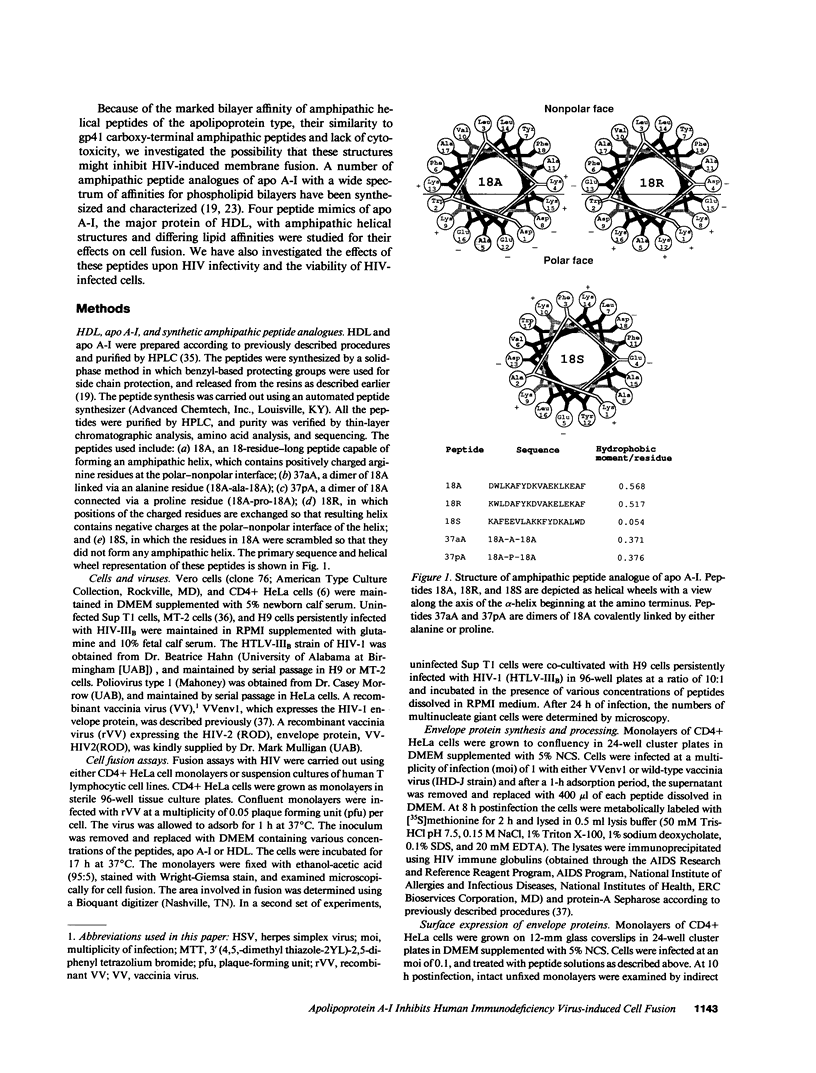
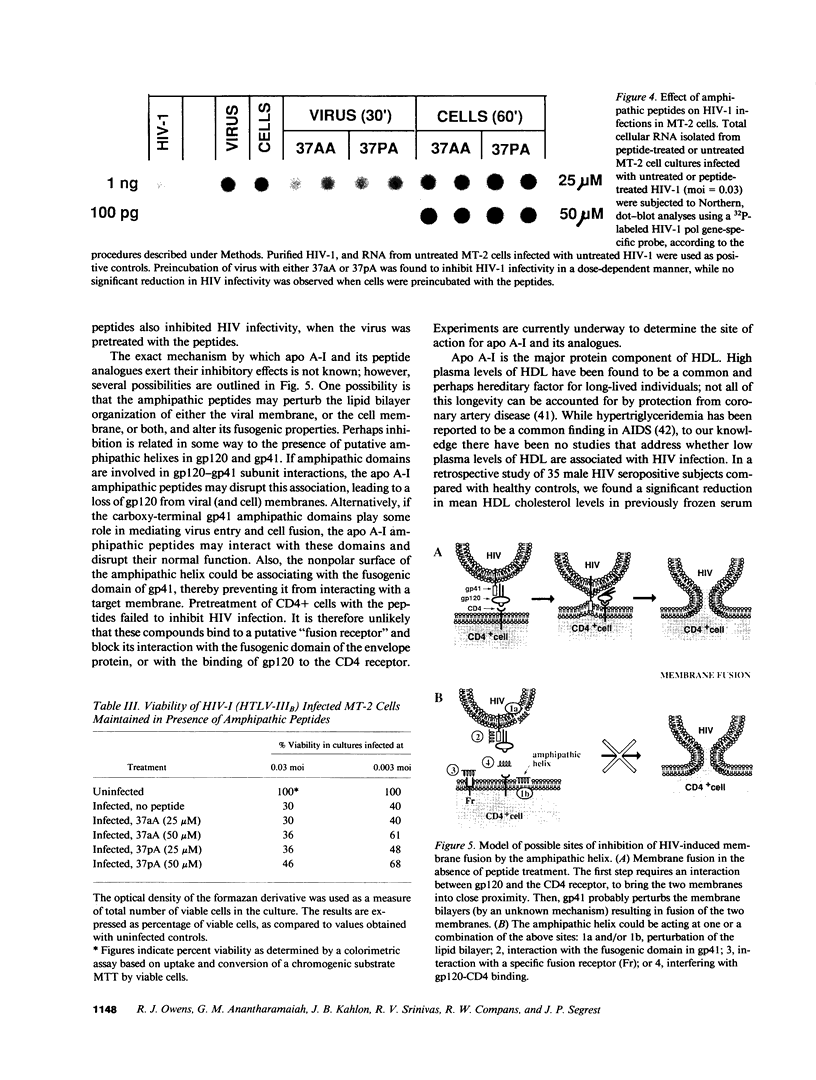
Abstract
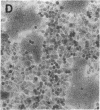
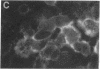
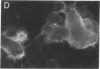
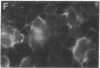
The envelope (membrane) glycoprotein of HIV is essential for virus attachment and entry into host cells. Additionally, when expressed on the plasma membrane of infected cells, the envelope protein is responsible for mediating cell-cell fusion which leads to the formation of multinucleated giant cells, one of the major cytopathic effects of HIV infections. The envelope glycoproteins of HIV contain regions that can fold into amphipathic alpha-helixes, and these regions have been suggested to play a role in subunit associations and in virus-induced cell fusion and cytopathic effects of HIV. We therefore tested the possibility that amphipathic helix-containing peptides and proteins may interfere with the HIV amphipathic peptides and inhibit those steps of HIV infection involving membrane fusion. Apolipoprotein A-I, the major protein component of high density lipoprotein, and its amphipathic peptide analogue were found to inhibit cell fusion, both in HIV-1-infected T cells and in recombinant vaccinia-virus-infected CD4+ HeLa cells expressing HIV envelope protein on their surfaces. The amphipathic peptides inhibited the infectivity of HIV-1. The inhibitory effects were manifest when the virus, but not cells, was pretreated with the peptides. Also, a reduction in HIV-induced cell killing was observed when virus-infected cell cultures were maintained in presence of amphipathic peptides. These results have potential implications for HIV biology and therapy.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharamaiah G. M., Hughes T. A., Iqbal M., Gawish A., Neame P. J., Medley M. F., Segrest J. P. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988 Mar;29(3):309–318. [PubMed] [Google Scholar]
- Anantharamaiah G. M., Jones J. L., Brouillette C. G., Schmidt C. F., Chung B. H., Hughes T. A., Bhown A. S., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem. 1985 Aug 25;260(18):10248–10255. [PubMed] [Google Scholar]
- Boman H. G., Faye I., von Hofsten P., Kockum K., Lee J. Y., Xanthopoulos K. G., Bennich H., Engström A., Merrifield R. B., Andreu D. On the primary structures of lysozyme, cecropins and attacins from Hyalophora cecropia. Dev Comp Immunol. 1985 Summer;9(3):551–558. doi: 10.1016/0145-305x(85)90018-7. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Robert-Guroff M., Wong-Staal F., Gallo R. C., Moss B. Expression of the HTLV-III envelope gene by a recombinant vaccinia virus. Nature. 1986 Apr 10;320(6062):535–537. doi: 10.1038/320535a0. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Anatharamaiah G. M., Brouillette C. G., Nishida T., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Correlation of structure with function. J Biol Chem. 1985 Aug 25;260(18):10256–10262. [PubMed] [Google Scholar]
- DeGrado W. F., Wasserman Z. R., Chowdhry V. Sequence and structural homologies among type I and type II interferons. Nature. 1982 Nov 25;300(5890):379–381. doi: 10.1038/300379a0. [DOI] [PubMed] [Google Scholar]
- Dice J. R., Rightsel W. A., Schabel F. M., Jr, McLean I. W., Jr Experiences in developing potential antiviral compounds. Ann N Y Acad Sci. 1965 Jul 30;130(1):24–30. doi: 10.1111/j.1749-6632.1965.tb12535.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Wesson M. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein 41. Biopolymers. 1990 Jan;29(1):171–177. doi: 10.1002/bip.360290122. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Epand R. F., McKenzie R. C. Effects of viral chemotherapeutic agents on membrane properties. Studies of cyclosporin A, benzyloxycarbonyl-D-Phe-L-Phe-Gly and amantadine. J Biol Chem. 1987 Feb 5;262(4):1526–1529. [PubMed] [Google Scholar]
- Epand R. M., Gawish A., Iqbal M., Gupta K. B., Chen C. H., Segrest J. P., Anantharamaiah G. M. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charge distribution, hydrophobicity, and secondary structure on lipid association and lecithin:cholesterol acyltransferase activation. J Biol Chem. 1987 Jul 5;262(19):9389–9396. [PubMed] [Google Scholar]
- Epand R. M. Virus replication inhibitory peptide inhibits the conversion of phospholipid bilayers to the hexagonal phase. Biosci Rep. 1986 Jul;6(7):647–653. doi: 10.1007/BF01114759. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Gartside P., Fallat R. W., Sielski J., Steiner P. M. Longevity syndromes: familial hypobeta and familial hyperalpha lipoproteinemia. J Lab Clin Med. 1976 Dec;88(6):941–957. [PubMed] [Google Scholar]
- Grunfeld C., Kotler D. P., Hamadeh R., Tierney A., Wang J., Pierson R. N. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989 Jan;86(1):27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- Haffar O. K., Dowbenko D. J., Berman P. W. Topogenic analysis of the human immunodeficiency virus type 1 envelope glycoprotein, gp160, in microsomal membranes. J Cell Biol. 1988 Nov;107(5):1677–1687. doi: 10.1083/jcb.107.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Kosowski S. G., Dalrymple J. M. Expression of AIDS virus envelope gene in recombinant vaccinia viruses. Nature. 1986 Apr 10;320(6062):537–540. doi: 10.1038/320537a0. [DOI] [PubMed] [Google Scholar]
- Kanellis P., Romans A. Y., Johnson B. J., Kercret H., Chiovetti R., Jr, Allen T. M., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Effect of charged amino acid residue topography on lipid affinity. J Biol Chem. 1980 Dec 10;255(23):11464–11472. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Miller F. A., Dixon G. J., Arnett G., Dice J. R., Rightsel W. A., Schabel F. M., Jr, McLean I. W., Jr Antiviral activity of carbobenzosy di- and tripeptides on measles virus. Appl Microbiol. 1968 Oct;16(10):1489–1496. doi: 10.1128/am.16.10.1489-1496.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides E., DeWald H., Lipnik M., Westland R., Posler J. Potential antiviral agents. Carbobenzoxy di- and tripeptides active against measles and herpes viruses. J Med Chem. 1968 Jan;11(1):74–79. doi: 10.1021/jm00307a016. [DOI] [PubMed] [Google Scholar]
- Norrby E. The effect of a carbobenzoxy tripeptide on the biological activities of measles virus. Virology. 1971 Jun;44(3):599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- Owens R. J., Compans R. W. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J Virol. 1989 Feb;63(2):978–982. doi: 10.1128/jvi.63.2.978-982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Choppin P. W. Oligopeptides that specifically inhibit membrane fusion by paramyxoviruses: studies on the site of action. Virology. 1983 Dec;131(2):518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. D., Scheid A., Choppin P. W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology. 1980 Aug;105(1):205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Sparrow J. T., Gotto A. M., Jr Apolipoprotein/lipid interactions: studies with synthetic polypeptides. CRC Crit Rev Biochem. 1982;13(1):87–107. doi: 10.3109/10409238209108710. [DOI] [PubMed] [Google Scholar]
- Srinivas R. V., Birkedal B., Owens R. J., Anantharamaiah G. M., Segrest J. P., Compans R. W. Antiviral effects of apolipoprotein A-I and its synthetic amphipathic peptide analogs. Virology. 1990 May;176(1):48–57. doi: 10.1016/0042-6822(90)90229-k. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Kaiser E. T. The structural characterization of beta-endorphin and related peptide hormones and neurotransmitters. Pharmacol Rev. 1986 Dec;38(4):291–319. [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]