Abstract
Rapid thermal carbonization in a dilute acetylene (C2H2) atmosphere has been used to chemically modify and precisely tune the pore size of ultrathin porous nanocrystalline silicon (pnc-Si). The magnitude of size reduction was controlled by varying the process temperature and time. Under certain conditions, the carbon coating displayed atomic ordering indicative of graphene layer formation conformal to the pore walls. Initial experiments show that carbonized membranes follow theoretical predictions for hydraulic permeability and retain the precise separation capabilities of untreated membranes.
Porous nanocrystalline silicon (pnc-Si) membranes are a promising material for a broad range of applications including filtration, cell culture substrates, and as a platform for electron microscopy and spectroscopy1, 2. This material is over an order of magnitude thinner than the thinnest electrochemically etched porous silicon (PSi) membranes3. Because the thickness of the membrane is only 15 nm, on the order of species to be separated, mass transport through the membrane is greatly enhanced as compared to traditional tortuous path polymer membranes that are typically many microns in thickness4, 5. Using these ultra-thin pnc-Si membranes, precise and low-loss separation of nanoparticles has been demonstrated in diffusion1 and convection6. These membranes use a bottom-up approach, rather than the expensive top-down approach used to manufacture other nanomembranes7–9.
Using standard microfabrication techniques, pnc-Si pore sizes can be tuned from being nonporous to having pores greater than 80 nm, however the resolution of control over pore size during manufacturing is limited to ± 5 nm. In order to optimize the quality of a separation procedure, finer control over pore sizes is desirable. Here we demonstrate that carbonization can be used as a post-production means to tune pore sizes with sub-nanometer precision thus allowing for further optimization of the sieving properties of the membrane. Under certain conditions, atomic ordering is observed along the pore walls suggesting graphene formation during carbonization. Given the high fluxes of water and gas through carbon nanotube (CNT) membranes, carbonization of pnc-Si may lead to novel silicon-carbon hybrid devices with enhanced transport properties over traditional technologies10–12. Consistent with our prior work involving untreated pnc-Si membranes6, the hydraulic permeability of a carbonized membrane is shown to follow theoretical predictions. We also demonstrate the separation of gold nanoparticles before and after carbonization and find that carbonized membranes retain the precision separation characteristics of untreated membranes6. Furthermore, we show that carbonized pnc-Si membranes can be used to effectively filter proteins of different sizes. Thus, carbonization of pnc-Si provides an additional path for pore size control without negative impact on the performance of pnc-Si as a size-selective filter. Finally, we find that carbonization can significantly improve chemical stability in highly alkaline solutions that rapidly etch silicon.
Previous techniques to control the size of nanoporous membranes involve coating pores with polymers13 or metal14. While polymer coatings are relatively easy to deposit, they are unable to withstand high temperatures and harsh chemical environments. Metal coatings are more resilient, but processing can be complicated and take a long time to complete15. Pore modification via rapid thermal carbonization suffers from neither of these drawbacks. Carbonization of PSi has been reported to improve chemical stability16 in extremely basic environments by displacing Si-H bonds with Si-C. Furthermore, a recent study has shown that thermally carbonized PSi is bio-compatible and non-toxic17. Functionalization of the PSi surface has been verified by various methods such as differential scanning calorimetry (DSC) and Fourier transform infrared spectrometry (FTIR)18 or photoluminescence measurements19. Although PSi and pnc-Si are different materials structurally, they both have Si-H terminations that can be replaced by Si-C during carbonization. Thus, we anticipated that pnc-Si would behave similarly during carbonization and inherit the improvements in stability observed with PSi. In this study, we relied on high-resolution transmission electron microscopy (HRTEM) and energy dispersive x-ray spectroscopy (EDX) for insight on how carbonization affects these ultrathin porous structures. Pore size distributions were obtained directly from the TEM micrographs using a custom pore recognition script written in MATLAB as previously described (available for download at nanomembranes.org/resources/software, Figure S-1)6.
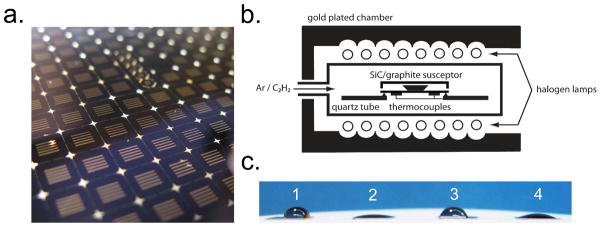
Carbonization of porous nanocrystalline silicon membranes was carried out in a Surface Science Integration Solaris 150 rapid thermal processor (El Mirage, AZ) inside a silicon carbide-coated graphite susceptor (Figure 1a). The rapid thermal processor is capable of reaching temperatures of 1200°C in less than 10 seconds and allows for the introduction of process gases via two mass flow controllers (Figure 1b). In this work, acetylene (C2H2) was chosen as the reactive hydrocarbon. This carbonization process was completed in less than 20 minutes as compared to the hours-long procedures using chemical vapor deposition reported in previous studies16, 19. A five-minute argon flush was performed before and after every thermal cycle to ensure the quartz chamber remained free of contaminant gases such as oxygen. After treatment, the contact angle of a carbonized membrane was measured (Figure 1c). Consistent with prior work, carbonization decreased the contact angle slightly, from 76° to 60°, after treatment16.
Figure 1.
Preparation of carbonized membranes. (a) Porous nanocrystalline silicon membrane chips fabricated on a 4-inch wafer. The active membrane area appears as five slits in the middle of the bulk silicon scaffold. The ultrathin porous nanocrystalline silicon membrane is 15 nm thick. (b) Schematic of the rapid thermal processing unit used for carbonization. Membrane chips are placed inside a SiC coated graphite susceptor. A mixture of argon and acetylene is introduced into the chamber and heated to high temperatures. Two thermocouples monitor the temperature uniformity of the process. (c) (1) Contact angles of an untreated (76°), (2) ozone treated (16°), (3) carbonized (60°), and (4) carbonized and ozone treated (22°) membrane.
Several groups have studied acetylene absorption onto silicon substrates through modeling20, 21 as well as experimentally22, 23. In these studies, it was determined that below 600°C, acetylene is chemisorbed at the surface. At 660°C, hydrogen disassociates and leaves the surface in the form of H2. At these elevated temperatures, carbon is free to diffuse into the subsurface and bond with silicon forming a Si1-xCx compound. At temperatures beyond 750°C graphitization occurs. Salonen et al. showed experimentally that carbonization was highly temperature dependent18. Below 600°C, FTIR measurements showed traces of C-Hx bonding in porous silicon. Above 600°C, the spectra indicated a transition to a SiC bonding configuration.
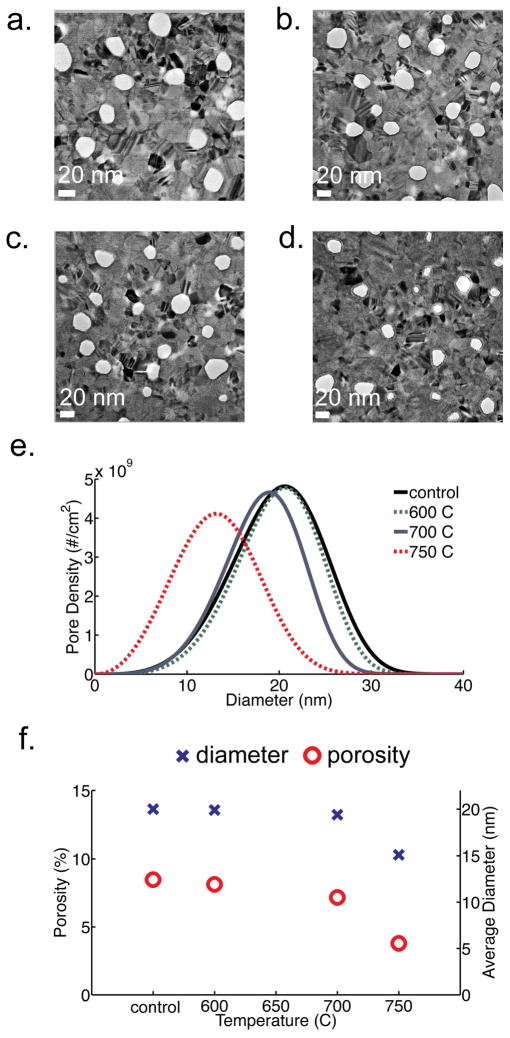
In this work, we have determined that the degree of pore closure from carbonization in pnc-Si is also highly temperature dependent (Figure 2). Membranes were fabricated identically, as described in Striemer et al.1, and each treated with acetylene at four different temperatures in the rapid thermal processor. To verify that the pore structure is only affected when acetylene is present, a control sample was annealed at 800°C for five minutes in argon only2. Electron microscopy and pore histogram analysis was performed on this membrane and we found that the mean diameter did not change by a thermal treatment in argon alone (Figure S-2). The soak time for all carbonization trials was five minutes and the argon and acetylene flow was fixed at 9 slm and 1 slm, respectively. Below 600°C, we did not observe a significant deposition of carbon along the pore walls. However, between 600°C and 700°C a noticeable carbon coating formed. At 750°C the rate of carbon growth increase nearly seven-fold and the average pore size and porosity of the membrane decreased considerably. At 800°C, the majority of the pores were occluded.
Figure 2.
TEM images of carbonized membranes at different temperatures: (a) untreated, (b) 600°C, (c) 700°C, (d) 750°C. Scale bar is 20 nm. A 10% acetylene in argon mixture was used with a five minute soak time. (e) Pore size distribution as calculated from TEM images (f) Graph showing size (blue ‘X’) and porosity dependence (red ‘O’) with soak temperature. The standard deviation of porosity and diameter is 0.25% and 0.5 nm, respectively.
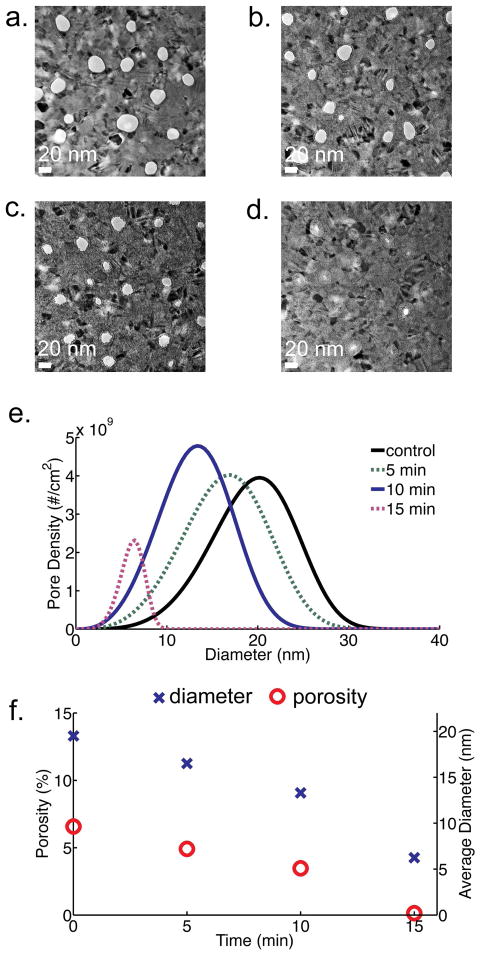
To investigate the effect of soak time on carbonization of pnc-Si membranes, three identically fabricated membranes were exposed to acetylene for 5, 10, and 15 minutes. The process temperature was fixed at 750°C with an argon to acetylene flow of 9 slm to 1 slm. By varying the soak time, we have found that pore diameters can be reduced by 5 nm after 5 minutes, 10 nm after 10 minutes, and greater than 15 nm after 15 minutes (Figure 3). The growth rate appears to be constant through 10 minutes except at the 15 minute time point where it increases slightly (Figure 3f). The approximate growth rate was calculated to be 3.1 Å/min by measuring the amount of carbon growth after 10 minutes. We hypothesize that after an initial seeding layer, carbon growth is eventually restricted by the mismatch strain due to the silicon and is governed by a new kinetic rate constant24. Carbon growth also appears to be independent of pore size (Figure S-3), suggesting that there is no self-limiting behavior in the process.
Figure 3.
TEM images of carbonized membranes with 10% of C2H2 at 750°C and various soak times: (a) untreated, (b) 5 minutes, (c) 10 minutes. A 15-minute soak time (d) occluded the majority of pores. Scale bar is 20 nm. (e) Pore size distribution as calculated from TEM images (f) Graph showing size (blue ‘X’) and porosity (red ‘O’) dependence with treatment time. The standard deviation of porosity and diameter is 0.3% and 0.7 nm, respectively.
Energy-dispersive x-ray spectroscopy (EDS) was performed on a carbonized pore and showed a high density of carbon coating the inner pore wall (Figure 4 inset). A closer look at the carbon coating reveals atomic ordering of a few nanometers from the edge of the pore (Figure 4). The lattice spacing was measured to be 3.6 Å, consistent with the spacing between graphene layers25. We hypothesize that the first carbon layers that adhere to the silicon pore are stressed due to the lattice mismatch and thus grow as an amorphous Si1-xCx film. At a critical thickness, the carbon atoms are able to arrange themselves at their characteristic bond length to form ordered layers of graphene. This supports the observation that the growth rate increases with longer carbonization times, possibly due to the transition from Si1-xCx to graphene formation. We envision that pnc-Si may be used as a platform to create novel hybrid carbon-silicon nanostructures with superior stability, transport and separation properties as compared to existing technologies.
Figure 4.
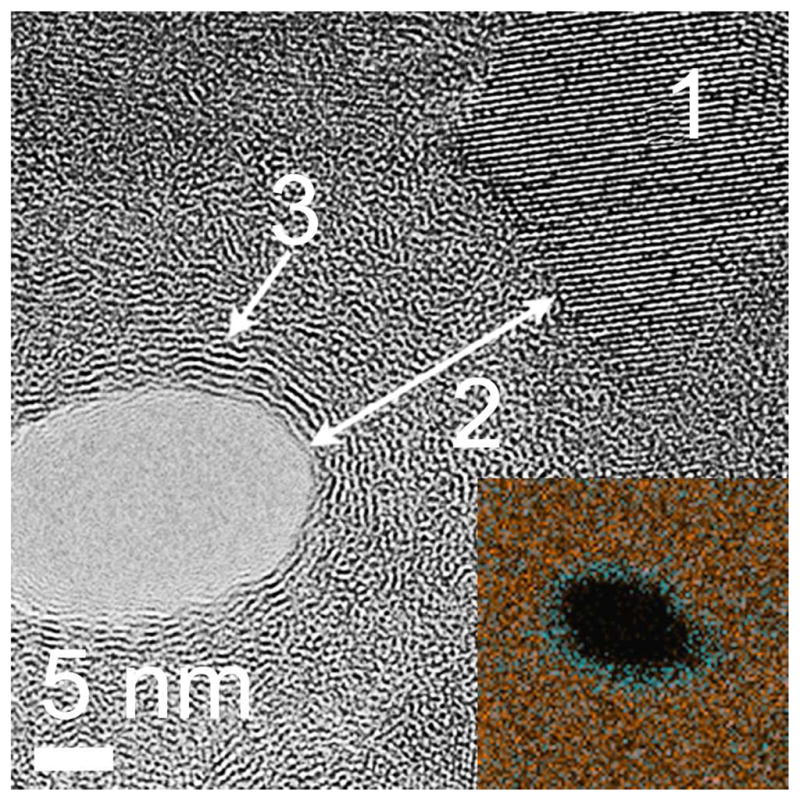
High-resolution bright field TEM images of a carbonized pore. The inset shows an x-ray energy dispersive x-ray (EDX) scan of a single carbonized pore. The EDX scan highlights areas of Si (red) and C (blue) composition. Label 1 marks a <111> oriented Si nanocrystal at the perimeter of the original pore wall. The carbon layer is highlighted by the arrow (2). Atomic ordering can be seen far from the original pore wall consistent with the spacing (3.6 Å) between sheets of graphite (3).
As previously demonstrated, carbonization of electrochemically etched porous silicon has improved chemical stability in highly basic etchants such as KOH26. We have also observed an improvement in pnc-Si membrane stability to harsh alkaline environments after carbonization. Carbonized and untreated samples were soaked in 5% (w/v) KOH (Mallinckrodt Baker, Phillipsburg, NJ) at 40°C for one hour. After this time, the untreated membrane had completely dissolved while the carbonized membrane remained intact (S-4). From these results, we can conclude that carbonization not only coats the inner pore walls of the pnc-Si membrane, but also the planar membrane surface, providing a high quality protective layer for high pH environments experiments and applications. This also confirms that carbonization coats the surface continuously as we did not observe any areas of chemical attack in the carbonized films.
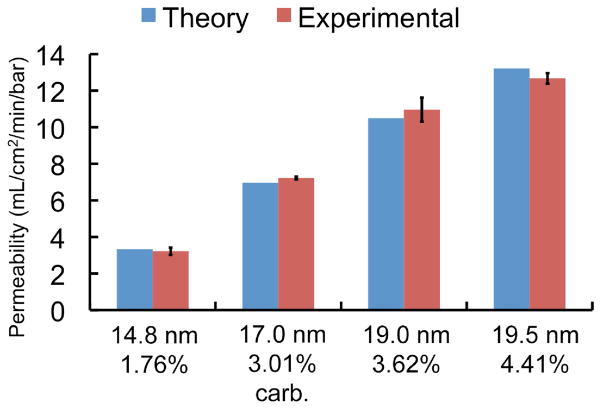
A hydraulic permeability study6 was performed to determine whether carbonization would impact water transport through the membranes (Figure 5). All samples were first ozone treated (Novascan PSD-UVT, Ames, IA) for ten minutes at 120°C in order to create a more hydrophilic surface that allowed complete wetting of the pores (Figure S-5)6. We have found that without ozone treatment, hydraulic permeability is hindered due to incomplete wetting of the nanopores. Specially fabricated membranes were installed into a custom plastic enclosure (SiMPore, Inc., West Henrietta, NY) and water was pressurized through the membrane. Three untreated membranes of varying porosities were first tested and compared to the theoretical prediction using the Dagan equation27 with pore distributions measured by TEM as outlined in Gaborski et al.6 A carbonized membrane was then tested and demonstrated permeability consistent with standard theory. In the calculation of theoretical permeability, we accounted for the slight increase in membrane thickness after carbonization. Unlike CNT membranes10, 12, we found that carbonized pnc-Si does not show an enhancement in water flow. We believe that the inherently high permeability of the untreated membranes can only be marginally improved by the very smooth graphene walls from which CNT membranes derive their enhancement.
Figure 5.
Hydraulic permeability of untreated and carbonized pnc-Si membranes. The permeability of a carbonized membrane matches the theoretical prediction. The average pore diameter and porosity for each membrane are shown under each bar. The error bars represent the measurement error in flow. It does not include the variability in the pore size calculation from TEM micrographs.
To demonstrate the separation capabilities of a carbonized pnc-Si membrane, carbonized and untreated membranes were tested for their ability to separate differently sized gold colloids (British BioCell International, Cardiff, UK) as previously shown for untreated membranes6. The same pressure cell used in the hydraulic permeability study was utilized for the nanoparticle separation. A 1:1 dilution of gold stock (0.01% w/v) to DI water was loaded into the pressure cell and the filtrate was collected for analysis using an absorbance plate reader (Tecan Group, Männedorf, Switzerland). As Table 1 demonstrates, a carbonized membrane has the ability to separate 6.6 nm from 7.9 nm gold nanoparticles while the untreated membrane passes both the 6.6 nm and 7.9 nm gold but retains 12.7 nm colloids. The sieving coefficients were experimentally calculated by taking the ratio between the filtrate and gold stock concentration. We were successful in reducing the effective membrane cutoff by coating the pore walls with 3 nm of carbon. The separation was not as sharp as the untreated membrane and may be due to the slight distribution in gold nanoparticle sizes and the non-optimized pore size distribution of the carbonized membrane.
Table 1.
Nanoparticle sieving coefficients (Cfiltrate/C0) of an untreated and carbonized membrane
| Avg. gold colloid size measured by TEM |
|||
|---|---|---|---|
| 6.6 nm | 7.9 nm | 12.7 nm | |
| untreated | |||
| avg. dia. 20.7 nm |
0.96 | 0.95 | 0.08 |
| carbonized | |||
| avg. dia. 17.0 nm |
0.68 | 0.22 | n.d.* |
Not detectable. Concentration was less than limit of detection (< 0.05 mg/mL)
To further demonstrate the potential of carbonized pnc-Si membranes for molecular sieving, an experiment involving three differently sized proteins was performed using a setup similar to that in the gold nanoparticle experiments. Each protein was filtered independently using identical carbonized membranes so that a sieving coefficient could be calculated using the same method employed in the nanoparticle trials. Cytochrome c, bovine serum albumin, or β-galactosidase at 1 mg/mL (w/v) concentration was placed inside the pressure cell and the solution on the filtrate side was recovered for analysis using the absorbance plate reader. Table 2 shows the protein sieving coefficient along with the molecular weight and hydrodynamic size of the proteins as determined by a dynamic light scattering system (Malvern ZetaSizer Nano ZS, Worcestershire, UK)28. The results indicate that the smallest protein, cytochrome c passed through relatively unhindered, while albumin transport was hindered, and β-galactosidase was completely held back.
Table 2.
Protein sieving coefficients (Cfiltrate/C0) of carbonized membranes with an average diameter of 17.8 nm
| Protein | MW | Diameter (nm) | Cfiltrate/C0 |
|---|---|---|---|
| Cytochrome c | 14 kD | 4.2 ± 1 | 0.73 |
| Albumin | 67 kD | 11.1 ± 4 | 0.39 |
| β-galactosidase | 460 kD | 15.5 ± 4.8 | n.d.* |
Not detectable. Concentration was less than limit of detection (< 0.05 mg/mL)
In this work, we have demonstrated a novel method for coating silicon nanopores with carbon. The thickness of the coating can be controlled with sub-nanometer precision by varying soak temperature and soak time. Pore diameters can be reduced by as little as 1 nm to greater than 25 nm for complete occlusion, thus allowing for more careful control over sieving characteristics. Hydraulic permeability studies have shown that carbonized membranes follow theoretical predictions for water transport and retain the nanoparticle separation capabilities of the untreated ultrathin membrane. Additionally, we have shown the potential use of carbonized pnc-Si membranes in molecular separations. These results demonstrate that carbonization is a simple and effective method for significantly improving the chemical stability of ultrathin silicon membranes, enables precise tuning of nanopores, and should have use in a wide range of sieving applications.
Supplementary Material
Acknowledgments
The authors wish to acknowledge J. Snyder for useful discussions and assistance with protein preparation; M. Bindschadler's work on the pore processing program (available for download at http://nanomembranes.org/resources/software); B. McIntyre and K. Bentley for microscopy assistance; P. Osborne for equipment fabrication assistance; J. P. DesOrmeaux and C. Chan of SiMPore for fabrication assistance; N. Nataraj of SiMPore for characterization work; and Y. Fu for spectroscopic ellipsometer data acquisition. Silicon microprocessing was conducted at the University of Rochester Hopeman Microfabrication Facility (HMF) and the Semiconductor and Microsystems Fabrication Laboratory (SMFL) at the Rochester Institute of Technology. This work was supported by the National Institutes of Health (R21EB006149; R21EB007480), NYSTAR (TTIP C020040), ARO (DAAD19-03-1-0267), and the National Science Foundation (DMR0722653; ECCS 0707795). As founders of SiMPore Inc., C.C.S., T.R.G., J.L.M., and P.M.F. declare a competing financial interest in this work.
Footnotes
Supporting Information Available: Supplemental information on pore size distribution calculation, ozone treatment of membranes, chemical stability, theoretical calculation of hydraulic permeability, and data on gold nanoparticle and protein filtration is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Nature. 2007;445(7129):749–753. doi: 10.1038/nature05532. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal AA, Nehilla BJ, Reisig KV, Gaborski TR, Fang DZ, Striemer CC, Fauchet PM, McGrath JL. Biomaterials. 2010;31(20):5408–5417. doi: 10.1016/j.biomaterials.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 3.von Behren J, Tsybeskov L, Fauchet PM. Appl Phys Lett. 1995;66(13):1662–1664. [Google Scholar]
- 4.Kim E, Xiong H, Striemer CC, Fang DZ, Fauchet PM, McGrath JL, Amemiya S. J Am Chem Soc. 2008;130(13):4230–4231. doi: 10.1021/ja711258w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishimatsu R, Kim J, Jing P, Striemer CC, Fang DZ, Fauchet PM, McGrath JL, Amemiya S. Anal Chem. 2010 doi: 10.1021/ac1005052. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaborski TR, Snyder JL, Striemer CC, Fang DZ, Hoffman M, Fauchet PM, McGrath JL. ACS Nano. 2010 doi: 10.1021/nn102064c. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong HD, Jansen HV, Gadgil VJ, Bostan CG, Berenschot E, van Rijn CJM, Elwenspoek M. Nano Lett. 2004;4(2):283–287. [Google Scholar]
- 8.Desai TA, Hansford D, Ferrari M. J Membrane Sci. 1999;159(1–2):221–231. [Google Scholar]
- 9.Storm AJ, Chen JH, Ling XS, Zandbergen HW, Dekker C. Nat Mater. 2003;2(8):537–40. doi: 10.1038/nmat941. [DOI] [PubMed] [Google Scholar]
- 10.Holt JK, Park HG, Wang Y, Stadermann M, Artyukhin AB, Grigoropoulos CP, Noy A, Bakajin O. Science. 2006;312(5776):1034–1037. doi: 10.1126/science.1126298. [DOI] [PubMed] [Google Scholar]
- 11.Yu M, Funke HH, Falconer JL, Noble RD. Nano Lett. 2009;9(1):225–229. doi: 10.1021/nl802816h. [DOI] [PubMed] [Google Scholar]
- 12.Whitby M, Cagnon L, Thanou M, Quirke N. Nano Lett. 2008;8(9):2632–2637. doi: 10.1021/nl080705f. [DOI] [PubMed] [Google Scholar]
- 13.Savariar EN, Krishnamoorthy K, Thayumanavan S. Nat Nano. 2008;3(2):112–117. doi: 10.1038/nnano.2008.6. [DOI] [PubMed] [Google Scholar]
- 14.Chen P, Mitsui T, Farmer DB, Golovchenko J, Gordon RG, Branton D. Nano Lett. 2004;4(7):1333–1337. doi: 10.1021/nl0494001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikko R, Markku L. Nanotechnology. 1999;10(1):19. [Google Scholar]
- 16.Salonen J, Björkqvist M, Laine E, Niinistö L. Appl Surf Sci. 2004;225(1–4):389–394. [Google Scholar]
- 17.Bimbo LM, Sarparanta M, Santos HA, Airaksinen AJ, Mäkilä E, Laaksonen T, Peltonen L, Lehto VP, Hirvonen J, Salonen J. ACS Nano. 2010;4(6):3023–3032. doi: 10.1021/nn901657w. [DOI] [PubMed] [Google Scholar]
- 18.Salonen J, Laine E, Niinistö L. J Appl Phys. 2002;91(1):456–461. [Google Scholar]
- 19.Lakshmikumar ST, Singh PK. J Appl Phys. 2002;92(6):3413–3415. [Google Scholar]
- 20.Pulci O, Silvestrelli PL, Palummo M, Ancilotto F, Sole RD. Phys Status Solidi C. 2003;0(8):2997–3001. [Google Scholar]
- 21.Mazzone AM. Surf Sci. 2007;601(1):218–226. [Google Scholar]
- 22.De Renzi V, Biagi R, del Pennino U. Appl Surf Sci. 2001;184(1–4):90–95. [Google Scholar]
- 23.Dufour G, Rochet F, Stedile FC, Poncey C, De Crescenzi M, Gunnella R, Froment M. Phys Rev B. 1997;56(7):4266. [Google Scholar]
- 24.Cimalla V, Karagodina KV, Pezoldt J, Eichhorn G. Mater Sci Eng B. 1995;29(1–3):170–175. [Google Scholar]
- 25.Bacon G. Acta Crystallogr. 1951;4(6):558–561. [Google Scholar]
- 26.Salonen J, Lehto VP, Björkqvist M, Laine E, Niinistö L. Stability Studies of Thermally-Carbonized Porous Silicon. Materials Research Society; 2000. [Google Scholar]
- 27.Dagan Z, Weinbaum S, Pfeffer R. J Fluidic Mech Digital Archive. 1982;115(1):505–523. [Google Scholar]
- 28.Snyder JL, Clark A, Jr, Fang DZ, Gaborski TR, Striemer CC, Fauchet PM, McGrath JL. J Membrane Sci. 2010 doi: 10.1016/j.memsci.2010.11.056. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.