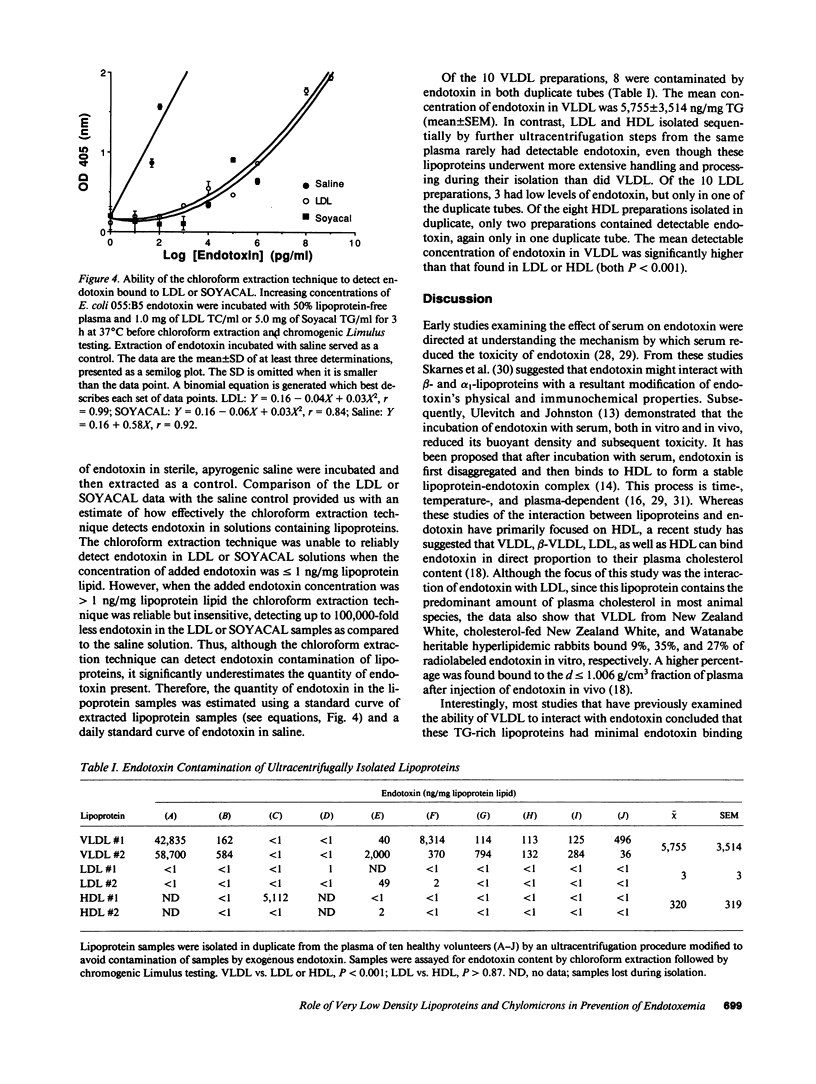
Abstract
Endotoxemia stimulates many physiologic responses including disturbances in lipid metabolism. We hypothesized that this lipemia may be part of a defensive mechanism by which the body combats the toxic effects of circulating endotoxin. We tested the effects of mixtures of endotoxin, lipoproteins, and lipoprotein-free plasma and determined the ability of varying concentrations of human very low density lipoproteins (VLDL) and chylomicrons, as well as low density lipoproteins (LDL) and high density lipoproteins (HDL), and of the synthetic lipid emulsion SOYACAL to prevent endotoxin-induced death in mice. This study demonstrates that the triglyceride-rich VLDL and chylomicrons, as well as cholesterol-rich LDL and HDL, and cholesterol-free SOYACAL can protect against endotoxin-induced death. Protection required small amounts of lipoprotein-free plasma, and depended on the incubation time and the concentration of lipoprotein lipid. Despite stringent techniques to prevent exogenous endotoxin contamination eight of ten duplicate VLDL preparations contained endotoxin (5,755 +/- 3,514 ng endotoxin/mg triglyceride, mean +/- SEM) making the isolation of endotoxin-free VLDL difficult. In contrast, simultaneous preparations of LDL and HDL were relatively free of endotoxin contamination (3 +/- 3 and 320 +/- 319 ng/mg total cholesterol, respectively), suggesting that the contamination of VLDL occurs in vivo and not during the isolation procedure. These observations suggest a possible role for increased triglyceride-rich lipoproteins in the host's defense against endotoxemia and infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez C., Ramos A. Lipids, lipoproteins, and apoproteins in serum during infection. Clin Chem. 1986 Jan;32(1 Pt 1):142–145. [PubMed] [Google Scholar]
- Alving C. R., Richardson E. C. Mitogenic activities of lipid A and liposome-associated lipid A: effects of epitope density. Rev Infect Dis. 1984 Jul-Aug;6(4):493–496. doi: 10.1093/clinids/6.4.493. [DOI] [PubMed] [Google Scholar]
- Amaral J. F., Shearer J. D., Mastrofrancesco B., Gann D. S., Caldwell M. D. The effect of endotoxin on glucose metabolism in skeletal muscle requires the presence of plasma. Arch Surg. 1989 Jun;124(6):727–732. doi: 10.1001/archsurg.1989.01410060099021. [DOI] [PubMed] [Google Scholar]
- Benedetto D. A., Shands J. W., Jr, Shah D. O. The interaction of bacterial lipopolysaccharide with phospholipid bilayers and monolayers. Biochim Biophys Acta. 1973 Mar 16;298(2):145–157. doi: 10.1016/0005-2736(73)90346-5. [DOI] [PubMed] [Google Scholar]
- Cabana V. G., Siegel J. N., Sabesin S. M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989 Jan;30(1):39–49. [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Serio M. K., Adi S., Moser A. H., Grunfeld C. Tumor necrosis factor stimulates hepatic lipid synthesis and secretion. Endocrinology. 1989 May;124(5):2336–2342. doi: 10.1210/endo-124-5-2336. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Serio M. K., Moser A. H., Dinarello C. A., Grunfeld C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology. 1989 Jul;125(1):267–274. doi: 10.1210/endo-125-1-267. [DOI] [PubMed] [Google Scholar]
- Fiser R. H., Denniston J. C., Beisel W. R. Infection with Diplococcus pneumoniae and Salmonella typhimurium in monkeys: changes in plasma lipids and lipoproteins. J Infect Dis. 1972 Jan;125(1):54–60. doi: 10.1093/infdis/125.1.54. [DOI] [PubMed] [Google Scholar]
- Fried V. A., Rothfield L. I. Interactions between lipopolysaccharide and phosphatidylethanolamine in molecular monolayers. Biochim Biophys Acta. 1978 Dec 4;514(1):69–82. doi: 10.1016/0005-2736(78)90077-9. [DOI] [PubMed] [Google Scholar]
- GILBERT R. P. Mechanisms of the hemodynamic effects of endotoxin. Physiol Rev. 1960 Apr;40:245–279. doi: 10.1152/physrev.1960.40.2.245. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. I., Stone P. C., Stuart J. An improved chromogenic substrate endotoxin assay for clinical use. J Clin Pathol. 1983 Oct;36(10):1145–1149. doi: 10.1136/jcp.36.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. I., Goldberg P. K., Bloom N., Degenshein G. A., Kozinn P. J. Endotoxin and bacteria in portal blood. Gastroenterology. 1977 Jun;72(6):1268–1270. [PubMed] [Google Scholar]
- Lanza-Jacoby S., Lansey S. C., Cleary M. P., Rosato F. E. Alterations in lipogenic enzymes and lipoprotein lipase activity during gram-negative sepsis in the rat. Arch Surg. 1982 Feb;117(2):144–147. doi: 10.1001/archsurg.1982.01380260028005. [DOI] [PubMed] [Google Scholar]
- Lees R. S., Fiser R. H., Jr, Beisel W. R., Bartelloni P. J. Effects of an experimental viral infection on plasma lipid and lipoprotein metabolism. Metabolism. 1972 Sep;21(9):825–833. doi: 10.1016/0026-0495(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Masoro E. J. Fat metabolism in normal and abnormal states. Am J Clin Nutr. 1977 Aug;30(8):1311–1320. doi: 10.1093/ajcn/30.8.1311. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. In vivo interaction of bacterial lipopolysaccharide (LPS) with rabbit platelets: modulation by C3 and high density lipoproteins. J Immunol. 1981 Apr;126(4):1575–1580. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Navab M., Hough G. P., Van Lenten B. J., Berliner J. A., Fogelman A. M. Low density lipoproteins transfer bacterial lipopolysaccharides across endothelial monolayers in a biologically active form. J Clin Invest. 1988 Feb;81(2):601–605. doi: 10.1172/JCI113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T. Addition of perchloric acid to blood samples for colorimetric limulus test using chromogenic substrate: comparison with conventional procedures and clinical applications. J Lab Clin Med. 1984 Sep;104(3):321–330. [PubMed] [Google Scholar]
- Pearson F. C., Weary M. E., Sargent H. E., Novitsky T. J., Lin H., Lindsay G., Berzofsky R. N., Lane A. L., Wilson J. D., Cooper J. F. Comparison of several control standard endotoxins to the National Reference Standard Endotoxin--an HIMA collaborative study. Appl Environ Microbiol. 1985 Jul;50(1):91–93. doi: 10.1128/aem.50.1.91-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN F. S., SKARNES R. C., LANDY M., SHEAR M. J. Inactivation of endotoxin by a humoral component. III. Role of divalent cation and a dialyzable component. J Exp Med. 1958 Nov 1;108(5):701–711. doi: 10.1084/jem.108.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Cerami A. Hypertriglyceridemia associated with Trypanosoma brucei brucei infection in rabbits: role of defective triglyceride removal. Mol Biochem Parasitol. 1980 Oct;2(1):31–38. doi: 10.1016/0166-6851(80)90046-8. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Glode L. M., Rosenstreich D. L. Lack of responsiveness of C3H/HeJ macrophages to lipopolysaccharide: the cellular basis of LPS-stimulated metabolism. J Immunol. 1979 Mar;122(3):932–935. [PubMed] [Google Scholar]
- SKARNES R. C., ROSEN F. S., SHEAR M. J., LANDY M. Inactivation of endotoxin by a humoral component. II. Interaction of endotoxin with serum and plasma. J Exp Med. 1958 Nov 1;108(5):685–699. doi: 10.1084/jem.108.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Metabolic disorders of serum lipoproteins in endotoxin-poisoned mice: the role of high density lipoprotein (HDL) and triglyceride-rich lipoproteins. Microbiol Immunol. 1982;26(11):1017–1034. doi: 10.1111/j.1348-0421.1982.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Sammalkorpi K., Valtonen V., Kerttula Y., Nikkilä E., Taskinen M. R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988 Sep;37(9):859–865. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr Affinity of endotoxin for membranes. J Infect Dis. 1973 Jul;128(Suppl):197–201. doi: 10.1093/infdis/128.supplement_1.s197. [DOI] [PubMed] [Google Scholar]
- Skarnes R. C. The inactivation of endotoxin after interaction with certain proteins of normal serum. Ann N Y Acad Sci. 1966 Jun 30;133(2):644–662. doi: 10.1111/j.1749-6632.1966.tb52395.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Nikaido H. Physical interaction between lipid A and phospholipids: a study with spin-labeled phospholipids. Rev Infect Dis. 1984 Jul-Aug;6(4):488–492. doi: 10.1093/clinids/6.4.488. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Haberland M. E., Edwards P. A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Knights C. V., Siber G. R. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986 Nov;154(5):784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985 Jun;248(6 Pt 1):E732–E740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]




