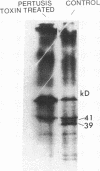
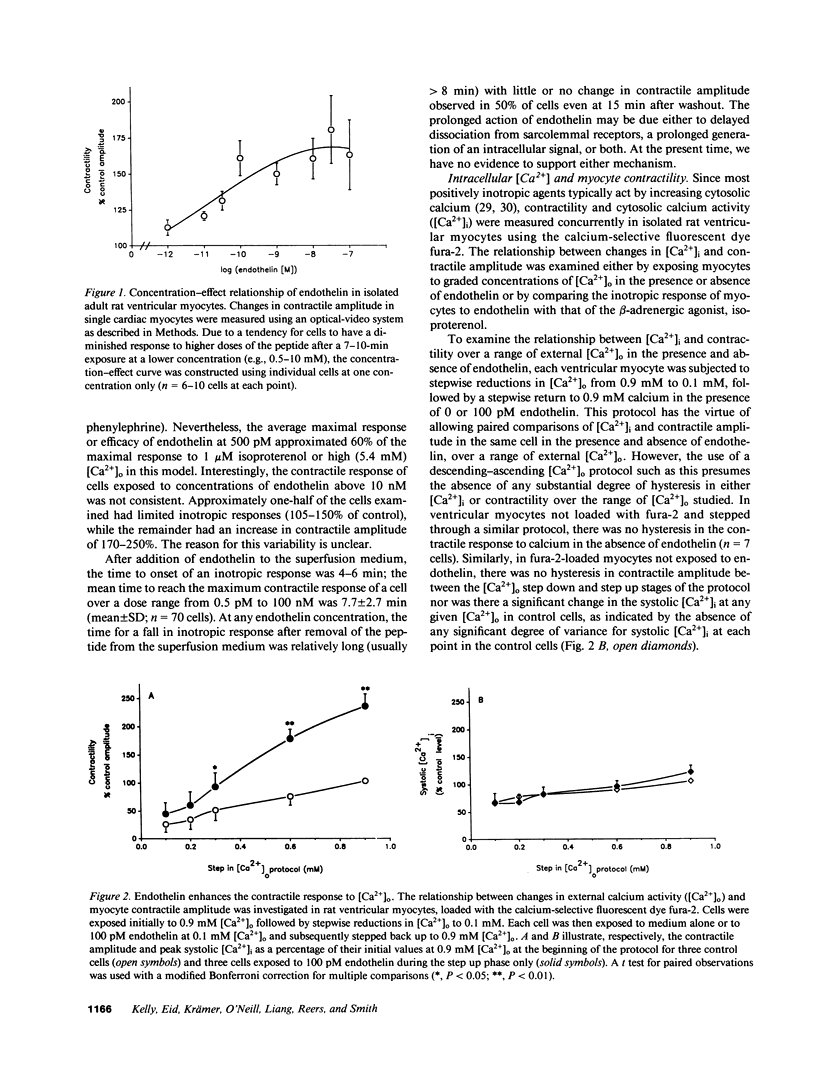
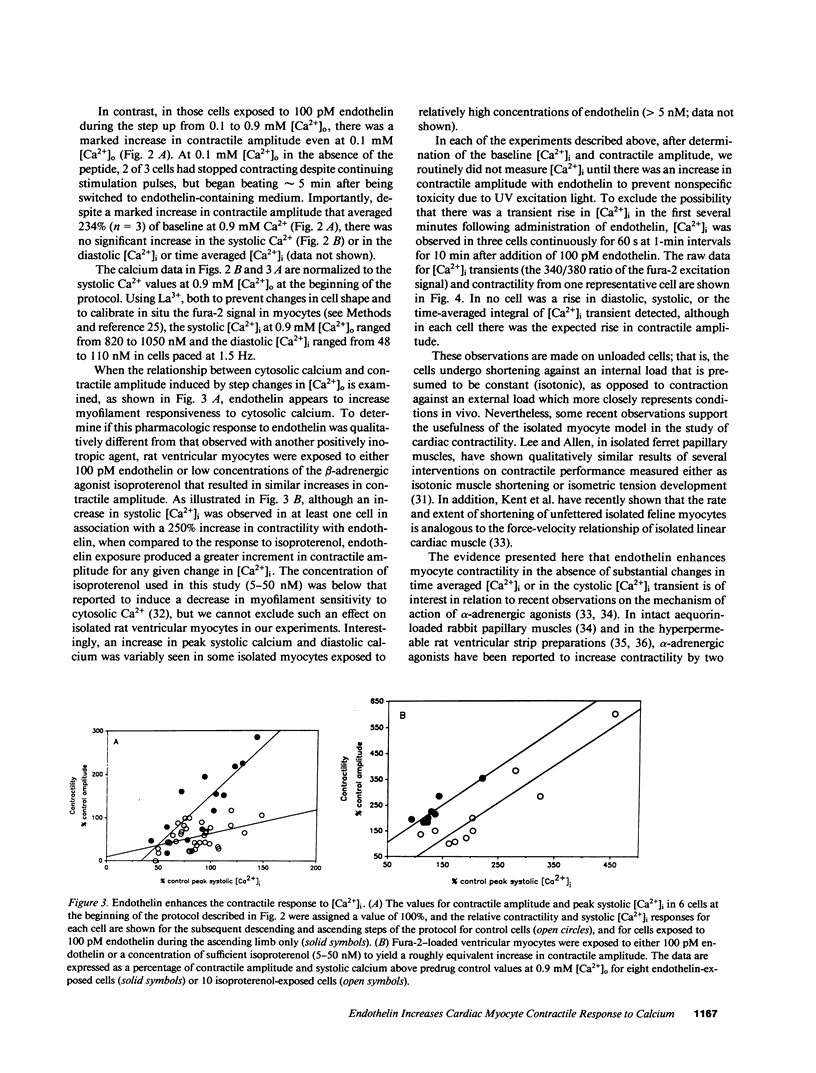
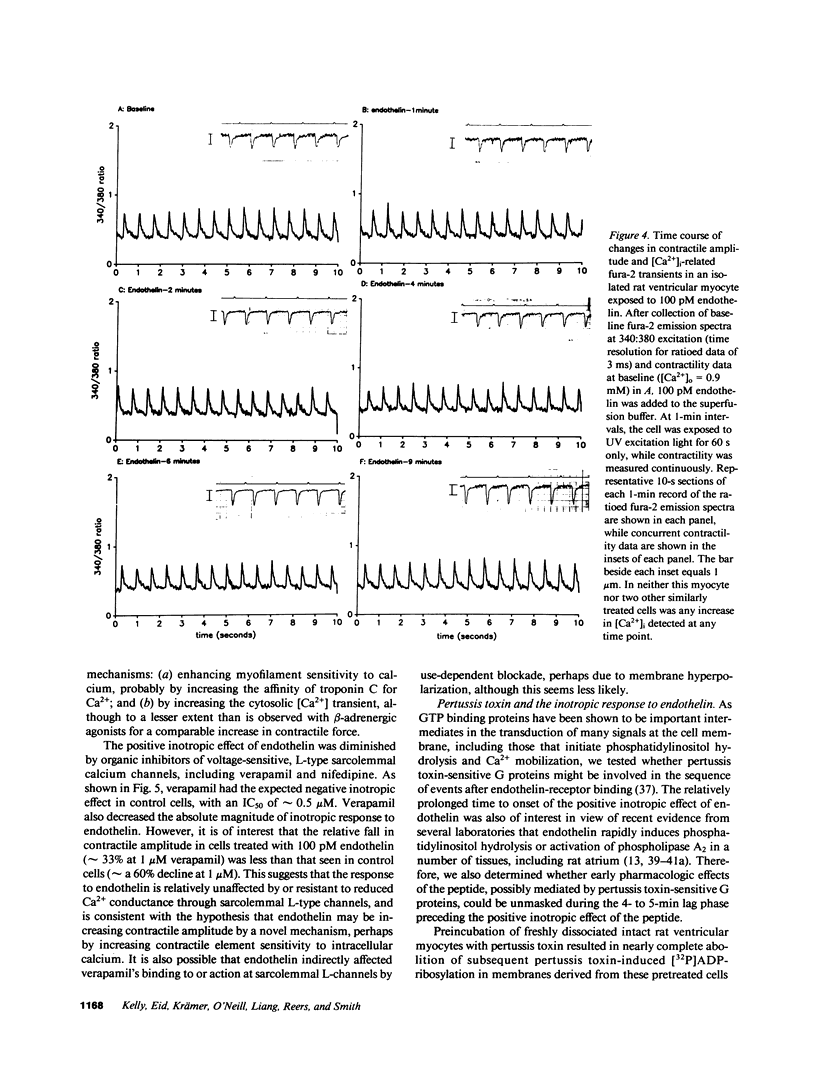
Abstract
It has long been assumed that the primary influences regulating cardiac contractility are the extent of mechanical loading of muscle fibers and the activity of the autonomic nervous system. However, the vasoactive peptide endothelin, initially found in vascular endothelium, is among the most potent positively inotropic agents yet described in mammalian myocardium. In isolated adult rat ventricular cells, endothelin's action was slow in onset but very long lasting with an EC50 of 50 pM that approximates the reported KD of the peptide for its receptor in rat heart. When the calcium activity of the buffer superfusing isolated single fura-2-loaded myocytes paced at 1.5 Hz was varied from 0.1 to 0.9 mM [Ca2+]o, 100 pM endothelin increased contractile amplitude with no significant change in diastolic or systolic [Ca2+]i, thus appearing to sensitize the myofilaments to intracellular calcium. Pertussis toxin, or prior exposure to a beta-adrenergic agonist, reduced or abolished the increase in myocyte contractility induced by endothelin. This novel and potent pharmacologic action of endothelin points to the potential importance of local, paracrine factors, perhaps derived from microvascular endothelium or endocardium, in the control of the contractile function of the heart.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banga H. S., Walker R. K., Winberry L. K., Rittenhouse S. E. Pertussis toxin can activate human platelets. Comparative effects of holotoxin and its ADP-ribosylating S1 subunit. J Biol Chem. 1987 Nov 5;262(31):14871–14874. [PubMed] [Google Scholar]
- Brutsaert D. L., Meulemans A. L., Sipido K. R., Sys S. U. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circ Res. 1988 Feb;62(2):358–366. doi: 10.1161/01.res.62.2.358. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Constantine J. M., Bonventre J. V. Cytosolic free calcium concentration and glucose transport in isolated cardiac myocytes. Am J Physiol. 1987 Feb;252(2 Pt 1):C163–C172. doi: 10.1152/ajpcell.1987.252.2.C163. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Blinks J. R. Actions of sympathomimetic amines on the Ca2+ transients and contractions of rabbit myocardium: reciprocal changes in myofibrillar responsiveness to Ca2+ mediated through alpha- and beta-adrenoceptors. Circ Res. 1988 Feb;62(2):247–265. doi: 10.1161/01.res.62.2.247. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Hirata Y., Yoshimi H., Kojima T., Kobayashi Y., Yanagisawa M., Masaki T. Endothelin is a potent secretagogue for atrial natriuretic peptide in cultured rat atrial myocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):167–172. doi: 10.1016/s0006-291x(88)81064-7. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Galron R., Kloog Y., Bdolah A., Sokolovsky M. Functional endothelin/sarafotoxin receptors in rat heart myocytes: structure-activity relationships and receptor subtypes. Biochem Biophys Res Commun. 1989 Sep 15;163(2):936–943. doi: 10.1016/0006-291x(89)92312-7. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. The isolation of Ca2+-resistant myocytes from the adult rat. J Mol Cell Cardiol. 1980 Jul;12(7):715–723. doi: 10.1016/0022-2828(80)90101-7. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Fukuda Y., Yoshimi H., Emori T., Shichiri M., Marumo F. Specific receptor for endothelin in cultured rat cardiocytes. Biochem Biophys Res Commun. 1989 May 15;160(3):1438–1444. doi: 10.1016/s0006-291x(89)80165-2. [DOI] [PubMed] [Google Scholar]
- Hu J. R., Berninger U. G., Lang R. E. Endothelin stimulates atrial natriuretic peptide (ANP) release from rat atria. Eur J Pharmacol. 1988 Dec 6;158(1-2):177–178. doi: 10.1016/0014-2999(88)90271-3. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Yanagisawa M., Kimura S., Goto K., Masaki T. Positive chronotropic effects of endothelin, a novel endothelium-derived vasoconstrictor peptide. Pflugers Arch. 1988 Nov;413(1):108–110. doi: 10.1007/BF00581239. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Yanagisawa M., Kimura S., Goto K., Masaki T. Positive inotropic action of novel vasoconstrictor peptide endothelin on guinea pig atria. Am J Physiol. 1988 Oct;255(4 Pt 2):H970–H973. doi: 10.1152/ajpheart.1988.255.4.H970. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Kent R. L., Mann D. L., Urabe Y., Hisano R., Hewett K. W., Loughnane M., Cooper G., 4th Contractile function of isolated feline cardiocytes in response to viscous loading. Am J Physiol. 1989 Nov;257(5 Pt 2):H1717–H1727. doi: 10.1152/ajpheart.1989.257.5.H1717. [DOI] [PubMed] [Google Scholar]
- Kitayoshi T., Watanabe T., Shimamoto N. Cardiovascular effects of endothelin in dogs: positive inotropic action in vivo. Eur J Pharmacol. 1989 Aug 3;166(3):519–522. doi: 10.1016/0014-2999(89)90367-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leatherman G. F., Kim D., Smith T. W. Effect of phorbol esters on contractile state and calcium flux in cultured chick heart cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H205–H209. doi: 10.1152/ajpheart.1987.253.1.H205. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Comparison of the effects of inotropic interventions on isometric tension and shortening in isolated ferret ventricular muscle. Cardiovasc Res. 1989 Sep;23(9):748–755. doi: 10.1093/cvr/23.9.748. [DOI] [PubMed] [Google Scholar]
- Lew R. A., Baertschi A. J. Endothelial cells stimulate ANF secretion from atrial myocytes in co-culture. Biochem Biophys Res Commun. 1989 Sep 15;163(2):701–709. doi: 10.1016/0006-291x(89)92280-8. [DOI] [PubMed] [Google Scholar]
- Liang B. T., Hellmich M. R., Neer E. J., Galper J. B. Development of muscarinic cholinergic inhibition of adenylate cyclase in embryonic chick heart. Its relationship to changes in the inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1986 Jul 5;261(19):9011–9021. [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- McClellan G. B., Winegrad S. The regulation of the calcium sensitivity of the contractile system in mammalian cardiac muscle. J Gen Physiol. 1978 Dec;72(6):737–764. doi: 10.1085/jgp.72.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moravec C. S., Reynolds E. E., Stewart R. W., Bond M. Endothelin is a positive inotropic agent in human and rat heart in vitro. Biochem Biophys Res Commun. 1989 Feb 28;159(1):14–18. doi: 10.1016/0006-291x(89)92397-8. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Blinks J. R. Intracellular Ca2+ transients in the cat papillary muscle. Can J Physiol Pharmacol. 1982 Apr;60(4):524–528. doi: 10.1139/y82-072. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Power R. F., Wharton J., Zhao Y., Bloom S. R., Polak J. M. Autoradiographic localization of endothelin-1 binding sites in the cardiovascular and respiratory systems. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S50–S74. doi: 10.1097/00005344-198900135-00013. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Activation of phospholipase A2 by endothelin in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):279–286. doi: 10.1016/s0006-291x(89)80209-8. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Lewis M. J., Henderson A. H. Inotropic effects of endothelin in ferret ventricular myocardium. Eur J Pharmacol. 1989 Apr 25;163(2-3):365–367. doi: 10.1016/0014-2999(89)90208-2. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Meulemans A. L., Brutsaert D. L. Myocardial inotropic responses to aggregating platelets and modulation by the endocardium. Circulation. 1989 Jun;79(6):1315–1323. doi: 10.1161/01.cir.79.6.1315. [DOI] [PubMed] [Google Scholar]
- Shattock M. J., Bers D. M. Rat vs. rabbit ventricle: Ca flux and intracellular Na assessed by ion-selective microelectrodes. Am J Physiol. 1989 Apr;256(4 Pt 1):C813–C822. doi: 10.1152/ajpcell.1989.256.4.C813. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch J. P., Hirth-Dietrich C., Kazda S., Neuser D. Endothelin stimulates release of atrial natriuretic peptides in vitro and in vivo. Life Sci. 1989;45(10):869–875. doi: 10.1016/0024-3205(89)90200-2. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Vigne P., Lazdunski M., Frelin C. The inotropic effect of endothelin-1 on rat atria involves hydrolysis of phosphatidylinositol. FEBS Lett. 1989 Jun 5;249(2):143–146. doi: 10.1016/0014-5793(89)80611-8. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Miyazaki H., Kondoh M., Masuda Y., Kimura S., Yanagisawa M., Masaki T., Murakami K. Two distinct types of endothelin receptors are present on chick cardiac membranes. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1252–1259. doi: 10.1016/0006-291x(89)91377-6. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kusumoto K., Kitayoshi T., Shimamoto N. Positive inotropic and vasoconstrictive effects of endothelin-1 in in vivo and in vitro experiments: characteristics and the role of L-type calcium channels. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S108–s123. doi: 10.1097/00005344-198900135-00027. [DOI] [PubMed] [Google Scholar]
- Winegrad S., McClellan G., Weisberg A., Lin L. E., Weindling S., Horowits R. Beta-adrenergic regulation of cardiac myosin. Can J Physiol Pharmacol. 1987 Apr;65(4):606–609. doi: 10.1139/y87-102. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]