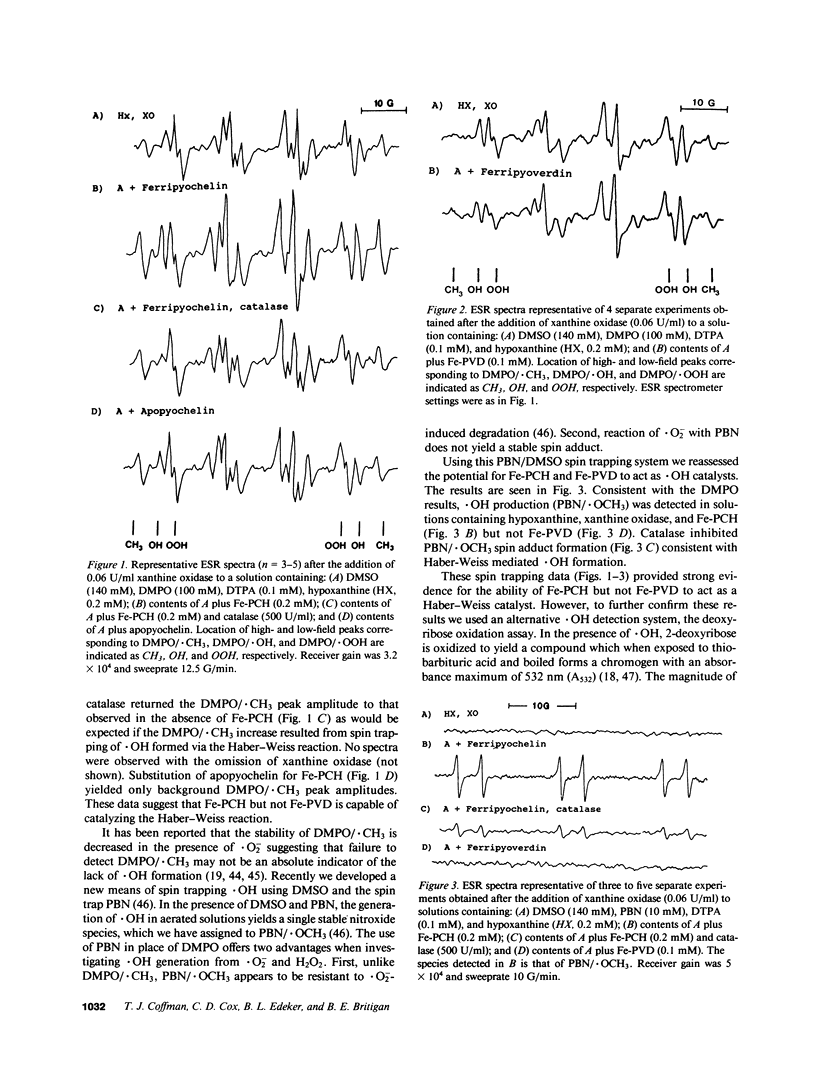
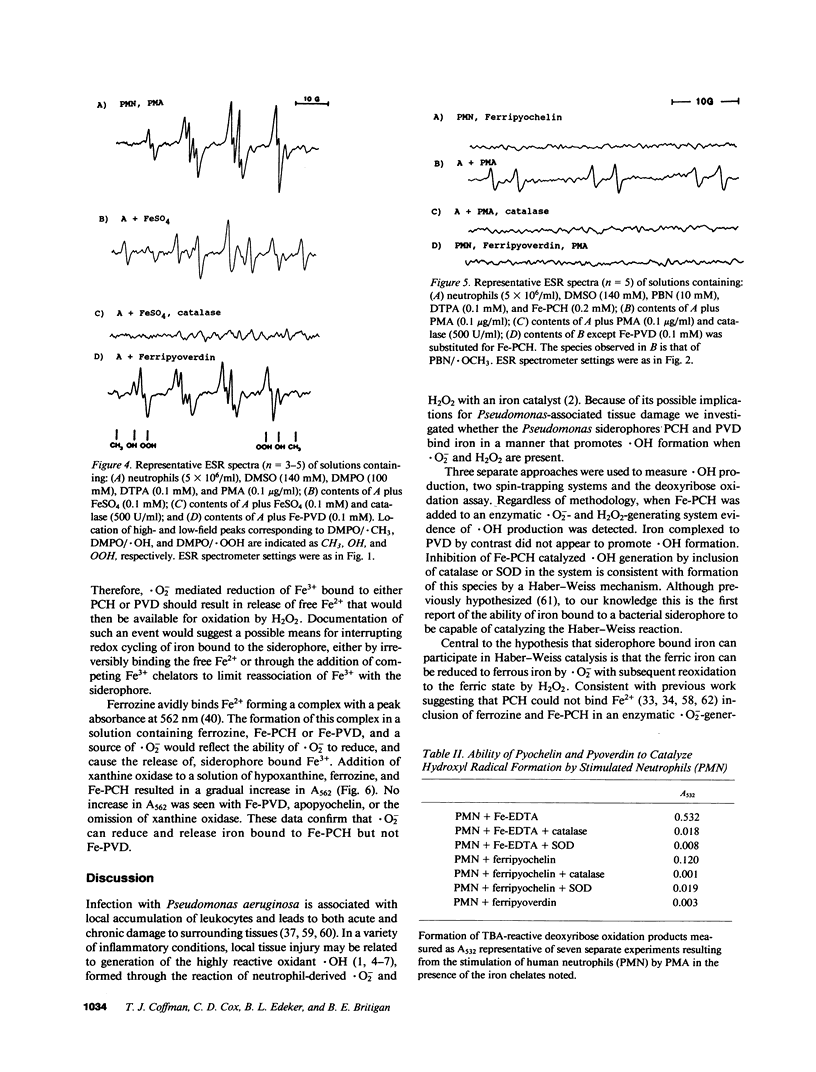
Abstract
Tissue injury has been linked to neutrophil associated hydroxyl radical (.OH) generation, a process that requires an exogenous transition metal catalyst such as iron. In vivo most iron is bound in a noncatalytic form. To obtain iron required for growth, many bacteria secrete iron chelators (siderophores). Since Pseudomonas aeruginosa infections are associated with considerable tissue destruction, we examined whether iron bound to the Pseudomonas siderophores pyochelin (PCH) and pyoverdin (PVD) could act as .OH catalysts. Purified PCH and PVD were iron loaded (Fe-PCH, Fe-PVD) and added to a hypoxanthine/xanthine oxidase superoxide- (.O2-) and hydrogen peroxide (H2O2)-generating system. Evidence for .OH generation was then sought using two different spin-trapping agents (5.5 dimethyl-pyrroline-1-oxide or N-t-butyl-alpha-phenylnitrone), as well as the deoxyribose oxidation assay. Regardless of methodology, .OH generation was detected in the presence of Fe-PCH but not Fe-PVD. Inhibition of the process by catalase and/or SOD suggested .OH formation with Fe-PCH occurred via the Haber-Weiss reaction. Similar results were obtained when stimulated neutrophils were used as the source of .O2- and H2O2. Addition of Fe-PCH but not Fe-PVD to stimulated neutrophils yielded .OH as detected by the above assay systems. Since PCH and PVD bind ferric (Fe3+) but not ferrous (Fe2+) iron, .OH catalysis with Fe-PCH would likely involve .O2(-)-mediated reduction of Fe3+ to Fe2+ with subsequent release of "free" Fe2+. This was confirmed by measuring formation of the Fe2(+)-ferrozine complex after exposure of Fe-PCH, but not Fe-PVD, to enzymatically generated .O2-. These data show that Fe-PCH, but not Fe-PVD, is capable of catalyzing generation of .OH. Such a process could represent as yet another mechanism of tissue injury at sites of infection with P. aeruginosa.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987 Jan 1;241(1):273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer R. F., McCleary C. J. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radic Biol Med. 1987;3(6):389–395. doi: 10.1016/0891-5849(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Buettner G. R. Spin trapping evidence for the lack of significant hydroxyl radical production during the respiration burst of human phagocytes using a spin adduct resistant to superoxide-mediated destruction. J Biol Chem. 1990 Feb 15;265(5):2650–2656. [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Rosen G. M. Detection of the production of oxygen-centered free radicals by human neutrophils using spin trapping techniques: a critical perspective. J Leukoc Biol. 1987 Apr;41(4):349–362. doi: 10.1002/jlb.41.4.349. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Hassett D. J., Rosen G. M., Hamill D. R., Cohen M. S. Neutrophil degranulation inhibits potential hydroxyl-radical formation. Relative impact of myeloperoxidase and lactoferrin release on hydroxyl-radical production by iron-supplemented neutrophils assessed by spin-trapping techniques. Biochem J. 1989 Dec 1;264(2):447–455. doi: 10.1042/bj2640447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Chai Y., Cohen M. S. Do human neutrophils make hydroxyl radical? Determination of free radicals generated by human neutrophils activated with a soluble or particulate stimulus using electron paramagnetic resonance spectrometry. J Biol Chem. 1986 Apr 5;261(10):4426–4431. [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Buettner G. R. The reaction of superoxide, formate radical, and hydrated electron with transferrin and its model compound, Fe(III)-ethylenediamine-N,N'-bis[2-(2-hydroxyphenyl)acetic acid] as studied by pulse radiolysis. J Biol Chem. 1987 Sep 5;262(25):11995–11998. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Boonchai S., Parry S. H., Väisänen-Rhen V., Korhonen T. K., Williams P. H. Aerobactin-mediated iron uptake by Escherichia coli isolates from human extraintestinal infections. Infect Immun. 1986 Mar;51(3):966–968. doi: 10.1128/iai.51.3.966-968.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Do humans neutrophils form hydroxyl radical? Evaluation of an unresolved controversy. Free Radic Biol Med. 1988;5(2):81–88. doi: 10.1016/0891-5849(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron reductases from Pseudomonas aeruginosa. J Bacteriol. 1980 Jan;141(1):199–204. doi: 10.1128/jb.141.1.199-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Pfestorf M., Botzenhart K., Abdallah M. A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun. 1988 Jan;56(1):291–293. doi: 10.1128/iai.56.1.291-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Production of hydroxyl radical by decomposition of superoxide spin-trapped adducts. Mol Pharmacol. 1982 Mar;21(2):262–265. [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Hill H. A., Okolow-Zubkowska M. J., Segal A. W. The production of hydroxyl and superoxide radicals by stimulated human neutrophils- measurements by EPR spectroscopy. FEBS Lett. 1979 Apr 1;100(1):23–26. doi: 10.1016/0014-5793(79)81123-0. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Rush S. W., Moak S. A., Weitz Z. Conversion of superoxide generated by polymorphonuclear leukocytes to hydroxyl radical: a direct spectrophotometric detection system based on degradation of deoxyribose. Free Radic Biol Med. 1989;6(4):385–392. doi: 10.1016/0891-5849(89)90084-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Hoepelman I. M., Bezemer W. A., Vandenbroucke-Grauls C. M., Marx J. J., Verhoef J. Bacterial iron enhances oxygen radical-mediated killing of Staphylococcus aureus by phagocytes. Infect Immun. 1990 Jan;58(1):26–31. doi: 10.1128/iai.58.1.26-31.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987 Dec 15;47(24 Pt 1):6522–6527. [PubMed] [Google Scholar]
- Kaur H., Fagerheim I., Grootveld M., Puppo A., Halliwell B. Aromatic hydroxylation of phenylalanine as an assay for hydroxyl radicals: application to activated human neutrophils and to the heme protein leghemoglobin. Anal Biochem. 1988 Aug 1;172(2):360–367. doi: 10.1016/0003-2697(88)90456-3. [DOI] [PubMed] [Google Scholar]
- Kluger M. J., Rothenburg B. A. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science. 1979 Jan 26;203(4378):374–376. doi: 10.1126/science.760197. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H. The reaction of ferrous EDTA with hydrogen peroxide: evidence against hydroxyl radical formation. J Free Radic Biol Med. 1985;1(4):281–285. doi: 10.1016/0748-5514(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Kuroda M., Murakami K., Ishikawa Y. Role of hydroxyl radicals derived from granulocytes in lung injury induced by phorbol myristate acetate. Am Rev Respir Dis. 1987 Dec;136(6):1435–1444. doi: 10.1164/ajrccm/136.6.1435. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Pulmonary disease associated with Pseudomonas aeruginosa in cystic fibrosis: current status of the host-bacterium interaction. J Infect Dis. 1985 Apr;151(4):575–580. doi: 10.1093/infdis/151.4.575. [DOI] [PubMed] [Google Scholar]
- Pou S., Cohen M. S., Britigan B. E., Rosen G. M. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J Biol Chem. 1989 Jul 25;264(21):12299–12302. [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J. 1988 Jan 1;249(1):185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M., Harada R. N. Effect of staphylococcal iron content on the killing of Staphylococcus aureus by polymorphonuclear leukocytes. Infect Immun. 1981 Apr;32(1):407–410. doi: 10.1128/iai.32.1.407-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Root R. K., Metcalf J. A. H2O2 release from human granulocytes during phagocytosis. Relationship to superoxide anion formation and cellular catabolism of H2O2: studies with normal and cytochalasin B-treated cells. J Clin Invest. 1977 Dec;60(6):1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Hydroxyl radical generation by polymorphonuclear leukocytes measured by electron spin resonance spectroscopy. J Clin Invest. 1979 Dec;64(6):1725–1729. doi: 10.1172/JCI109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]
- Sagone A. L., Jr, Husney R. M. Oxidation of salicylates by stimulated granulocytes: evidence that these drugs act as free radical scavengers in biological systems. J Immunol. 1987 Apr 1;138(7):2177–2183. [PubMed] [Google Scholar]
- Samuni A., Black C. D., Krishna C. M., Malech H. L., Bernstein E. F., Russo A. Hydroxyl radical production by stimulated neutrophils reappraised. J Biol Chem. 1988 Sep 25;263(27):13797–13801. [PubMed] [Google Scholar]
- Samuni A., Krishna C. M., Riesz P., Finkelstein E., Russo A. Superoxide reaction with nitroxide spin-adducts. Free Radic Biol Med. 1989;6(2):141–148. doi: 10.1016/0891-5849(89)90111-1. [DOI] [PubMed] [Google Scholar]
- Sriyosachati S., Cox C. D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986 Jun;52(3):885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tero-Kubota S., Ikegami Y., Kurokawa T., Sasaki R., Sugioka K., Nakano M. Generation of free radicals and initiation of radical reactions in nitrones - Fe2+ - phosphate buffer systems. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1025–1031. doi: 10.1016/0006-291x(82)92102-7. [DOI] [PubMed] [Google Scholar]
- Thomas M. J., Shirley P. S., Hedrick C. C., DeChatelet L. R. Role of free radical processes in stimulated human polymorphonuclear leukocytes. Biochemistry. 1986 Dec 2;25(24):8042–8048. doi: 10.1021/bi00372a037. [DOI] [PubMed] [Google Scholar]
- Till G. O., Hatherill J. R., Tourtellotte W. W., Lutz M. J., Ward P. A. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am J Pathol. 1985 Jun;119(3):376–384. [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Winterbourn C. C. High-affinity iron binding by xanthine oxidase. J Free Radic Biol Med. 1986;2(5-6):393–396. doi: 10.1016/s0748-5514(86)80041-1. [DOI] [PubMed] [Google Scholar]
- Walling C., Partch R. E., Weil T. Kinetics of the decomposition of hydrogen peroxide catalyzed by ferric ethylenediaminetetraacetate complex. Proc Natl Acad Sci U S A. 1975 Jan;72(1):140–142. doi: 10.1073/pnas.72.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Iverson P., Pryzwansky K. B., Spitznagel J. K., Cooney M. H. Bactericidal capacity of phorbol myristate acetate-treated human polymorphonuclear leukocytes. Infect Immun. 1978 Dec;22(3):945–955. doi: 10.1128/iai.22.3.945-955.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Carbonetti N. H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986 Mar;51(3):942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Myeloperoxidase as an effective inhibitor of hydroxyl radical production. Implications for the oxidative reactions of neutrophils. J Clin Invest. 1986 Aug;78(2):545–550. doi: 10.1172/JCI112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Radic Biol Med. 1987;3(1):33–39. doi: 10.1016/0891-5849(87)90037-2. [DOI] [PubMed] [Google Scholar]


