Abstract
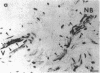
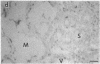
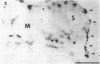
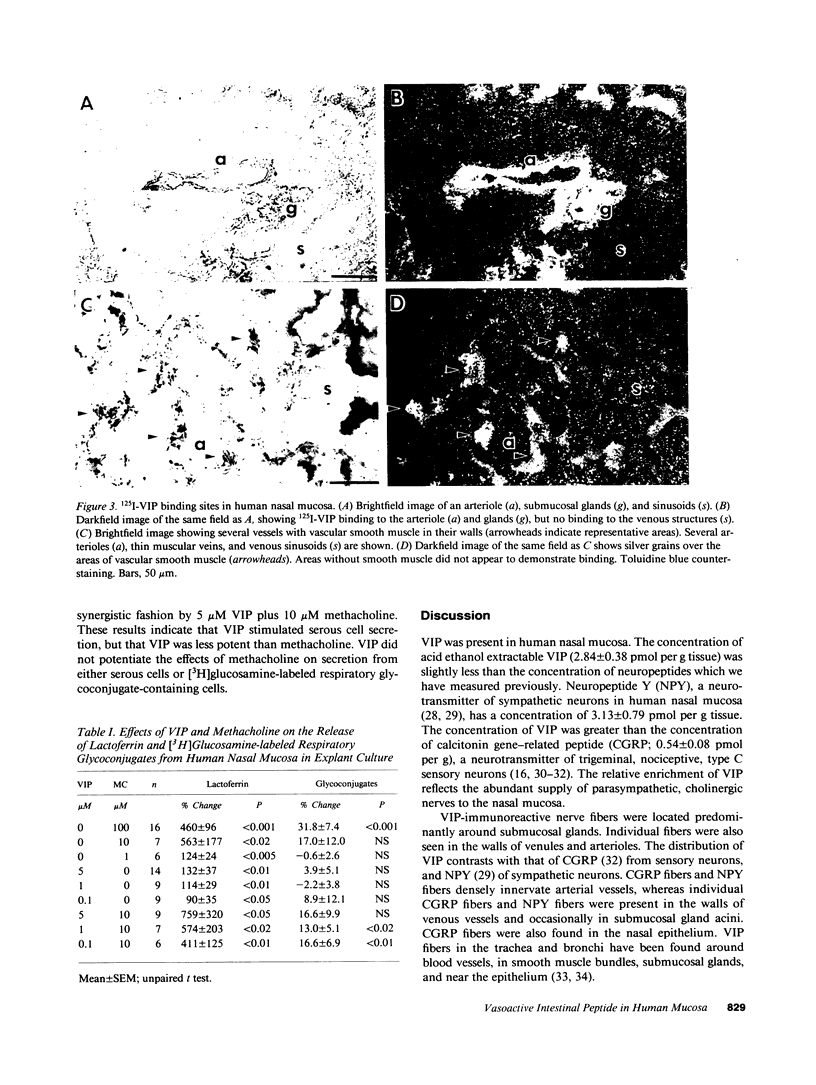
Vasoactive intestinal peptide (VIP), which is present with acetylcholine in parasympathetic nerve fibers, may have important regulatory functions in mucous membranes. The potential roles for VIP in human nasal mucosa were studied using an integrated approach. The VIP content of human nasal mucosa was determined to be 2.84 +/- 0.47 pmol/g wet weight (n = 8) by RIA. VIP-immunoreactive nerve fibers were found to be most concentrated in submucosal glands adjacent to serous and mucous cells. 125I-VIP binding sites were located on submucosal glands, epithelial cells, and arterioles. In short-term explant culture, VIP stimulated lactoferrin release from serous cells but did not stimulate [3H]glucosamine-labeled respiratory glycoconjugate secretion. Methacholine was more potent than VIP, and methacholine stimulated both lactoferrin and respiratory glycoconjugate release. The addition of VIP plus methacholine to explants resulted in additive increases in lactoferrin release. Based upon the autoradiographic distribution of 125I-VIP binding sites and the effects on explants, VIP derived from parasympathetic nerve fibers may function in the regulation of serous cell secretion in human nasal mucosa. VIP may also participate in the regulation of vasomotor tone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carstairs J. R., Barnes P. J. Visualization of vasoactive intestinal peptide receptors in human and guinea pig lung. J Pharmacol Exp Ther. 1986 Oct;239(1):249–255. [PubMed] [Google Scholar]
- Cauna N., Hinderer K. H. Fine structure of blood vessels of the human nasal respiratory mucosa. Ann Otol Rhinol Laryngol. 1969 Aug;78(4):865–879. doi: 10.1177/000348946907800418. [DOI] [PubMed] [Google Scholar]
- Coexistence of neuronal messengers: a new principle in chemical transmission. Proceedings of the Marcus Wallenberg Symposium. Stockholm, 26-28 June, 1985. Prog Brain Res. 1986;68:1–411. [PubMed] [Google Scholar]
- Coles S. J., Bhaskar K. R., O'Sullivan D. D., Neill K. H., Reid L. M. Airway mucus: composition and regulation of its secretion by neuropeptides in vitro. Ciba Found Symp. 1984;109:40–60. doi: 10.1002/9780470720905.ch4. [DOI] [PubMed] [Google Scholar]
- Coles S. J., Said S. I., Reid L. M. Inhibition by vasoactive intestinal peptide of glycoconjugate and lysozyme secretion by human airways in vitro. Am Rev Respir Dis. 1981 Nov;124(5):531–536. doi: 10.1164/arrd.1981.124.5.531. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Marsden C. A., Bennett G. W. Measurement of catecholamines, indoleamines, thyrotrophin releasing hormone and substance P in rat and human spinal cord using a common extraction method. J Neurosci Methods. 1987 Nov;22(1):31–39. doi: 10.1016/0165-0270(87)90086-0. [DOI] [PubMed] [Google Scholar]
- Costa M., Furness J. B., Gibbins I. L., Morris J. L., Bornstein J. C., Llewellyn-Smith I. J., Murphy R. Colocalization of VIP with other neuropeptides and neurotransmitters in the autonomic nervous system. Ann N Y Acad Sci. 1988;527:103–109. doi: 10.1111/j.1749-6632.1988.tb26976.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J., Bek T., Lundberg J. M., Hökfelt T. VIP and PHI in cat neurons: co-localization but variable tissue content possible due to differential processing. Regul Pept. 1985 Sep;12(1):21–34. doi: 10.1016/0167-0115(85)90183-1. [DOI] [PubMed] [Google Scholar]
- Gashi A. A., Borson D. B., Finkbeiner W. E., Nadel J. A., Basbaum C. B. Neuropeptides degranulate serous cells of ferret tracheal glands. Am J Physiol. 1986 Aug;251(2 Pt 1):C223–C229. doi: 10.1152/ajpcell.1986.251.2.C223. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Hua X. Y. Tachykinins and calcitonin gene-related peptide in relation to peripheral functions of capsaicin-sensitive sensory neurons. Acta Physiol Scand Suppl. 1986;551:1–45. [PubMed] [Google Scholar]
- Itoh N., Obata K., Yanaihara N., Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983 Aug 11;304(5926):547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Khalil Z., Andrews P. V., Helme R. D. VIP modulates substance P-induced plasma extravasation in vivo. Eur J Pharmacol. 1988 Jul 7;151(2):281–287. doi: 10.1016/0014-2999(88)90809-6. [DOI] [PubMed] [Google Scholar]
- Klaassen A. B., van Megen Y. J., Kuijpers W., van den Broek P. Autonomic innervation of the nasal mucosa. ORL J Otorhinolaryngol Relat Spec. 1988;50(1):32–41. doi: 10.1159/000275967. [DOI] [PubMed] [Google Scholar]
- Konno A., Togawa K. Role of the vidian nerve in nasal allergy. Ann Otol Rhinol Laryngol. 1979 Mar-Apr;88(2 Pt 1):258–266. doi: 10.1177/000348947908800219. [DOI] [PubMed] [Google Scholar]
- Larsson O., Dunér-Engström M., Lundberg J. M., Fredholm B. B., Anggård A. Effects of VIP, PHM and substance P on blood vessels and secretory elements of the human submandibular gland. Regul Pept. 1986 Feb;13(3-4):319–326. doi: 10.1016/0167-0115(86)90049-2. [DOI] [PubMed] [Google Scholar]
- Login G. R., Schnitt S. J., Dvorak A. M. Rapid microwave fixation of human tissues for light microscopic immunoperoxidase identification of diagnostically useful antigens. Lab Invest. 1987 Nov;57(5):585–591. [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Fahrenkrug J. Complementary role of vasoactive intestinal polypeptide (VIP) and acetylcholine for cat submandibular gland blood flow and secretion. II. Effects of cholinergic antagonists and VIP antiserum. Acta Physiol Scand. 1981;113(3):329–336. doi: 10.1111/j.1748-1716.1981.tb06903.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Lundblad L., Martling C. R., Saria A., Stjärne P., Anggård A. Coexistence of multiple peptides and classic transmitters in airway neurons: functional and pathophysiologic aspects. Am Rev Respir Dis. 1987 Dec;136(6 Pt 2):S16–S22. doi: 10.1164/ajrccm/136.6_Pt_2.S16. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- Lundblad L. Protective reflexes and vascular effects in the nasal mucosa elicited by activation of capsaicin-sensitive substance P-immunoreactive trigeminal neurons. Acta Physiol Scand Suppl. 1984;529:1–42. [PubMed] [Google Scholar]
- Lundgren J. D., Wiedermann C. J., Logun C., Plutchok J., Kaliner M., Shelhamer J. H. Substance P receptor-mediated secretion of respiratory glycoconjugate from feline airways in vitro. Exp Lung Res. 1989;15(1):17–29. doi: 10.3109/01902148909069606. [DOI] [PubMed] [Google Scholar]
- Malm L., Sundler F., Uddman R. Effects of vasoactive intestinal polypeptide on resistance and capacitance vessels in the nasal mucosa. Acta Otolaryngol. 1980 Sep-Oct;90(3-4):304–308. doi: 10.3109/00016488009131730. [DOI] [PubMed] [Google Scholar]
- Malm L., Sundler F., Uddman R. Effects of vasoactive intestinal polypeptide on resistance and capacitance vessels in the nasal mucosa. Acta Otolaryngol. 1980 Sep-Oct;90(3-4):304–308. doi: 10.3109/00016488009131730. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Christofi F. L., Brooks B. D., Barnett W., Cook M. A. Characterization of content and chromatographic forms of neuropeptides in purified nerve varicosities prepared from guinea pig myenteric plexus. Regul Pept. 1988 May;21(1-2):69–83. doi: 10.1016/0167-0115(88)90092-4. [DOI] [PubMed] [Google Scholar]
- Ollerenshaw S., Jarvis D., Woolcock A., Sullivan C., Scheibner T. Absence of immunoreactive vasoactive intestinal polypeptide in tissue from the lungs of patients with asthma. N Engl J Med. 1989 May 11;320(19):1244–1248. doi: 10.1056/NEJM198905113201904. [DOI] [PubMed] [Google Scholar]
- Patow C. A., Shelhamer J., Marom Z., Logun C., Kaliner M. Analysis of human nasal mucous glycoproteins. Am J Otolaryngol. 1984 Sep-Oct;5(5):334–343. doi: 10.1016/s0196-0709(84)80003-4. [DOI] [PubMed] [Google Scholar]
- Peatfield A. C., Barnes P. J., Bratcher C., Nadel J. A., Davis B. Vasoactive intestinal peptide stimulates tracheal submucosal gland secretion in ferret. Am Rev Respir Dis. 1983 Jul;128(1):89–93. doi: 10.1164/arrd.1983.128.1.89. [DOI] [PubMed] [Google Scholar]
- Potter E. K. Neuropeptide Y as an autonomic neurotransmitter. Pharmacol Ther. 1988;37(2):251–273. doi: 10.1016/0163-7258(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Druce H. M., Baraniuk J. N., Kaliner M. A. Pathophysiology of rhinitis. 1. Assessment of the sources of protein in methacholine-induced nasal secretions. Am Rev Respir Dis. 1988 Aug;138(2):413–420. doi: 10.1164/ajrccm/138.2.413. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Meredith S. D., Baraniuk J. N., Druce H. M., Banks S. M., Kaliner M. A. The pathophysiology of rhinitis. II. Assessment of the sources of protein in histamine-induced nasal secretions. Am Rev Respir Dis. 1989 Mar;139(3):791–800. doi: 10.1164/ajrccm/139.3.791. [DOI] [PubMed] [Google Scholar]
- Raphael G., Raphael M. H., Kaliner M. Gustatory rhinitis: a syndrome of food-induced rhinorrhea. J Allergy Clin Immunol. 1989 Jan;83(1):110–115. doi: 10.1016/0091-6749(89)90484-3. [DOI] [PubMed] [Google Scholar]
- Richardson P. S., Webber S. E. The control of mucous secretion in the airways by peptidergic mechanisms. Am Rev Respir Dis. 1987 Dec;136(6 Pt 2):S72–S76. doi: 10.1164/ajrccm/136.6_Pt_2.S72. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive peptides in the lung, with special reference to vasoactive intestinal peptide. Exp Lung Res. 1982 Nov;3(3-4):343–348. doi: 10.3109/01902148209069662. [DOI] [PubMed] [Google Scholar]
- Shimura S., Sasaki T., Ikeda K., Sasaki H., Takishima T. VIP augments cholinergic-induced glycoconjugate secretion in tracheal submucosal glands. J Appl Physiol (1985) 1988 Dec;65(6):2537–2544. doi: 10.1152/jappl.1988.65.6.2537. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Quantitative distribution of calcitonin gene-related peptide in the rat central nervous system. Peptides. 1985 Nov-Dec;6(6):1069–1073. doi: 10.1016/0196-9781(85)90429-2. [DOI] [PubMed] [Google Scholar]
- Sundler F., Ekblad E., Grunditz T., Håkanson R., Uddman R. Vasoactive intestinal peptide in the peripheral nervous system. Ann N Y Acad Sci. 1988;527:143–167. doi: 10.1111/j.1749-6632.1988.tb26979.x. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E., Hemsén A., Lundberg J. M. Radioimmunoassay for neuropeptide Y (NPY): chromatographic characterization of immunoreactivity in plasma and tissue extracts. Scand J Clin Lab Invest. 1985 Jun;45(4):355–365. doi: 10.3109/00365518509161019. [DOI] [PubMed] [Google Scholar]
- Uddman R., Grunditz T., Sundler F. Calcitonin gene related peptide: a sensory transmitter in dental pulps? Scand J Dent Res. 1986 Jun;94(3):219–224. doi: 10.1111/j.1600-0722.1986.tb01756.x. [DOI] [PubMed] [Google Scholar]
- Uddman R., Sundler F. Neuropeptides in the airways: a review. Am Rev Respir Dis. 1987 Dec;136(6 Pt 2):S3–S8. doi: 10.1164/ajrccm/136.6_Pt_2.S3. [DOI] [PubMed] [Google Scholar]