Abstract
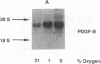
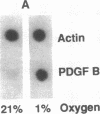
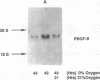
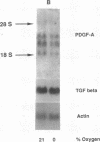
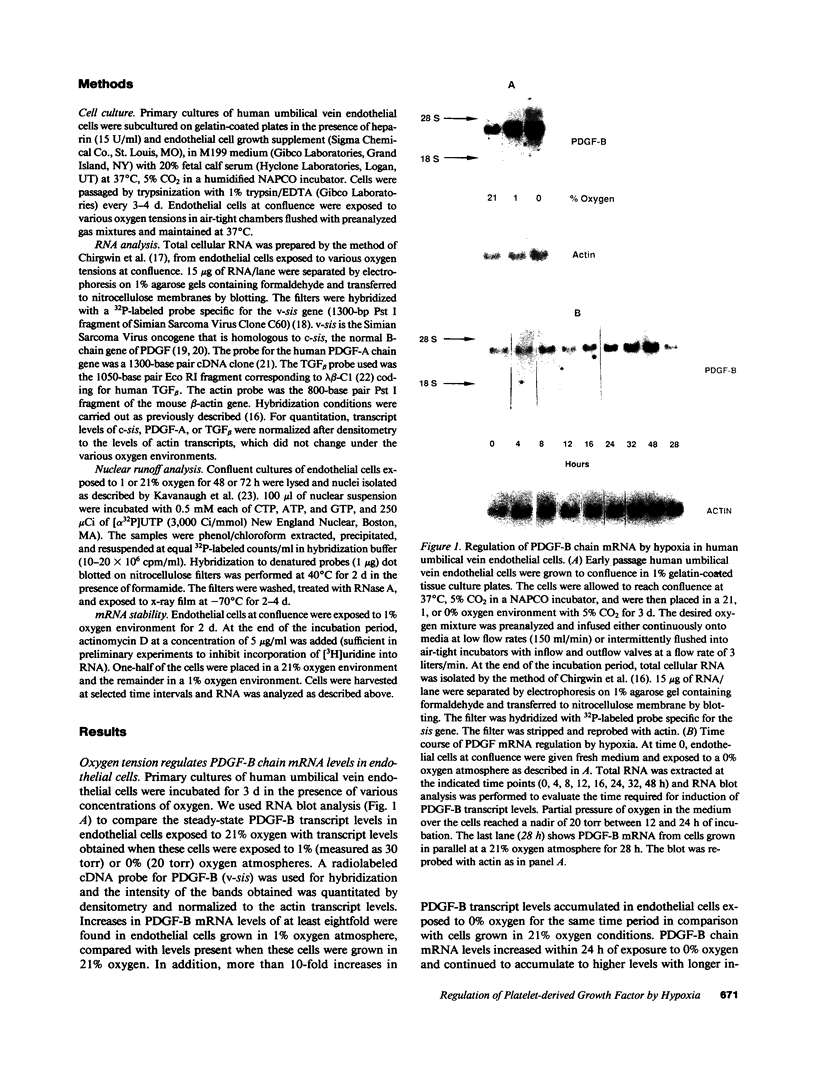
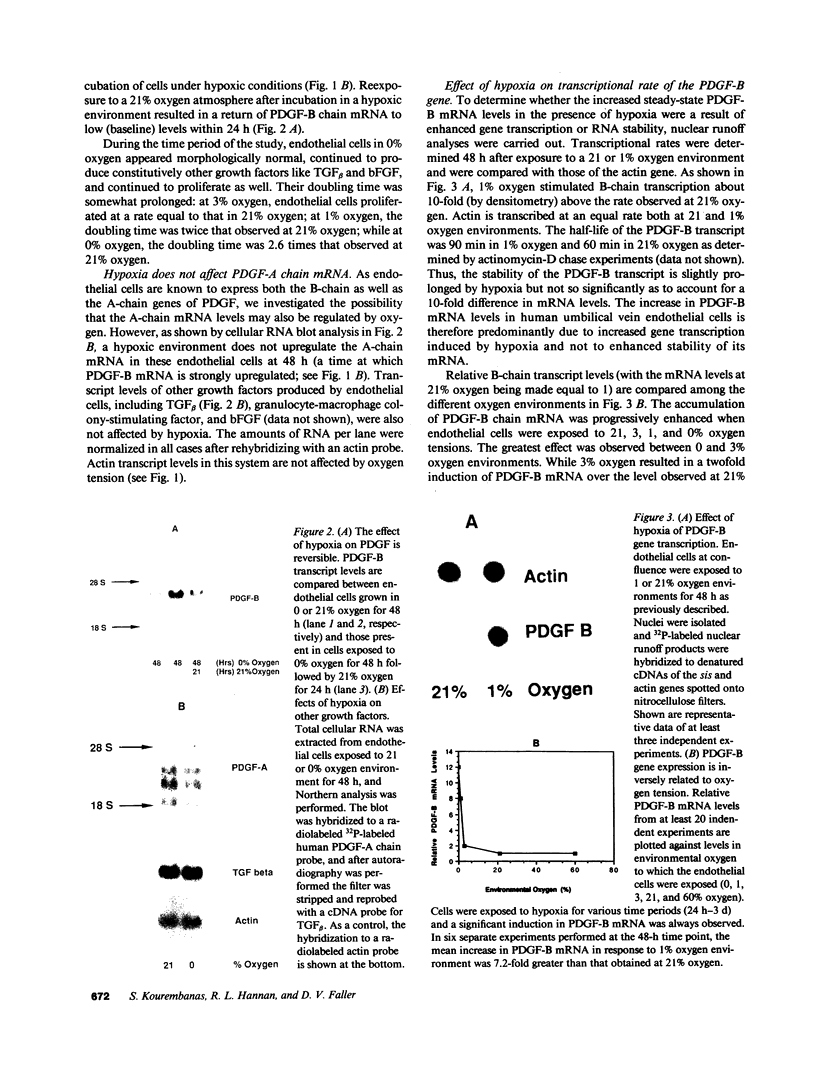
Hypoxic states are associated with abnormal proliferation and constriction of the smooth muscle cells surrounding the distal vessels of the lung. In hypoxic as well as in normal states, the endothelial cell layer may play a key role in controlling smooth muscle tone by secreting a number of vasoactive agents. Platelet-derived growth factor (PDGF), produced by endothelial cells, is a major growth factor for vascular smooth muscle cells and a powerful vasoconstrictor. It consists of a disulfide-linked dimer of two related peptides, A and B, that are products of two different genes. We found that hypoxic conditions (0-3% oxygen environments) significantly increased PDGF-B mRNA in cultured human umbilical vein endothelial cells by enhancing the transcriptional rate of this gene. This increase was inversely proportional to oxygen tension and was reversible upon reexposure of cells to a 21% oxygen atmosphere. mRNA levels of PDGF-A were not affected nor was the overall rate of cellular gene transcription increased in response to hypoxia. These studies indicate that endothelial cells are not only capable of sensing oxygen tension, but are also able to discriminate and respond to even small differences in oxygen tension resulting in dramatic upregulation of the PDGF-B chain gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Brown A., Colen A. H., Fisher H. F. Effect of ammonia on the glutamate dehydrogenase catalyzed oxidative deamination of L-glutamate. The steady state. Biochemistry. 1979 Dec 25;18(26):5924–5928. doi: 10.1021/bi00593a025. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Williams L. T. Agents that increase cAMP accumulation block endothelial c-sis induction by thrombin and transforming growth factor-beta. J Biol Chem. 1987 Sep 5;262(25):11893–11896. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S. Roles of growth factor activities in oncogenesis. Blood. 1984 Nov;64(5):951–958. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Petri E., Cetta A., Wong-Staal F. Deletions of specific regions of the simian sarcoma-associated virus genome are found in defective viruses and in the simian sarcoma virus. J Virol. 1982 Feb;41(2):593–604. doi: 10.1128/jvi.41.2.593-604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Chang T., Seppä H. E., Kleinman H. K., Martin G. R. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982 Nov;113(2):261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey K. A., Rubanyi G., Paul R. J., Highsmith R. F. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985 May;248(5 Pt 1):C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Humphries D. E., Lee S. L., Fanburg B. L., Silbert J. E. Effects of hypoxia and hyperoxia on proteoglycan production by bovine pulmonary artery endothelial cells. J Cell Physiol. 1986 Feb;126(2):249–253. doi: 10.1002/jcp.1041260214. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Harsh G. R., 4th, Starksen N. F., Rocco C. M., Williams L. T. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J Biol Chem. 1988 Jun 15;263(17):8470–8472. [PubMed] [Google Scholar]
- Kourembanas S., Faller D. V. Platelet-derived growth factor production by human umbilical vein endothelial cells is regulated by basic fibroblast growth factor. J Biol Chem. 1989 Mar 15;264(8):4456–4459. [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res. 1987 May;60(5):653–658. doi: 10.1161/01.res.60.5.653. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol. 1986 May;250(5 Pt 1):C766–C770. doi: 10.1152/ajpcell.1986.250.5.C766. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Hypoxia releases a vasoconstrictor substance from the canine vascular endothelium. J Physiol. 1985 Jul;364:45–56. doi: 10.1113/jphysiol.1985.sp015728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starksen N. F., Harsh G. R., 4th, Gibbs V. C., Williams L. T. Regulated expression of the platelet-derived growth factor A chain gene in microvascular endothelial cells. J Biol Chem. 1987 Oct 25;262(30):14381–14384. [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]