Abstract
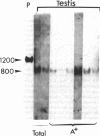
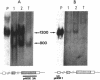
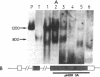
Proopiomelanocortin is a polypeptide precursor molecule, the processing of which generates ACTH, beta-endorphin, the beta- and gamma-lipotropins, the joining peptide, and the NH2-terminal fragment. Anterior pituitary corticotrophs are the major site of proopiomelanocortin gene expression in man and the predominant, if not sole source of circulating ACTH. Recent data have established that proopiomelanocortin gene expression also occurs in various normal nonpituitary tissues, one of the best studied being the testis. In this latter organ the dominant gene products are short transcripts of approximately 800 nucleotides, which lack the first two exons of the gene and cannot encode a complete proopiomelanocortin molecule. In this report we show that the mode of proopiomelanocortin gene expression is occasionally modified in human Leydig cell tumors: a 1,200-nucleotide mRNA species identical to that in the pituitary is produced. It results from the usual (pituitary) start site of transcription and thus can encode the complete proopiomelanocortin molecule. In two out of six tumors, large amounts of the 1,200-nucleotide transcript led to a dramatic increase of approximately 1,000-fold in proopiomelanocortin peptide concentrations as compared with the normal and peritumoral testis. Proopiomelanocortin processing in these tumors generates various peptide fragments including ACTH. These results may help to understand the mechanism of proopiomelanocortin expression in nonpituitary tumors and have implications for the more general phenomenon of ectopic hormone secretion.
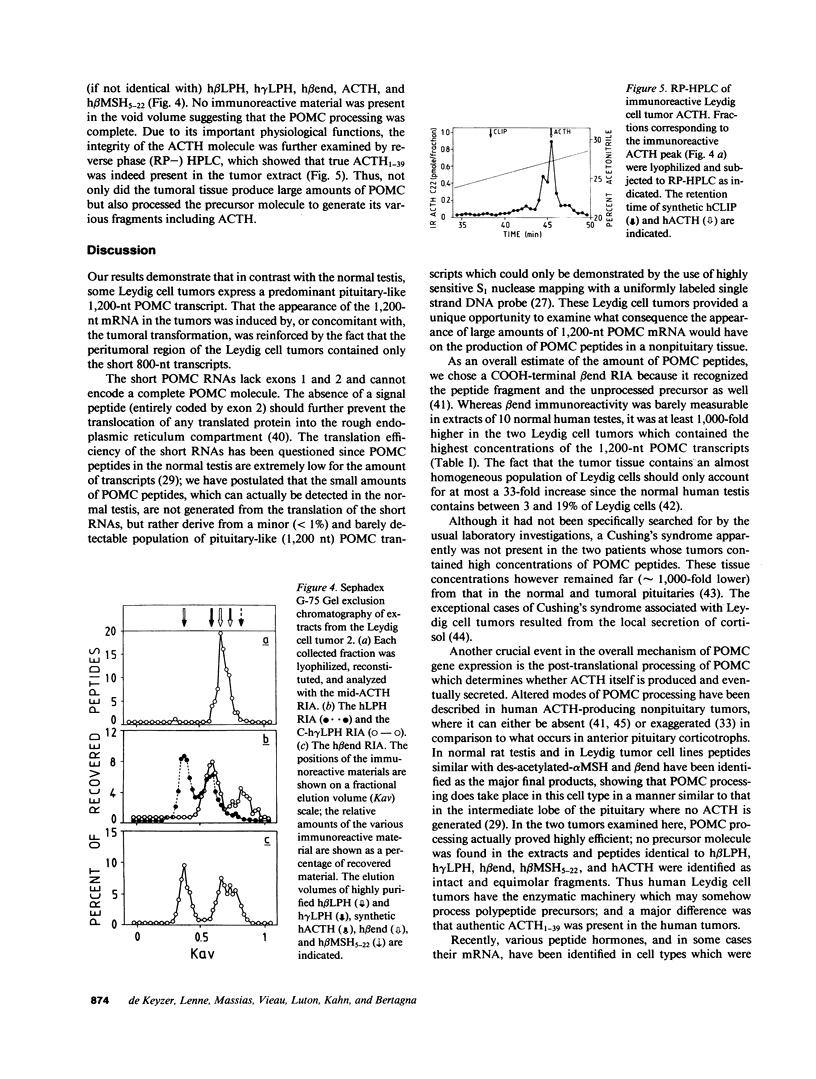
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Orwoll E., Kendall J. W., Herbert E., Paxton H. The distribution of forms of adrenocorticotropin and beta-endorphin in normal, tumorous, and autopsy human anterior pituiary tissue: virtual absence of 13K adrenocorticotropin. J Clin Endocrinol Metab. 1980 Aug;51(2):376–380. doi: 10.1210/jcem-51-2-376. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Shaha C., Mather J., Salomon Y., Margioris A. N., Liotta A. S., Gerendai I., Chen C. L., Krieger D. T. Identification and possible function of pro-opiomelanocortin-derived peptides in the testis. Ann N Y Acad Sci. 1984;438:346–364. doi: 10.1111/j.1749-6632.1984.tb38296.x. [DOI] [PubMed] [Google Scholar]
- Bertagna X. Y., Nicholson W. E., Sorenson G. D., Pettengill O. S., Mount C. D., Orth D. N. Corticotropin, lipotropin, and beta-endorphin production by a human nonpituitary tumor in culture: evidence for a common precursor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5160–5164. doi: 10.1073/pnas.75.10.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X. Y., Stone W. J., Nicholson W. E., Mount C. D., Orth D. N. Simultaneous assay of immunoreactive beta-lipotropin, gamma-lipotropin, and beta-endorphin in plasma of normal human subjects, patients with ACTH/lipotropin hypersecretory syndromes, and patients undergoing chronic hemodialysis. J Clin Invest. 1981 Jan;67(1):124–133. doi: 10.1172/JCI110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X., Camus F., Lenne F., Girard F., Luton J. P. Human joining peptide: a proopiomelanocortin product secreted as a homodimer. Mol Endocrinol. 1988 Nov;2(11):1108–1114. doi: 10.1210/mend-2-11-1108. [DOI] [PubMed] [Google Scholar]
- Bertagna X., Lenne F., Comar D., Massias J. F., Wajcman H., Baudin V., Luton J. P., Girard F. Human beta-melanocyte-stimulating hormone revisited. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9719–9723. doi: 10.1073/pnas.83.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X., Seurin D., Pique L., Luton J. P., Bricaire H., Girard F. Peptides related to the NH2-terminal end of proopiocortin in man. J Clin Endocrinol Metab. 1983 Mar;56(3):489–495. doi: 10.1210/jcem-56-3-489. [DOI] [PubMed] [Google Scholar]
- Birnberg N. C., Lissitzky J. C., Hinman M., Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6982–6986. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzetti R., McLoughlin L., Lavender P. M., Clark A. J., Rees L. H. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropic hormone-like immunoreactivity in human normal peripheral mononuclear cells and lymphoid and myeloid malignancies. J Clin Invest. 1989 Feb;83(2):733–737. doi: 10.1172/JCI113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Dionne F. T., Roberts J. L. Regulation of the pro-opiomelanocortin mRNA levels in rat pituitary by dopaminergic compounds. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2211–2215. doi: 10.1073/pnas.80.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. A., Funder J. W. Arginine vasopressin (AVP) and AVP-like immunoreactivity in peripheral tissues. Endocr Rev. 1986 Nov;7(4):449–460. doi: 10.1210/edrv-7-4-449. [DOI] [PubMed] [Google Scholar]
- DeBold C. R., Nicholson W. E., Orth D. N. Immunoreactive proopiomelanocortin (POMC) peptides and POMC-like messenger ribonucleic acid are present in many rat nonpituitary tissues. Endocrinology. 1988 Jun;122(6):2648–2657. doi: 10.1210/endo-122-6-2648. [DOI] [PubMed] [Google Scholar]
- DeBold C. R., Schworer M. E., Connor T. B., Bird R. E., Orth D. N. Ectopic pro-opiolipomelanocortin: sequence of cDNA coding for beta-melanocyte-stimulating hormone and beta-endorphin. Science. 1983 May 13;220(4598):721–723. doi: 10.1126/science.6301015. [DOI] [PubMed] [Google Scholar]
- Deschepper C. F., Mellon S. H., Cumin F., Baxter J. D., Ganong W. F. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y. S., Sherrod A., Lobo R. A., Paulson R. J., Shinagawa T., Chen S. W., Kjos S., Hsueh W. A. Human ovarian theca cells are a source of renin. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1957–1961. doi: 10.1073/pnas.85.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Chamberland M., Charron J., Jeannotte L., Nemer M. Structure of the rat pro-opiomelanocortin (POMC) gene. FEBS Lett. 1985 Nov 25;193(1):54–58. doi: 10.1016/0014-5793(85)80078-8. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Garrett J. E., Douglass J. O. Human chorionic gonadotropin regulates expression of the proenkephalin gene in adult rat Leydig cells. Mol Endocrinol. 1989 Dec;3(12):2093–2100. doi: 10.1210/mend-3-12-2093. [DOI] [PubMed] [Google Scholar]
- Germain G., Ferre F. Hormones et parturition chez les primates. Ann Endocrinol (Paris) 1987;48(4):311–321. [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Expression of the proopiomelanocortin gene is developmentally regulated and affected by germ cells in the male mouse reproductive system. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1600–1604. doi: 10.1073/pnas.84.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Localization of mRNAs in mouse testes by in situ hybridization: distribution of alpha-tubulin and developmental stage specificity of pro-opiomelanocortin transcripts. Dev Biol. 1985 Oct;111(2):293–305. doi: 10.1016/0012-1606(85)90484-1. [DOI] [PubMed] [Google Scholar]
- Jeannotte L., Burbach J. P., Drouin J. Unusual proopiomelanocortin ribonucleic acids in extrapituitary tissues: intronless transcripts in testes and long poly(A) tails in hypothalamus. Mol Endocrinol. 1987 Oct;1(10):749–757. doi: 10.1210/mend-1-10-749. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Daegelen D., Dreyfus J. C. Cell-free translation of messenger RNAs from adult and fetal human muscle. Characterization of neosynthesized glycogen phosphorylase, phosphofructokinase and glucose phosphate isomerase. Eur J Biochem. 1981 May;116(1):7–12. doi: 10.1111/j.1432-1033.1981.tb05293.x. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyrim K., Higi M., Hossfeld D. K., Seeber S., Schmidt C. G. Autonomous cortisol secretion by a metastatic Leydig cell carcinoma associated with Klinefelter's syndrome. J Cancer Res Clin Oncol. 1981;100(1):85–93. doi: 10.1007/BF00405905. [DOI] [PubMed] [Google Scholar]
- Kuhn J. M., Mahoudeau J. A., Billaud L., Joly J., Rieu M., Gancel A., Archambeaud-Mouveroux F., Steg A., Luton J. P. Evaluation of diagnostic criteria for Leydig cell tumours in adult men revealed by gynaecomastia. Clin Endocrinol (Oxf) 1987 Apr;26(4):407–416. doi: 10.1111/j.1365-2265.1987.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Lacaze-Masmonteil T., de Keyzer Y., Luton J. P., Kahn A., Bertagna X. Characterization of proopiomelanocortin transcripts in human nonpituitary tissues. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7261–7265. doi: 10.1073/pnas.84.20.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Hagn C., Fischer-Colbrie R., Winkler H. Presence of chromogranin A, B and C in bovine endocrine and nervous tissues: a comparative immunohistochemical study. Histochem J. 1986 Jul;18(7):380–386. doi: 10.1007/BF01675219. [DOI] [PubMed] [Google Scholar]
- Lee W., Mason A. J., Schwall R., Szonyi E., Mather J. P. Secretion of activin by interstitial cells in the testis. Science. 1989 Jan 20;243(4889):396–398. doi: 10.1126/science.2492117. [DOI] [PubMed] [Google Scholar]
- Li H., Risbridger G. P., Funder J. W., Clements J. A. Effect of ethane dimethane sulphonate on proopiomelanocortin (POMC) mRNA and POMC-derived peptides in the rat testis. Mol Cell Endocrinol. 1989 Aug;65(1-2):203–207. doi: 10.1016/0303-7207(89)90181-0. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., Clements J. A., Markwick A. J., Cheng C., McNally M., Smith A. I., Funder J. W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986 Jun;77(6):1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad J. R., Roberts J. L. Regulation of proopiomelanocortin gene expression in pituitary. Endocr Rev. 1988 Feb;9(1):135–158. doi: 10.1210/edrv-9-1-135. [DOI] [PubMed] [Google Scholar]
- Mori H., Hiromoto N., Nakahara M., Shiraishi T. Stereological analysis of Leydig cell ultrastructure in aged humans. J Clin Endocrinol Metab. 1982 Oct;55(4):634–641. doi: 10.1210/jcem-55-4-634. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Kita T., Taii S., Imura H., Numa S. Glucocorticoid effect on the level of corticotropin messenger RNA activity in rat pituitary. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3283–3286. doi: 10.1073/pnas.74.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Teranishi Y., Watanabe Y., Notake M., Noda M., Kakidani H., Jingami H., Numa S. Isolation and characterization of the bovine corticotropin/beta-lipotropin precursor gene. Eur J Biochem. 1981 Apr;115(3):429–438. doi: 10.1111/j.1432-1033.1981.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Notake M., Tobimatsu T., Watanabe Y., Takahashi H., Mishina M., Numa S. Isolation and characterization of the mouse corticotropin-beta-lipotropin precursor gene and a related pseudogene. FEBS Lett. 1983 May 30;156(1):67–71. doi: 10.1016/0014-5793(83)80250-6. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Immunoreactive human chromogranin A in diverse polypeptide hormone producing human tumors and normal endocrine tissues. J Clin Endocrinol Metab. 1983 Nov;57(5):1084–1086. doi: 10.1210/jcem-57-5-1084. [DOI] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Seeburg P. H., Shine J., Herbert E., Baxter J. D., Goodman H. M. Corticotropin and beta-endorphin: construction and analysis of recombinant DNA complementary to mRNA for the common precursor. Proc Natl Acad Sci U S A. 1979 May;76(5):2153–2157. doi: 10.1073/pnas.76.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter B. S., Johnson L. K., Baxter J. D., Roberts J. L. Differential regulation by glucocorticoids of proopiomelanocortin mRNA levels in the anterior and intermediate lobes of the rat pituitary. Endocrinology. 1982 Apr;110(4):1442–1444. doi: 10.1210/endo-110-4-1442. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J., Ratcliffe J. G., Rees L. H., Landon J. Corticotrophin-like peptides in the rat pituitary. J Endocrinol. 1974 Jun;61(3):355–367. doi: 10.1677/joe.0.0610355. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Rochemont J., Hamelin J., Lis M., Chrétien M. Primary structure of the major human pituitary pro-opiomelanocortin NH2-terminal glycopeptide. Evidence for an aldosterone-stimulating activity. J Biol Chem. 1981 Aug 10;256(15):7977–7984. [PubMed] [Google Scholar]
- Shu-Dong T., Phillips D. M., Halmi N., Krieger D., Bardin C. W. Beta-endorphin is present in the male reproductive tract of five species. Biol Reprod. 1982 Oct;27(3):755–764. doi: 10.1095/biolreprod27.3.755. [DOI] [PubMed] [Google Scholar]
- Suda T., Demura H., Demura R., Jibiki K., Tozawa F., Shizume K. Anterior pituitary hormones in plasma and pituitaries from patients with Cushing's disease. J Clin Endocrinol Metab. 1980 Nov;51(5):1048–1053. doi: 10.1210/jcem-51-5-1048. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Hakamata Y., Watanabe Y., Kikuno R., Miyata T., Numa S. Complete nucleotide sequence of the human corticotropin-beta-lipotropin precursor gene. Nucleic Acids Res. 1983 Oct 11;11(19):6847–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. The nature of the immunoreactive lipotropins in human plasma and tissue extracts. J Clin Invest. 1978 Jul;62(1):94–104. doi: 10.1172/JCI109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay Y., Tretjakoff I., Peterson A., Antakly T., Zhang C. X., Drouin J. Pituitary-specific expression and glucocorticoid regulation of a proopiomelanocortin fusion gene in transgenic mice. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8890–8894. doi: 10.1073/pnas.85.23.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Nakai Y., Jingami H., Imura H., Taii S., Nakanishi S., Numa S. Identification of the mRNA coding for the ACTH-beta-lipotropin precursor in a human ectopic ACTH-producing tumor. Biochem Biophys Res Commun. 1981 Jan 30;98(2):535–540. doi: 10.1016/0006-291x(81)90873-1. [DOI] [PubMed] [Google Scholar]
- Uhler M., Herbert E., D'Eustachio P., Ruddle F. D. The mouse genome contains two nonallelic pro-opiomelanocortin genes. J Biol Chem. 1983 Aug 10;258(15):9444–9453. [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]
- Ying S. Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988 May;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- de Keyzer Y., Bertagna X., Lenne F., Girard F., Luton J. P., Kahn A. Altered proopiomelanocortin gene expression in adrenocorticotropin-producing nonpituitary tumors. Comparative studies with corticotropic adenomas and normal pituitaries. J Clin Invest. 1985 Nov;76(5):1892–1898. doi: 10.1172/JCI112184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keyzer Y., Rousseau-Merck M. F., Luton J. P., Girard F., Kahn A., Bertagna X. Pro-opiomelanocortin gene expression in human phaeochromocytomas. J Mol Endocrinol. 1989 May;2(3):175–181. doi: 10.1677/jme.0.0020175. [DOI] [PubMed] [Google Scholar]