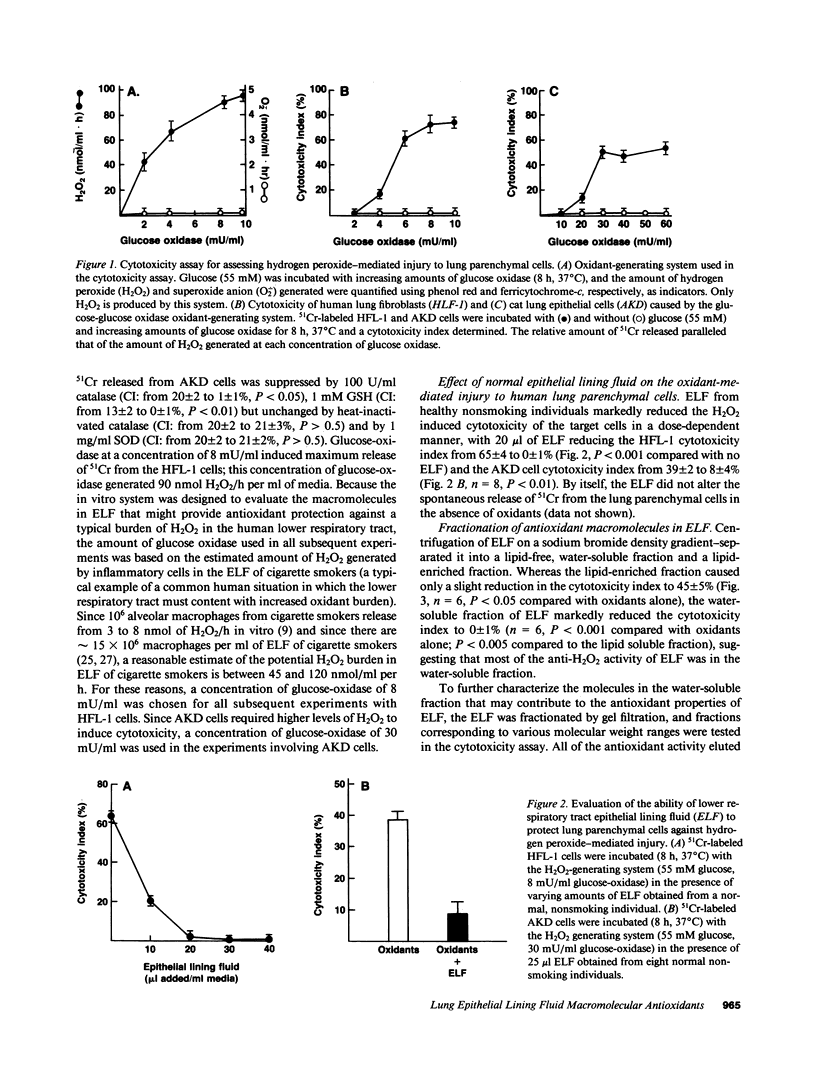
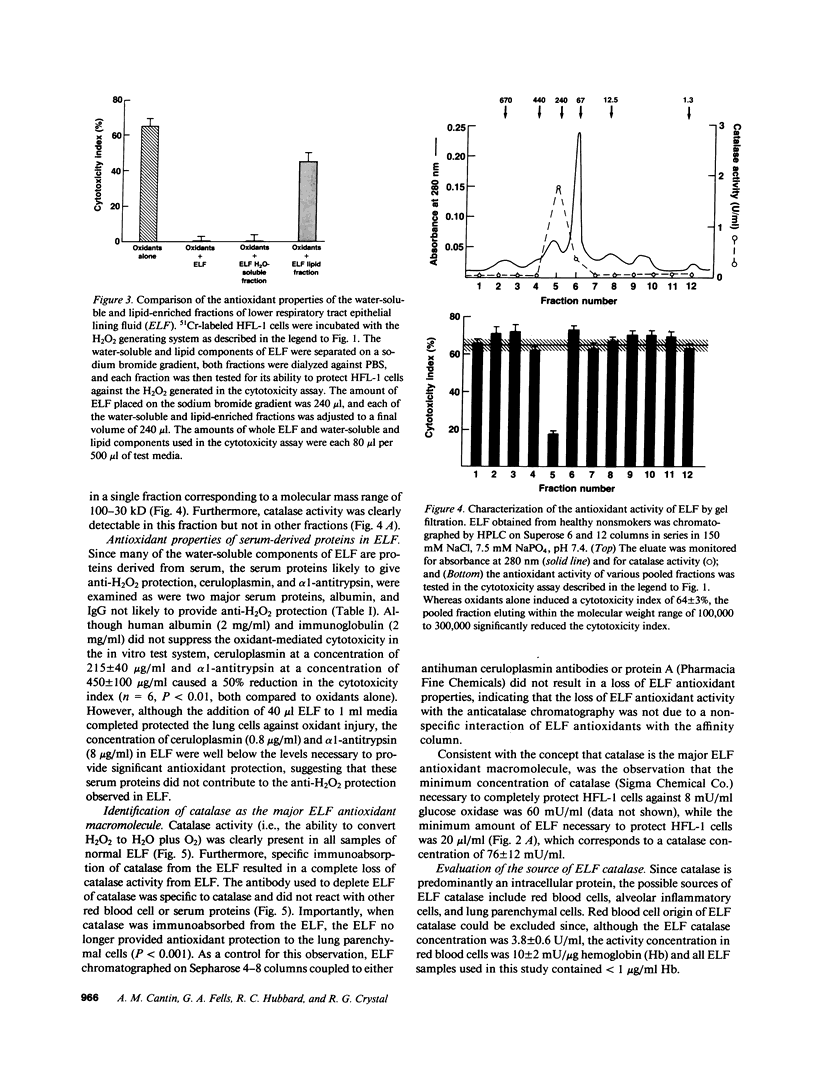
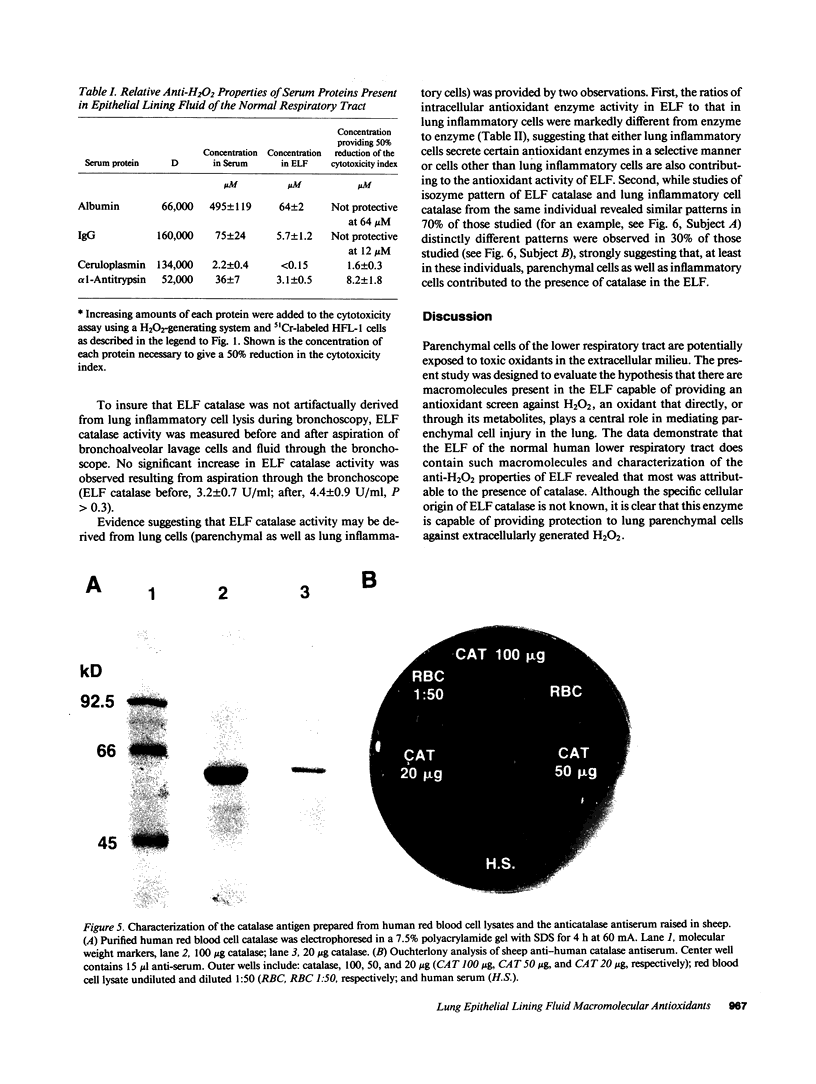
Abstract
We hypothesized that the alveolar structures may contain extracellular macromolecules with antioxidant properties to defend against oxidants. To evaluate this 51Cr-labeled human lung fibroblasts (HFL-1) and cat lung epithelial cells (AKD) were exposed to a H2O2-generating system and alveolar epithelial lining fluid (ELF) from healthy nonsmokers was tested for its ability to protect the lung cells from H2O2-mediated injury. The ELF provided marked antioxidant protection, with most from a H2O-soluble fraction in the 100-300-kD range. Plasma proteins with anti-H2O2 properties were in insufficient concentrations to provide the antioxidant protection observed. However, catalase, a normal intracellular antioxidant, was present in sufficient concentration to account for most of the observed anti-H2O2 properties of ELF. Depletion of ELF with an anticatalase antibody abolished the anti-H2O2 macromolecular defenses of ELF. Since catalase is not normally released by cells, a likely explanation for its presence in high concentrations in normal ELF is that it is released by lung inflammatory and parenchymal cells onto the epithelial surface of the lower respiratory tract during their normal turnover and collects there due to the slow turnover of ELF. It is likely that catalase in the ELF of normal individuals plays a role in protecting lung parenchymal cells against oxidants present in the extracellular milieu.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Lauterburg B. H., Mitchell J. R. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983 Dec;227(3):749–754. [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Al-Timimi D. J., Dormandy T. L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977 Nov 15;168(2):283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Schroeder W. A., Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys. 1972 Jun;150(2):606–617. doi: 10.1016/0003-9861(72)90080-x. [DOI] [PubMed] [Google Scholar]
- Bradley K. H., Kawanami O., Ferrans V. J., Crystal R. G. The fibroblast of human lung alveolar structures: a differentiated cell with a major role in lung structure and function. Methods Cell Biol. 1980;21A:37–64. doi: 10.1016/s0091-679x(08)60757-8. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Davies P., Drath D. B., Engel E. E., Huber G. L. The localization of catalase in the pulmonary alveolar macrophage. Lab Invest. 1979 Feb;40(2):221–226. [PubMed] [Google Scholar]
- Davis W. B., Fells G. A., Sun X. H., Gadek J. E., Venet A., Crystal R. G. Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix. A possible role for eosinophils in chronic inflammatory disorders of the lower respiratory tract. J Clin Invest. 1984 Jul;74(1):269–278. doi: 10.1172/JCI111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- FLEISHER G. A., WAKIM K. G. The fate of enzymes in body fluids-an experimental study. I. Disappearance rates of glutamic-pyruvic transaminase under various conditions. J Lab Clin Med. 1963 Jan;61:76–85. [PubMed] [Google Scholar]
- Frank L., Massaro D. Oxygen toxicity. Am J Med. 1980 Jul;69(1):117–126. doi: 10.1016/0002-9343(80)90509-4. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Goldenberg H., Hüttinger M., Kollner U., Kramar R., Pavelka M. Catalase positive particles from pig lung. Biochemical preparations and morphological studies. Histochemistry. 1978 Jul 12;56(3-4):253–264. doi: 10.1007/BF00495987. [DOI] [PubMed] [Google Scholar]
- Greening A. P., Lowrie D. B. Extracellular release of hydrogen peroxide by human alveolar macrophages: the relationship to cigarette smoking and lower respiratory tract infections. Clin Sci (Lond) 1983 Dec;65(6):661–664. doi: 10.1042/cs0650661. [DOI] [PubMed] [Google Scholar]
- Góth L., Németh H., Mészáros I. Serum catalase activity for detection of hemolytic diseases. Clin Chem. 1983 Apr;29(4):741–743. [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hook G. E. Extracellular hydrolases of the lung. Biochemistry. 1978 Feb 7;17(3):520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Huber G. L., Edmunds L. H., Jr, Finley T. N. Effect of experimental saline lavage on pulmonary mechanics and morphology. Am Rev Respir Dis. 1971 Sep;104(3):337–347. doi: 10.1164/arrd.1971.104.3.337. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Kniazeff A. J., Stoner G. D., Terry L., Wagner R. M., Hoppenstand R. D. Characteristics of epithelial cella cultured from feline lung. Lab Invest. 1976 May;34(5):495–500. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Arcinue E. L., Partin J. C., Partin J. S., Ram M. L., Chang C. H., Smialek J., Sarnaik A. Quantitative evaluation of the extent of hepatic enzyme changes in Reye syndrome compared with normal liver or with non-Reye liver disorders: objective criteria for animal models. Pediatr Res. 1985 Jan;19(1):19–22. doi: 10.1203/00006450-198501000-00006. [DOI] [PubMed] [Google Scholar]
- Monboisse J. C., Braquet P., Randoux A., Borel J. P. Non-enzymatic degradation of acid-soluble calf skin collagen by superoxide ion: protective effect of flavonoids. Biochem Pharmacol. 1983 Jan 1;32(1):53–58. doi: 10.1016/0006-2952(83)90651-2. [DOI] [PubMed] [Google Scholar]
- Moriguchi K., Higashi N., Kitagawa S., Takase K., Ohya N., Uyeda T., Hirai K. Differentiation of human pulmonary alveolar epithelial cells revealed by peroxisome changes in pulmonary proteinosis. Exp Mol Pathol. 1984 Apr;40(2):262–270. doi: 10.1016/0014-4800(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Tierney D. F. Biochemical and metabolic changes in the lung with oxygen, ozone, and nitrogen dioxide toxicity. Am Rev Respir Dis. 1978 Dec;118(6):1061–1090. doi: 10.1164/arrd.1978.118.6.1061. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., Kaseki H., Mohammed J. R., Cornwell D. G., Davis W. B. Deficiency of vitamin E in the alveolar fluid of cigarette smokers. Influence on alveolar macrophage cytotoxicity. J Clin Invest. 1986 Mar;77(3):789–796. doi: 10.1172/JCI112376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Petrik P. Fine structural identification of peroxisomes in mouse and rat bronchiolar and alveolar epithelium. J Histochem Cytochem. 1971 Jun;19(6):339–348. doi: 10.1177/19.6.339. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Terauchi K., Davis W. H., Jr Electron spin resonance (ESR) study of cigarette smoke by use of spin trapping techniques. Environ Health Perspect. 1976 Aug;16:161–176. doi: 10.1289/ehp.7616161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T. R., Weatherall D. J. Deficient heme synthesis as the cause of noninducibility of hemoglobin synthesis in a Friend erythroleukemia cell line. Cell. 1979 Feb;16(2):415–423. doi: 10.1016/0092-8674(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley S. A., Paciga J. E., Balis J. U. Purification of surfactant from lung washings and washings contaminated with blood constituents. Lipids. 1977 Jun;12(6):505–510. doi: 10.1007/BF02535450. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Skoza L., Snyder A., Kikkawa Y. Ascorbic acid in bronchoalveolar wash. Lung. 1983;161(2):99–109. doi: 10.1007/BF02713848. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Pollard T. D., Vaughan M. Isolation and properties of phagocytic vesicles. II. Alveolar macrophages. J Clin Invest. 1972 Mar;51(3):604–614. doi: 10.1172/JCI106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury W., Spencer A. K., Stahman M. A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971 Nov;44(1):301–305. doi: 10.1016/0003-2697(71)90375-7. [DOI] [PubMed] [Google Scholar]