Abstract
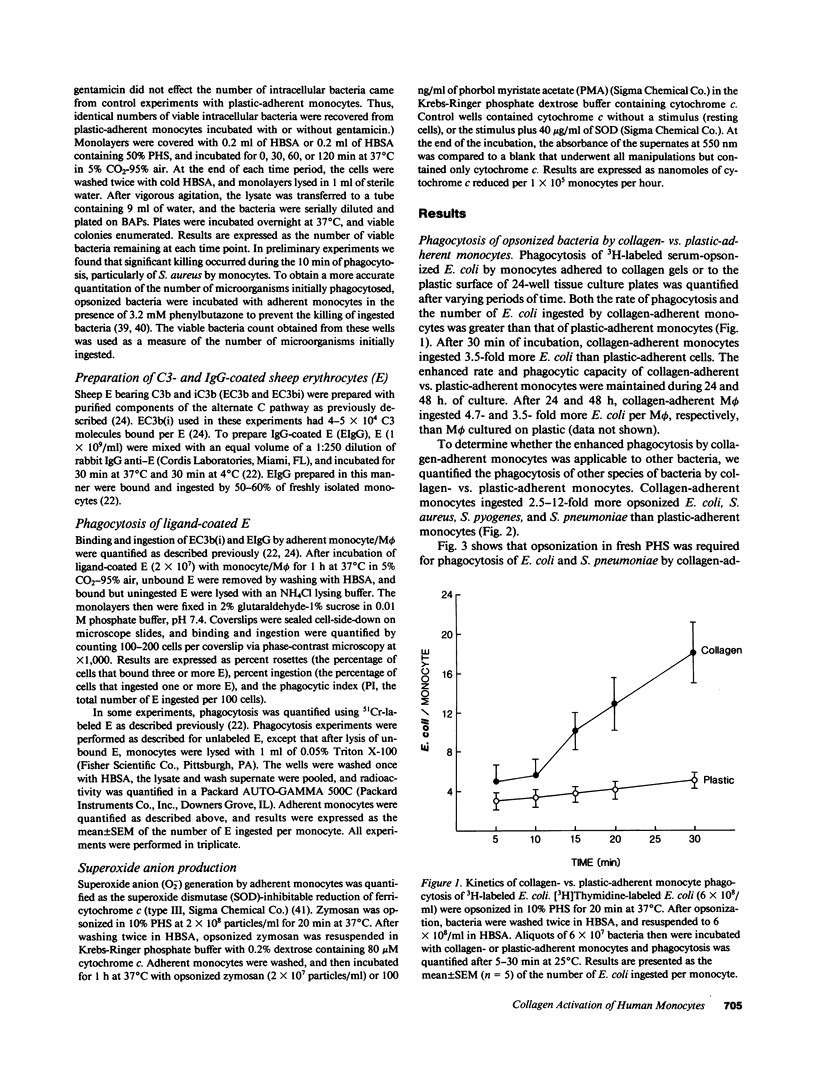
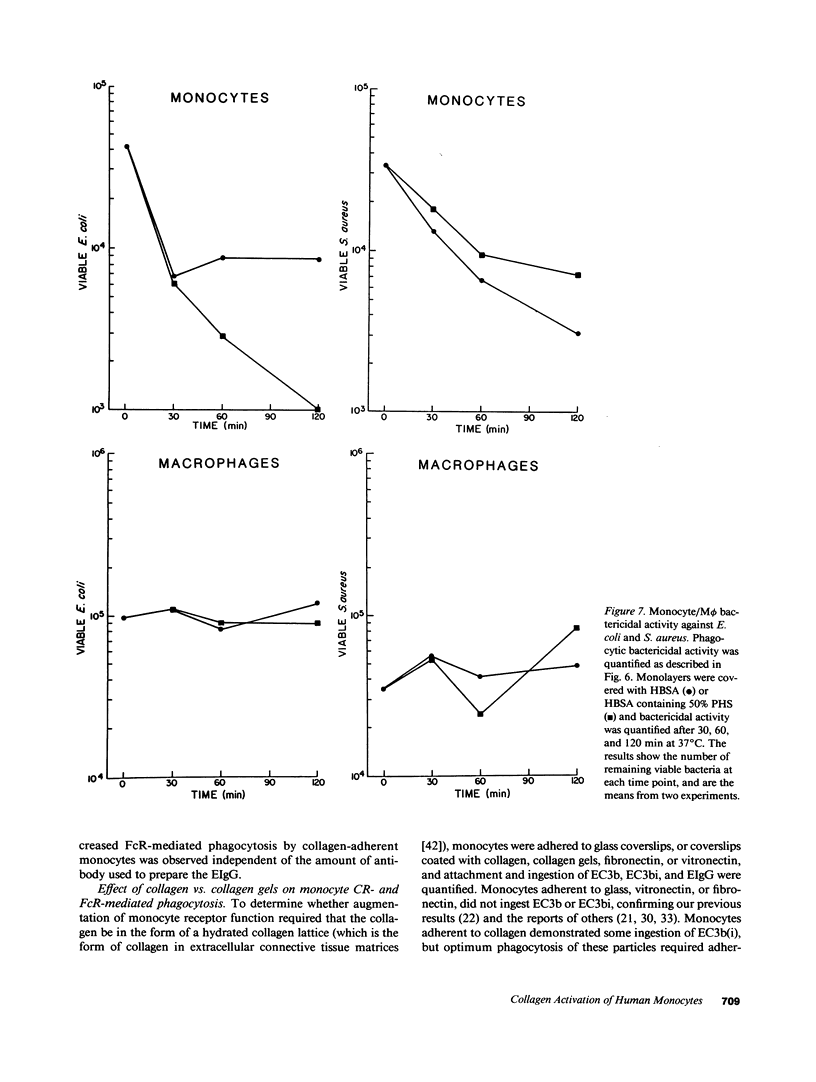
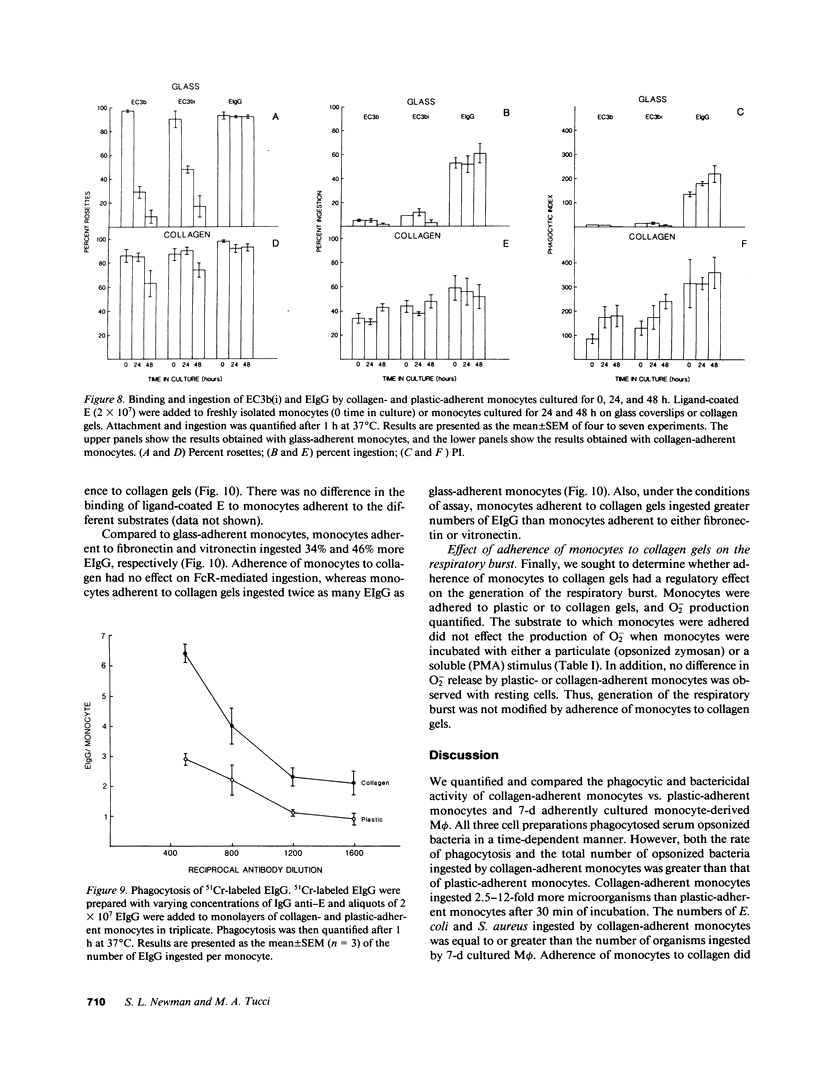
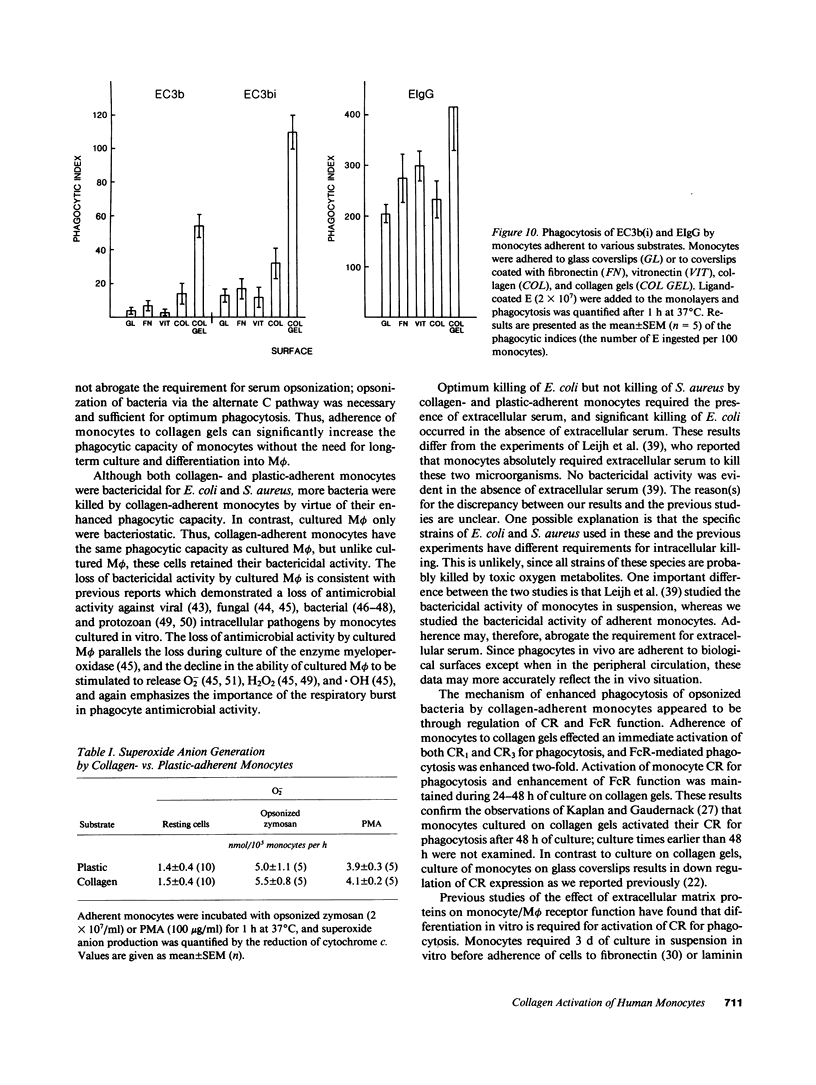
In inflammation monocytes emigrate from the peripheral circulation into an extravascular area rich in extracellular matrix proteins. In this milieu, phagocytes ingest and kill invading pathogens. In the present studies, we found that monocytes adhered to type I collagen gels phagocytized 2.5-12-fold more opsonized Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, and Streptococcus pneumoniae than plastic-adherent monocytes. The rate of phagocytosis and the number of bacteria ingested by collagen-adherent monocytes was equal to, or greater than, the number of bacteria ingested by 7-d cultured macrophages (M phi). Although both collagen- and plastic-adherent monocytes were bactericidal for E. coli and S. aureus, more bacteria were killed by collagen-adherent monocytes by virtue of their enhanced phagocytic capacity. Cultured M phi only were bacteriostatic. Adherence of monocytes to collagen gels activated C receptors (CR) types 1 and 3 for phagocytosis, and enhanced Fc receptor (FcR)-mediated phagocytosis. Collagen- and plastic-adherent monocytes produced equivalent amounts of superoxide anion in response to phorbol myristate acetate and opsonized zymosan. Thus, the enhanced phagocytosis and killing of opsonized bacteria by collagen-adherent monocytes appear to be by regulation of the function of membrane CR and FcR, without apparent enhancement of the respiratory burst. These data suggest that adherence of monocytes to the extracellular matrix during inflammation may rapidly activate these cells for enhanced phagocytic bactericidal activity.
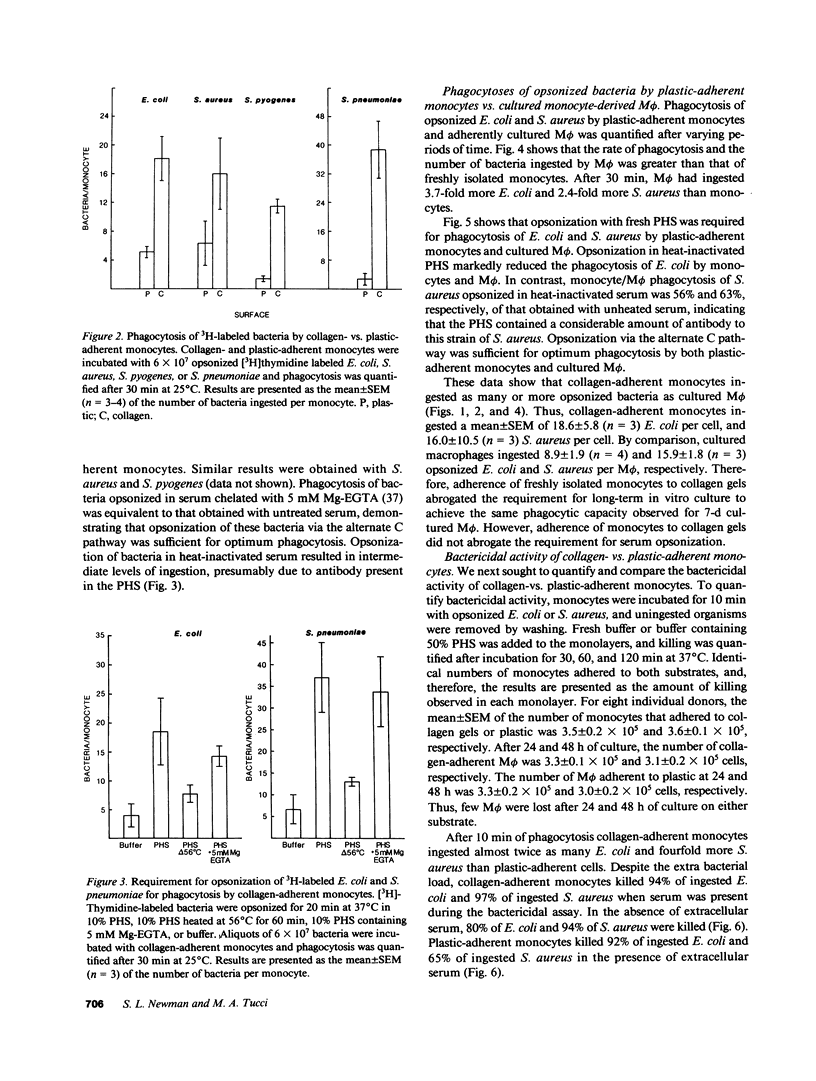
Full text
PDF

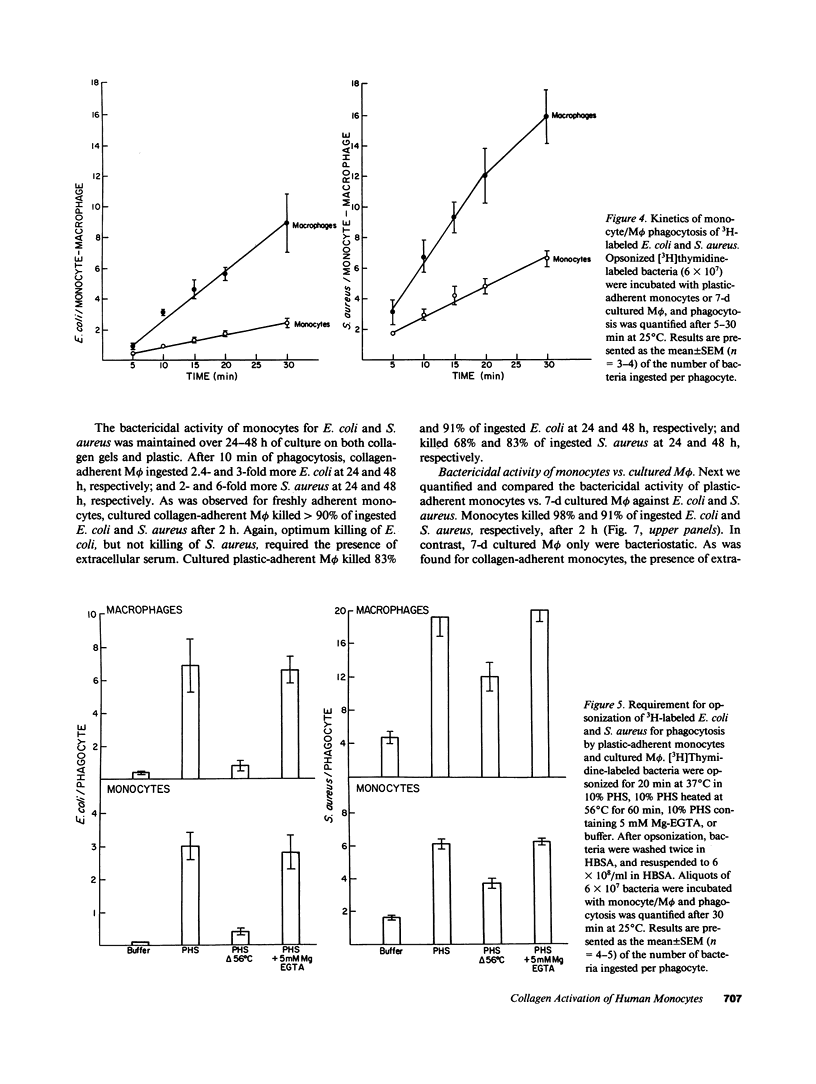



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
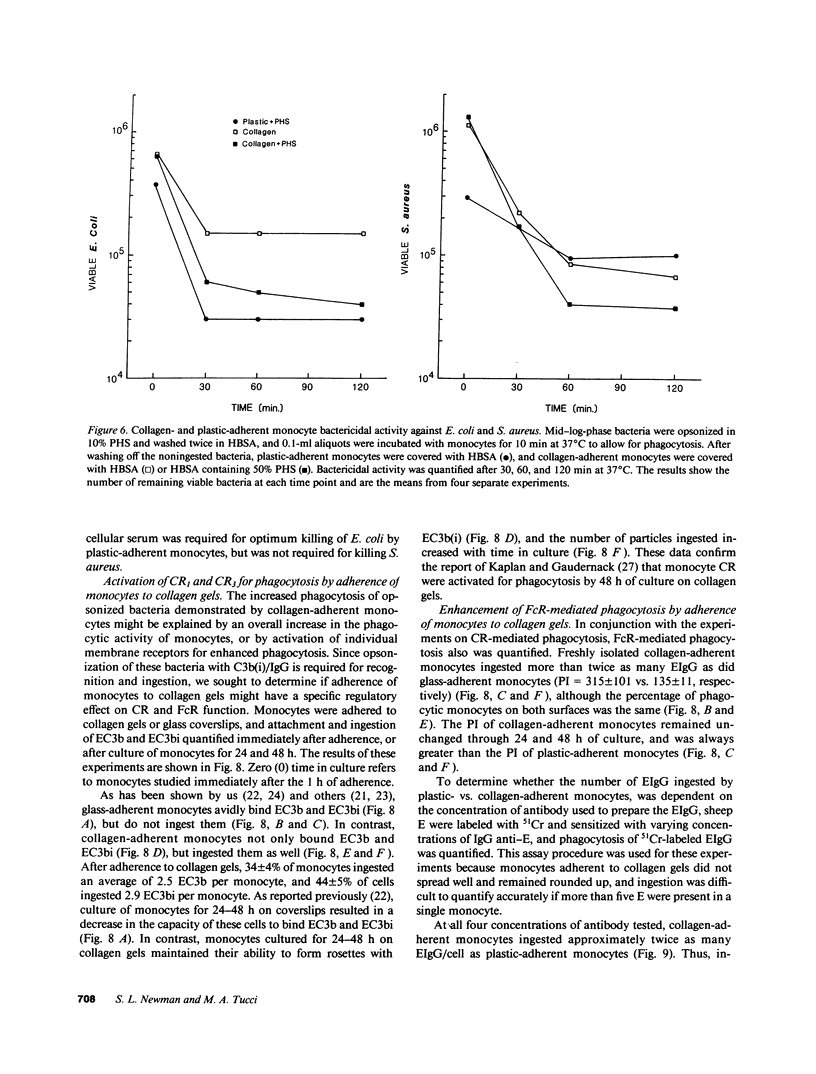
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., Kleinman H. K., Takahashi T., O'Shea J. J., Brown E. J. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J Exp Med. 1985 May 1;161(5):912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Goodwin J. L. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988 Mar 1;167(3):777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Gaither T. A., Hammer C. H., Frank M. M. The interaction of C3b bound to pneumococci with factor H (beta 1H globulin), factor I (C3b/C4b inactivator), and properdin factor B of the human complement system. J Immunol. 1983 Jul;131(1):409–415. [PubMed] [Google Scholar]
- Colten H. R. Molecular basis of complement deficiency syndromes. Lab Invest. 1985 May;52(5):468–474. [PubMed] [Google Scholar]
- Czuprynski C. J., Campbell P. A., Henson P. M. Killing of Listeria monocytogenes by human neutrophils and monocytes, but not by monocyte-derived macrophages. J Reticuloendothel Soc. 1983 Jul;34(1):29–44. [PubMed] [Google Scholar]
- Daniels C. A., Kleinerman E. S., Snyderman R. Abortive and productive infections of human mononuclear phagocytes by type I herpes simplex virus. Am J Pathol. 1978 Apr;91(1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Ricard-Blum S., Kaufmann M. T., Herbage D. Type IX collagen is a potent inducer of PGE2 and interleukin 1 production by human monocyte macrophages. FEBS Lett. 1986 Mar 31;198(2):208–212. doi: 10.1016/0014-5793(86)80406-9. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers P. A., Wright S. D., Olsen E., Kimball B., Cohn Z. A. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987 Sep;105(3):1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973 Feb;7(2):231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenberger A. G., Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977 Feb 1;145(2):357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierman D. F., Johnson C. E., Haskill J. S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989 Mar 15;142(6):1970–1976. [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Wong W. W. Complement ligand-receptor interactions that mediate biological responses. Annu Rev Immunol. 1983;1:243–271. doi: 10.1146/annurev.iy.01.040183.001331. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Gordon D. L., Rice J., Finlay-Jones J. J., McDonald P. J., Hostetter M. K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988 Apr;157(4):697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- Gresham H. D., Clement L. T., Lehmeyer J. E., Griffin F. M., Jr, Volanakis J. E. Stimulation of human neutrophil Fc receptor-mediated phagocytosis by a low molecular weight cytokine. J Immunol. 1986 Aug 1;137(3):868–875. [PubMed] [Google Scholar]
- Gresham H. D., Goodwin J. L., Allen P. M., Anderson D. C., Brown E. J. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989 May;108(5):1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Mullinax P. J. Effects of differentiation in vivo and of lymphokine treatment in vitro on the mobility of C3 receptors of human and mouse mononuclear phagocytes. J Immunol. 1985 Nov;135(5):3394–3397. [PubMed] [Google Scholar]
- Grossman N., Joiner K. A., Frank M. M., Leive L. C3b binding, but not its breakdown, is affected by the structure of the O-antigen polysaccharide in lipopolysaccharide from Salmonellae. J Immunol. 1986 Mar 15;136(6):2208–2215. [PubMed] [Google Scholar]
- Hemler M. E. Adhesive protein receptors on hematopoietic cells. Immunol Today. 1988 Apr;9(4):109–113. doi: 10.1016/0167-5699(88)91280-7. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The roles of the Fc and C3 receptors in the phagocytosis and killing of bacteria by human phagocytes. J Reticuloendothel Soc. 1980 Dec;28(Suppl):17s–26s. [PubMed] [Google Scholar]
- Hostetter M. K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986 Apr;153(4):682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G. In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. J Exp Med. 1983 Jun 1;157(6):2061–2072. doi: 10.1084/jem.157.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B. Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol. 1975 Jul;115(1):15–17. [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Newman S. L., Devery-Pocius J. E., Ross G. D., Henson P. M. Phagocytosis by human monocyte-derived macrophages. Independent function of receptors for C3b (CR1) and iC3b (CR3). Complement. 1984;1(4):213–227. [PubMed] [Google Scholar]
- Newman S. L., Johnston R. B., Jr Role of binding through C3b and IgG in polymorphonuclear neutrophil function: studies with trypsin-generated C3b. J Immunol. 1979 Oct;123(4):1839–1846. [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K. Deposition of C3b and iC3b onto particulate activators of the human complement system. Quantitation with monoclonal antibodies to human C3. J Exp Med. 1985 Jun 1;161(6):1414–1431. doi: 10.1084/jem.161.6.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S. L., Musson R. A., Henson P. M. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Parker C. J., Frame R. N., Elstad M. R. Vitronectin (S protein) augments the functional activity of monocyte receptors for IgG and complement C3b. Blood. 1988 Jan;71(1):86–93. [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Yancey K., Frank M. M., Takahashi T., Brown E. J. Studies on the fibronectin receptors of human peripheral blood leukocytes. Morphologic and functional characterization. J Exp Med. 1984 Jan 1;159(1):137–151. doi: 10.1084/jem.159.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen F. S., Janeway C. A. The gamma globulins. 3. The antibody deficiency syndromes. N Engl J Med. 1966 Sep 29;275(13):709–contd. doi: 10.1056/NEJM196609292751307. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Thompson R. A., Walport M. J., Springer T. A., Watson J. V., Ward R. H., Lida J., Newman S. L., Harrison R. A., Lachmann P. J. Characterization of patients with an increased susceptibility to bacterial infections and a genetic deficiency of leukocyte membrane complement receptor type 3 and the related membrane antigen LFA-1. Blood. 1985 Oct;66(4):882–890. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Jaffe E. A., Murray H. W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-gamma-activated macrophages. J Immunol. 1986 Jul 15;137(2):689–692. [PubMed] [Google Scholar]
- Sasada M., Kubo A., Nishimura T., Kakita T., Moriguchi T., Yamamoto K., Uchino H. Candidacidal activity of monocyte-derived human macrophages: relationship between Candida killing and oxygen radical generation by human macrophages. J Leukoc Biol. 1987 Apr;41(4):289–294. doi: 10.1002/jlb.41.4.289. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Nguyen B. Y., Sisson S. P., Kim Y. Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement. J Immunol. 1982 Oct;129(4):1681–1687. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J. J., Law S. K., Levine R. P., Cleary P. P. Resistance to phagocytosis by group A streptococci: failure of deposited complement opsonins to interact with cellular receptors. J Immunol. 1985 Jan;134(1):500–505. [PubMed] [Google Scholar]
- Winkelstein J. A. Opsonins: their function, identity, and clinical significance. J Pediatr. 1973 May;82(5):747–753. doi: 10.1016/s0022-3476(73)80062-9. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Licht M. R., Craigmyle L. S., Silverstein S. C. Communication between receptors for different ligands on a single cell: ligation of fibronectin receptors induces a reversible alteration in the function of complement receptors on cultured human monocytes. J Cell Biol. 1984 Jul;99(1 Pt 1):336–339. doi: 10.1083/jcb.99.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Fibronectin receptor of human macrophages recognizes the sequence Arg-Gly-Asp-Ser. J Exp Med. 1985 Aug 1;162(2):762–767. doi: 10.1084/jem.162.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986 Mar 1;136(5):1759–1764. [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Tumor-promoting phorbol esters stimulate C3b and C3b' receptor-mediated phagocytosis in cultured human monocytes. J Exp Med. 1982 Oct 1;156(4):1149–1164. doi: 10.1084/jem.156.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


