Abstract
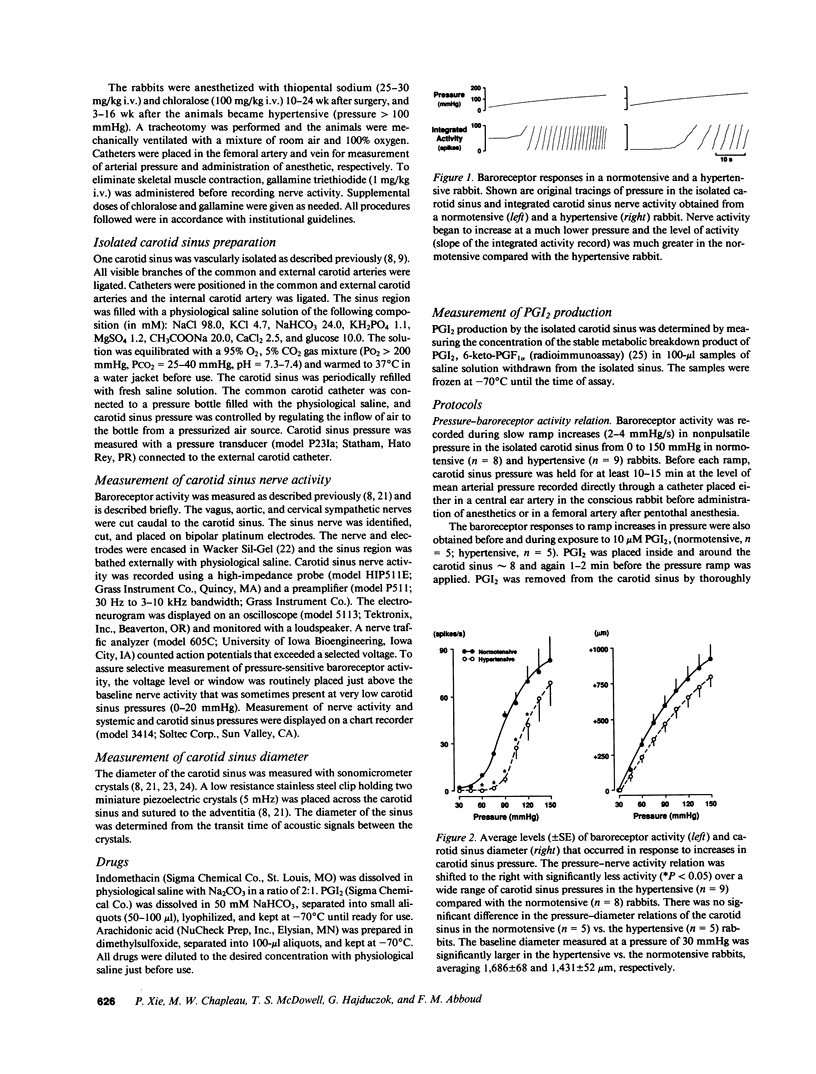
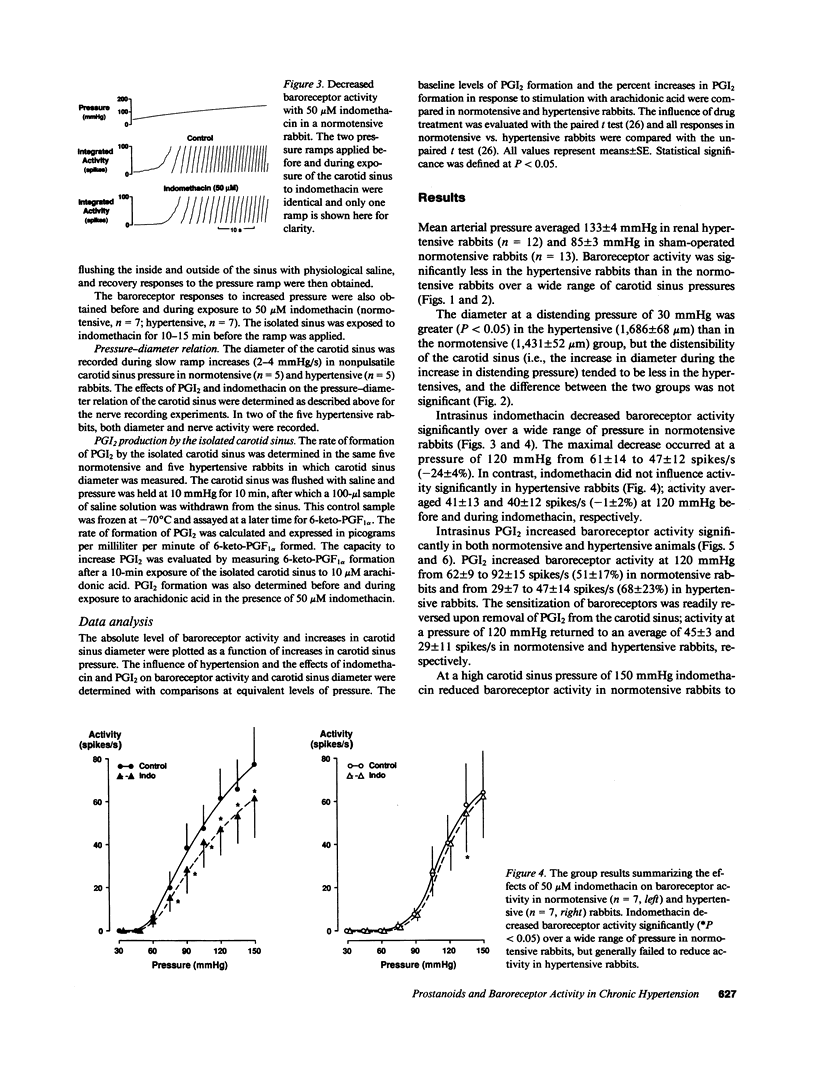
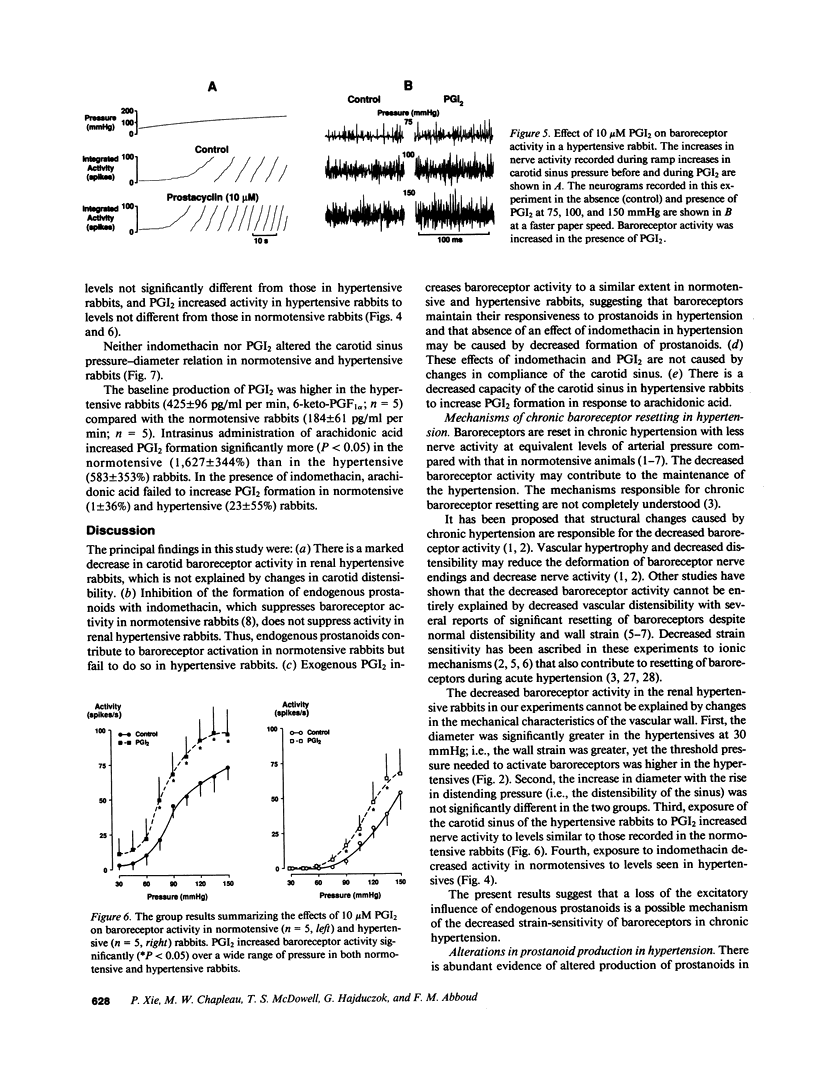
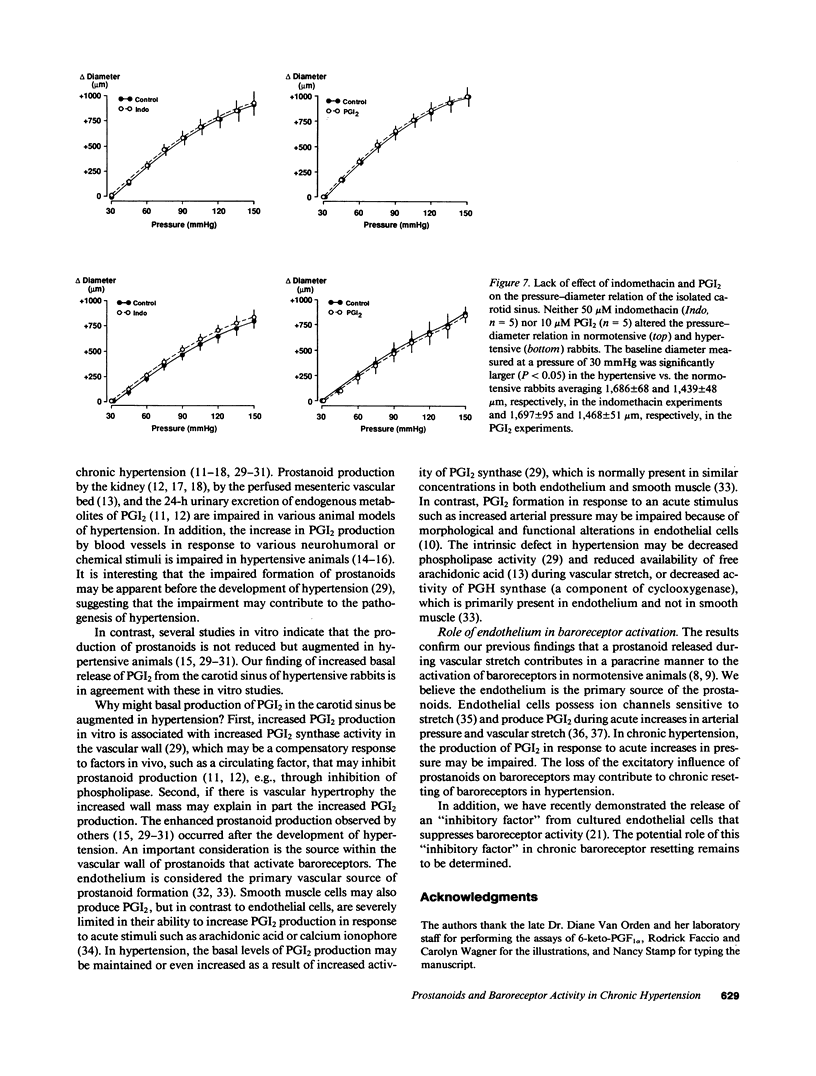
We examined the contribution of endogenous prostanoids to baroreceptor activation in chronic renal hypertension. Baroreceptor activity was recorded from the vascularly isolated carotid sinus during slow ramp increases in pressure in rabbits anesthetized with pentothal and chloralose. Mean arterial pressure averaged 133 +/- 4 mmHg in hypertensive rabbits (one kidney, one wrap, n = 12) and 85 +/- 3 mmHg in normotensive rabbits (one kidney, no wrap, n = 13). Baroreceptor activity was decreased significantly (P less than 0.05) in the hypertensive compared with the normotensive rabbits. The decreased baroreceptor activity could not be explained by decreased distensibility of the carotid sinus (sonomicrometers). Inhibition of the endogenous formation of prostanoids with intrasinus administration of indomethacin (50 microM) decreased baroreceptor activity in normotensive (P less than 0.05) but not in hypertensive rabbits over a wide range of pressures. At a pressure of 120 mmHg, activity declined from 61 +/- 14 spikes/s before indomethacin to 47 +/- 12 spikes/s with indomethacin, i.e., a drop of 24 +/- 4%. In contrast, corresponding values in hypertensive rabbits averaged 41 +/- 13 and 40 +/- 12 spikes/s (-1 +/- 2%). Intrasinus prostacyclin, on the other hand, increased activity in both groups: at 120 mmHg activity increased from 62 +/- 9 to 92 +/- 15 spikes/s (51 +/- 17%) in normotensive rabbits and from 29+/- 7 to 47 +/- 14 spikes/s (68 +/- 23%) in hypertensive rabbits. Neither indomethacin nor prostacyclin (n = 5) influenced the pressure-diameter relation of the carotid sinus. The increase in prostacyclin (6-keto-PGF 1 alpha) formation by the sinus in response to its exposure to arachidonic acid (10 microM) was significant (P less than 0.05) in the normotensives (1,627 +/- 344%; n = 5) but not in the hypertensives (583 +/- 353%; n = 5). We conclude that the decreased baroreceptor activity in chronic hypertension may not be caused by decreased distensibility of the vascular wall of the sinus and that endogenous prostanoids that contribute to baroreceptor activation in normotensive rabbits fail to do so in hypertensive rabbits. This appears to be due to decreased formation of prostacyclin rather than decreased sensitivity of the baroreceptors to prostacyclin. The results suggest a new mechanism that contributes to chronic baroreceptor resetting in hypertension.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andresen M. C., Kuraoka S., Brown A. M. Baroreceptor function and changes in strain sensitivity in normotensive and spontaneously hypertensive rats. Circ Res. 1980 Dec;47(6):821–828. doi: 10.1161/01.res.47.6.821. [DOI] [PubMed] [Google Scholar]
- Andresen M. C. Short- and long-term determinants of baroreceptor function in aged normotensive and spontaneously hypertensive rats. Circ Res. 1984 Jun;54(6):750–759. doi: 10.1161/01.res.54.6.750. [DOI] [PubMed] [Google Scholar]
- Angell-James J. E. Characteristics of single aortic and right subclavian baroreceptor fiber activity in rabbits with chronic renal hypertension. Circ Res. 1973 Feb;32(2):149–161. doi: 10.1161/01.res.32.2.149. [DOI] [PubMed] [Google Scholar]
- Armstrong J. M., Blackwell G. J., Flower R. J., McGiff J. C., Mullane K. M., Vane J. R. Genetic hypertension in rats is accompanied by a defect in renal prostaglandin catabolism. Nature. 1976 Apr 15;260(5552):582–586. doi: 10.1038/260582a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Saum W. R., Tuley F. H. A comparison of aortic baroreceptor discharge in normotensive and spontaneously hypertensive rats. Circ Res. 1976 Oct;39(4):488–496. doi: 10.1161/01.res.39.4.488. [DOI] [PubMed] [Google Scholar]
- Chapleau M. W., Hajduczok G., Abboud F. M. Mechanisms of resetting of arterial baroreceptors: an overview. Am J Med Sci. 1988 Apr;295(4):327–334. doi: 10.1097/00000441-198804000-00019. [DOI] [PubMed] [Google Scholar]
- Chapleau M. W., Hajduczok G., Shasby D. M., Abboud F. M. Activated endothelial cells in culture suppress baroreceptors in the carotid sinus of dog. Hypertension. 1988 Jun;11(6 Pt 2):586–590. doi: 10.1161/01.hyp.11.6.586. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Day J. S., Sonnenburg W. K., Smith W. L. Concentrations of prostaglandin endoperoxide synthase and prostaglandin I2 synthase in the endothelium and smooth muscle of bovine aorta. J Clin Invest. 1983 Dec;72(6):1882–1888. doi: 10.1172/JCI111151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demolle D., Boeynaems J. M. Prostacyclin production by the bovine aortic smooth muscle. Prostaglandins. 1986 Jul;32(1):155–159. doi: 10.1016/0090-6980(86)90160-7. [DOI] [PubMed] [Google Scholar]
- Falardeau P., Martineau A. In vivo production of prostaglandin I2 in Dahl salt-sensitive and salt-resistant rats. Hypertension. 1983 Sep-Oct;5(5):701–705. doi: 10.1161/01.hyp.5.5.701. [DOI] [PubMed] [Google Scholar]
- Farley D. B., Van Orden D. E. Effect of prostacyclin inhibition by tranylcypromine on uterine 6-keto-pgf 1 alpha levels during estrogen hyperemia in rats. Prostaglandins. 1982 May;23(5):657–674. doi: 10.1016/s0090-6980(82)80005-1. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Grant R. T., Rothschild P. A device for estimating blood-pressure in the rabbit. J Physiol. 1934 May 21;81(2):265–269. doi: 10.1113/jphysiol.1934.sp003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesch C. M., Abboud F. M., Thames M. D. Acute resetting of carotid sinus baroreceptors. II. Possible involvement of electrogenic Na+ pump. Am J Physiol. 1984 Nov;247(5 Pt 2):H833–H839. doi: 10.1152/ajpheart.1984.247.5.H833. [DOI] [PubMed] [Google Scholar]
- Heesch C. M., Thames M. D., Abboud F. M. Acute resetting of carotid sinus baroreceptors. I. Dissociation between discharge and wall changes. Am J Physiol. 1984 Nov;247(5 Pt 2):H824–H832. doi: 10.1152/ajpheart.1984.247.5.H824. [DOI] [PubMed] [Google Scholar]
- Koushanpour E., Behnia R. Partition of carotid baroreceptor response in two-kidney renal hypertensive dogs. Am J Physiol. 1987 Oct;253(4 Pt 2):R568–R575. doi: 10.1152/ajpregu.1987.253.4.R568. [DOI] [PubMed] [Google Scholar]
- Lamping K. G., Marcus M. L., Dole W. P. Removal of the endothelium potentiates canine large coronary artery constrictor responses to 5-hydroxytryptamine in vivo. Circ Res. 1985 Jul;57(1):46–54. doi: 10.1161/01.res.57.1.46. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hallam T. J., Rink T. J. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? 1987 Feb 26-Mar 4Nature. 325(6107):811–813. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- Leary W. P., Ledingham J. G., Vane J. R. Impaired prostaglandin release from the kidneys of salt-loaded and hypertensive rats. Prostaglandins. 1974 Sep 10;7(5):425–432. doi: 10.1016/0090-6980(74)90107-5. [DOI] [PubMed] [Google Scholar]
- Lennon E. A., Poyser N. L. Production of prostaglandins I2, E2 and F2 alpha by blood vessels of normotensive and hypertensive, male and female rats. Prostaglandins Leukot Med. 1986 Dec;25(2-3):71–89. doi: 10.1016/0262-1746(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Limas C. J., Limas C. Vascular prostaglandin synthesis in the spontaneously hypertensive rat. Am J Physiol. 1977 Oct;233(4):H493–H499. doi: 10.1152/ajpheart.1977.233.4.H493. [DOI] [PubMed] [Google Scholar]
- Lukacsko P. Effect of arachidonic acid on the basal release of prostaglandins E2 and I2 by rat arteries during the development of hypertension. Clin Exp Hypertens A. 1983;5(9):1471–1483. doi: 10.3109/10641968309069505. [DOI] [PubMed] [Google Scholar]
- MCCUBBIN J. W., GREEN J. H., PAGE I. H. Baroceptor function in chronic renal hypertension. Circ Res. 1956 Mar;4(2):205–210. doi: 10.1161/01.res.4.2.205. [DOI] [PubMed] [Google Scholar]
- McDowell T. S., Axtelle T. S., Chapleau M. W., Abboud F. M. Prostaglandins in carotid sinus enhance baroreflex in rabbits. Am J Physiol. 1989 Aug;257(2 Pt 2):R445–R450. doi: 10.1152/ajpregu.1989.257.2.R445. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Pagani M., Baig H., Sherman A., Manders W. T., Quinn P., Patrick T., Franklin D., Vatner S. F. Measurement of multiple simultaneous small dimensions and study of arterial pressure-dimension relations in conscious animals. Am J Physiol. 1978 Nov;235(5):H610–H617. doi: 10.1152/ajpheart.1978.235.5.H610. [DOI] [PubMed] [Google Scholar]
- Pipili E., Poyser N. L. Release of prostaglandins I2 and E2 from the perfused mesenteric arterial bed of normotensive and hypertensive rats. Effects of sympathetic nerve stimulation and norepinephrine administration. Prostaglandins. 1982 Apr;23(4):543–549. doi: 10.1016/0090-6980(82)90114-9. [DOI] [PubMed] [Google Scholar]
- Ricksten S. E., Thoren P. Reflex inhibition of sympathetic activity during volume load in awake normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1980 Sep;110(1):77–82. doi: 10.1111/j.1748-1716.1980.tb06632.x. [DOI] [PubMed] [Google Scholar]
- Rioux F., Quirion R., Regoli D. The role of prostaglandins in hypertension. I. The release of prostaglandins by aorta strips of renal, DOCA-salt, and spontaneously hypertensive rats. Can J Physiol Pharmacol. 1977 Dec;55(6):1330–1338. doi: 10.1139/y77-178. [DOI] [PubMed] [Google Scholar]
- Soma M., Manku M. S., Jenkins D. K., Horrobin D. F. Prostaglandins and thromboxane outflow from the perfused mesenteric vascular bed in spontaneously hypertensive rats. Prostaglandins. 1985 Feb;29(2):323–333. doi: 10.1016/0090-6980(85)90212-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Numabe A., Hirawa N., Ishimitsu T., Takada S., Sugimoto T., Yagi S. Alterations to the vascular vasodepressor prostaglandin system in DOCA-salt hypertensive rats and their enzymatic analysis. J Hypertens Suppl. 1988 Dec;6(4):S392–S394. doi: 10.1097/00004872-198812040-00123. [DOI] [PubMed] [Google Scholar]


