P, Clamme JP, Schaub E, Muriaux D, Cabanne C, Morellet N, Bouaziz S, Darlix JL, Mely Y, de Rocquigny H. Direct Vpr-Vpr interaction in cells monitored by two photon fluorescence correlation spectroscopy and fluorescence lifetime imaging. Retrovirology. 2008;5:87. doi: 10.1186/1742-4690-5-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Otsuka M, Miyoshi M, Khamsri B, Nomaguchi M, Adachi A. Vpx is critical for reverse transcription of the human immunodeficiency virus type 2 genome in macrophages. J Virol. 2008;82(15):7752–6. doi: 10.1128/JVI.01003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Sciena. 1986;233(4760):215–9. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436(7054):1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman HE. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72(4):3340–50. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4(1):65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res. 2005;3(1):53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- Goujon C, Riviere L, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, Cimarelli A. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology. 2007;4:2. doi: 10.1186/1742-4690-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279(5357):1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NR, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M, Emerman M, Montagnier L, Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989;8(4):1169–75. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Michaels F, Fargnoli K, Marcon L, Gallo RC, Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci U S A. 1990;87(20):8080–4. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69(11):6705–11. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YJ, McCall CM, Hu J, Zeng Y, Xiong Y. DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 2006;20(21):2949–54. doi: 10.1101/gad.1483206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Mihaylov IS, Banks DP, Zheng J, Zhang H. Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol. 2003;5(11):1008–15. doi: 10.1038/ncb1061. [DOI] [PubMed] [Google Scholar]
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol. 2006a;8(11):1277–83. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- Higa LA, Yang X, Zheng J, Banks D, Wu M, Ghosh P, Sun H, Zhang H. Involvement of CUL4 ubiquitin E3 ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E degradation. Cell Cycle. 2006b;5(1):71–7. doi: 10.4161/cc.5.1.2266. [DOI] [PubMed] [Google Scholar]
- Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173(4):1017–20. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, Washburn MP, Skowronski J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci U S A. 2007;104(28):11778–83. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrimech M, Yao XJ, Bachand F, Rougeau N, Cohen EA. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J Virol. 1999;73(5):4101–9. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol. 2004;6(10):1003–9. doi: 10.1038/ncb1172. [DOI] [PubMed] [Google Scholar]
- Huang J, Chen J. VprBP targets Merlin to the Roc1-Cul4A-DDB1 E3 ligase complex for degradation. Oncogene. 2008;27(29):4056–64. doi: 10.1038/onc.2008.44. [DOI] [PubMed] [Google Scholar]
- Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N, Costantini P, Druillennec S, Hoebeke J, Briand JP, Irinopoulou T, Daugas E, Susin SA, Cointe D, Xie ZH, Reed JC, Roques BP, Kroemer G. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med. 2000;191(1):33–46. doi: 10.1084/jem.191.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot G, Le Rouzic E, David A, Mazzolini J, Bouchet J, Bouaziz S, Niedergang F, Pancino G, Benichou S. Localization of HIV-1 Vpr to the nuclear envelope: impact on Vpr functions and virus replication in macrophages. Retrovirology. 2007;4:84. doi: 10.1186/1742-4690-4-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23(5):709–21. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Jonjic S, Polic B, Krmpotic A. Viral inhibitors of NKG2D ligands: friends or foes of immune surveillance? Eur J Immunol. 2008;38(11):2952–6. doi: 10.1002/eji.200838823. [DOI] [PubMed] [Google Scholar]
- Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69(10):6304–13. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe. 2009;6(1):68–80. doi: 10.1016/j.chom.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9(21):1893–903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- Kichler A, Pages JC, Leborgne C, Druillennec S, Lenoir C, Coulaud D, Delain E, Le Cam E, Roques BP, Danos O. Efficient DNA transfection mediated by the C-terminal domain of human immunodeficiency virus type 1 viral protein R. J Virol. 2000;74(12):5424–31. doi: 10.1128/jvi.74.12.5424-5431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427(6973):461–5. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin M, Cassiano L, Chalupny J, Chin W, Cosman D, Fanslow W, Mullberg J, Rousseau AM, Ulrich D, Armitage R. ULBP1, 2, 3: novel MHC class I-related molecules that bind to human cytomegalovirus glycoprotein UL16, activate NK cells. Eur J Immunol. 2001;31(5):1428–37. doi: 10.1002/1521-4141(200105)31:5<1428::AID-IMMU1428>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E, Belaidouni N, Estrabaud E, Morel M, Rain JC, Transy C, Margottin-Goguet F. HIV1 Vpr Arrests the Cell Cycle by Recruiting DCAF1/VprBP, a Receptor of the Cul4-DDB1 Ubiquitin Ligase. Cell Cycle. 2007;6(2):182–188. doi: 10.4161/cc.6.2.3732. [DOI] [PubMed] [Google Scholar]
- Le Rouzic E, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2005;2(1):11. doi: 10.1186/1742-4690-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner DB. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71(9):6339–47. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan CH, Russell P. The DNA damage response: sensing and signaling. Curr Opin Cell Biol. 2004;16(6):629–33. doi: 10.1016/j.ceb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Morellet N, Bouaziz S, Petitjean P, Roques BP. NMR structure of the HIV-1 regulatory protein VPR. J Mol Biol. 2003;327(1):215–27. doi: 10.1016/s0022-2836(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Hwang DS, Desai BM, Zhang D, Dayes N, Green DR, Weiner DB. HIV-1 Vpr induces apoptosis through caspase 9 in T cells and peripheral blood mononuclear cells. J Biol Chem. 2002;277(40):37820–31. doi: 10.1074/jbc.M205313200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Shibata R, Kiyomasu T, Higuchi I, Kishida Y, Ishimoto A, Adachi A. Mutational analysis of the human immunodeficiency virus vpr open reading frame. J Virol. 1989;63(9):4110–4. doi: 10.1128/jvi.63.9.4110-4114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276(5320):1857–61. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- Pancio HA, Vander Heyden N, Ratner L. The C-terminal proline-rich tail of human immunodeficiency virus type 2 Vpx is necessary for nuclear localization of the viral preintegration complex in nondividing cells. J Virol. 2000;74(13):6162–7. doi: 10.1128/jvi.74.13.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62(21):6178–86. [PubMed] [Google Scholar]
- Planelles V, Benichou S. Vpr and its interactions with cellular proteins. Curr Top Microbiol Immunol. 2010;339:177–200. doi: 10.1007/978-3-642-02175-6_9. [DOI] [PubMed] [Google Scholar]
- Planelles V, Jowett JB, Li QX, Xie Y, Hahn B, Chen IS. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70(4):2516–24. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon B, Jowett JB, Stewart SA, Armstrong RW, Rishton GM, Chen IS. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71(5):3961–71. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69(11):6859–64. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr upregulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2009 doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel ME, Wu LI, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69(2):882–8. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshal M, Kim B, Zhu Y, Nghiem P, Planelles V. Activation of the ATR-mediated DNA damage response by the HIV-1 viral protein R. J Biol Chem. 2003;278(28):25879–86. doi: 10.1074/jbc.M303948200. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B, Hakata Y, Landau NR. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc Natl Acad Sci U S A. 2007;104(10):4130–5. doi: 10.1073/pnas.0610167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W, Wecker K, de Rocquigny H, Baudat Y, Sire J, Roques BP. NMR structure of the (52-96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J Mol Biol. 1999;285(5):2105–17. doi: 10.1006/jmbi.1998.2381. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Felber BK, Pavlakis GN. Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing. Virology. 1991;183(2):677–86. doi: 10.1016/0042-6822(91)90996-o. [DOI] [PubMed] [Google Scholar]
- Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, Stevenson M. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4(5):e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Bailes E, Stevenson M, Emerman M, Hahn BH. Gene acquisition in HIV and SIV. Nature. 1996;383(6601):586–7. doi: 10.1038/383586a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MP, Schubert U, Williams SA, de Noronha CM, Kreisberg JF, Henklein P, Greene WC. HIV-1 Vpr displays natural protein-transducing properties: implications for viral pathogenesis. Virology. 2002;302(1):95–105. doi: 10.1006/viro.2002.1576. [DOI] [PubMed] [Google Scholar]
- Shostak LD, Ludlow J, Fisk J, Pursell S, Rimel BJ, Nguyen D, Rosenblatt JD, Planelles V. Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr. Exp Cell Res. 1999;251(1):156–65. doi: 10.1006/excr.1999.4568. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Swanson SK, Manel N, Florens L, Washburn MP, Skowronski J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008;4(5):e1000059. doi: 10.1371/journal.ppat.1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74(7):3105–11. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Tanaka K, Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell. 2005;121(3):387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Tan L, Ehrlich E, Yu XF. DDB1 and Cul4A are required for human immunodeficiency virus type 1 Vpr-induced G2 arrest. J Virol. 2007;81(19):10822–30. doi: 10.1128/JVI.01380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 1992;11(9):3405–12. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12(5):835–40. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- Tungaturthi PK, Sawaya BE, Singh SP, Tomkowicz B, Ayyavoo V, Khalili K, Collman RG, Amini S, Srinivasan A. Role of HIV-1 Vpr in AIDS pathogenesis: relevance and implications of intravirion, intracellular and free Vpr. Biomed Pharmacother. 2003;57(1):20–4. doi: 10.1016/s0753-3322(02)00328-1. [DOI] [PubMed] [Google Scholar]
- Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, Kroemer G. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7(12):1146–54. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhai L, Xu J, Joo HY, Jackson S, Erdjument-Bromage H, Tempst P, Xiong Y, Zhang Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol Cell. 2006;22(3):383–94. doi: 10.1016/j.molcel.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukherjee S, Narayan O, Zhao LJ. Characterization of a leucine-zipper-like domain in Vpr protein of human immunodeficiency virus type 1. Gene. 1996;178(1-2):7–13. doi: 10.1016/0378-1119(96)00312-5. [DOI] [PubMed] [Google Scholar]
- Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110(4):1207–14. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5(10):e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Duus KM, Friedrich TD, de Noronha CM. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and Cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J Biol Chem. 2007;282(37):27046–57. doi: 10.1074/jbc.M703955200. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F, Chanda PK, Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987;3(1):33–9. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- Yu XF, Yu QC, Essex M, Lee TH. The vpx gene of simian immunodeficiency virus facilitates efficient viral replication in fresh lymphocytes and macrophage. J Virol. 1991;65(9):5088–91. doi: 10.1128/jvi.65.9.5088-5091.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Xie YM, Chen IS. Depletion of Wee-1 kinase is necessary for both human immunodeficiency virus type 1 Vpr- and gamma irradiation-induced apoptosis. J Virol. 2003;77(3):2063–70. doi: 10.1128/JVI.77.3.2063-2070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LJ, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994;269(22):15577–82. [PubMed] [Google Scholar]
- Zhu Y, Gelbard HA, Roshal M, Pursell S, Jamieson BD, Planelles V. Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes. J Virol. 2001;75(8):3791–801. doi: 10.1128/JVI.75.8.3791-3801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ES, Sherman MP, Blackett JL, Neidleman JA, Kreis C, Mundt P, Williams SA, Warmerdam M, Kahn J, Hecht FM, Grant RM, de Noronha CM, Weyrich AS, Greene WC, Planelles V. Human immunodeficiency virus type 1 Vpr induces DNA replication stress in vitro and in vivo. J Virol. 2006;80(21):10407–18. doi: 10.1128/JVI.01212-06. [DOI] [PMC free article] [PubMed] [Google Scholar]