Abstract
In the crystal structure of the title salt, C6H6NO2 +·C6HCl2O4 −, the pyridine ring and the mean plane of the hydrogen chloranilate anion form a dihedral angle of 77.40 (8)°. The ionic components are held together by N—H⋯O and O—H⋯O hydrogen bonds, forming a supramolecular ladder. C—H⋯O interactions are also present.
Related literature
For the structures of related carboxypyridinium hydrogen chloranilates, see: Gotoh et al. (2006 ▶); Tabuchi et al. (2005 ▶); Ishida (2009 ▶).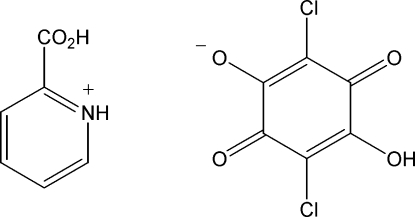
Experimental
Crystal data
C6H6NO2 +·C6HCl2O4 −
M r = 332.10
Monoclinic,
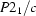
a = 9.4166 (8) Å
b = 19.6900 (16) Å
c = 6.7089 (6) Å
β = 99.043 (3)°
V = 1228.45 (18) Å3
Z = 4
Mo Kα radiation
μ = 0.56 mm−1
T = 103 K
0.30 × 0.30 × 0.23 mm
Data collection
Rigaku R-AXIS RAPID-II diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.847, T max = 0.880
9710 measured reflections
3433 independent reflections
2228 reflections with I > 2σ(I)
R int = 0.047
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.144
S = 1.10
3433 reflections
202 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.47 e Å−3
Δρmin = −0.92 e Å−3
Data collection: PROCESS-AUTO (Rigaku/MSC, 2004 ▶); cell refinement: PROCESS-AUTO; data reduction: CrystalStructure (Rigaku/MSC, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: CrystalStructure and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809006412/tk2376sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809006412/tk2376Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O1 | 0.92 (4) | 2.11 (3) | 2.932 (2) | 147 (3) |
| O2—H2⋯O5i | 0.79 (3) | 2.05 (3) | 2.746 (2) | 148 (3) |
| O6—H6⋯O4ii | 0.90 (3) | 1.63 (3) | 2.528 (2) | 177.1 (15) |
| C8—H8⋯O4iii | 0.95 | 2.50 | 3.338 (3) | 147 |
| C9—H9⋯O3iv | 0.95 | 2.33 | 3.227 (3) | 156 |
| C11—H11⋯O1v | 0.95 | 2.46 | 3.374 (3) | 162 |
Symmetry codes: (i) 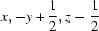 ; (ii)
; (ii) 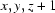 ; (iii)
; (iii) 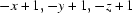 ; (iv)
; (iv) 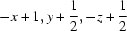 ; (v)
; (v) 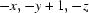 .
.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 19550018) from the Japan Society for the Promotion of Science.
supplementary crystallographic information
Comment
The title salt, (I), was prepared in order to extend our study on D—H···A hydrogen bonding (D = N, O or C; A = N, O or Cl) in chloranilic acid – substituted-pyridine systems (Gotoh et al., 2006; Tabuchi et al., 2005).
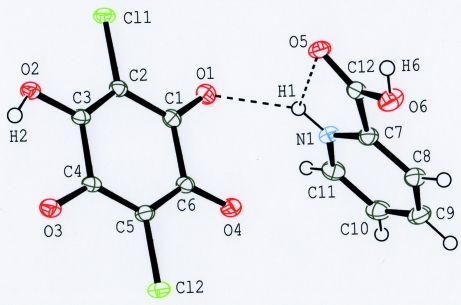
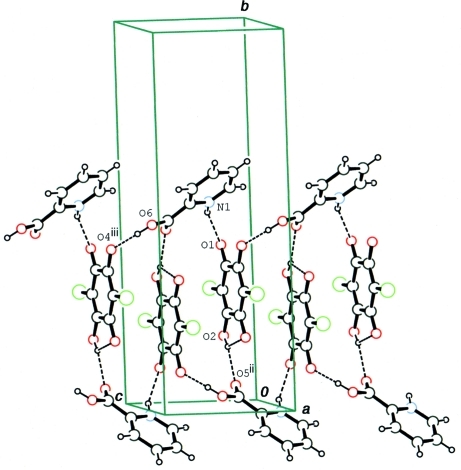
Compound (I) comprises 2-carboxypyridinium cations and hydrogen chloranilate anions in the ratio 1:1. Ions directly connected by an N—H···O hydrogen bond, Fig. 1, form a dihedral angle between their respective mean planes of 77.40 (8)°. In the cation, the carboxy O5/O6/C12 plane forms a dihedral angle of 11.44 (6)° with the pyridine ring, which is similar to those of 2.74 (6) and 10.01 (3)° observed in 3-carboxypyridinium hydrogen chloranilate and 4-carboxypyridinium hydrogen chloranilate monohydrate, respectively (Ishida, 2009). The ions are further connected by O—H···O hydrogen bonds (Table 1) to afford a supramolecular ladder running along the c axis (Fig. 2). The ladders are linked by weaker N—H···O hydrogen bonds and C—H···O contacts to form a 3-D network (Table 1).
Experimental
Crystals were obtained by slow evaporation from a methanol solution (ca 30 ml) containing a 1:1 molar ratio of chloranilic acid (0.302 g) and picolinic acid (0.179 g).
Refinement
The H atoms attached to O and N were located from a difference Fourier map and refined isotropically to O—H = 0.79 (3) & 0.90 (3) Å and N—H = 0.92 (4) Å. The remaining H atoms were included in the riding approximation with C—H = 0.95 Å, and with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
Molecular components of (I) showing the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level. Intra- and inter-molecular N—H···O hydrogen bonds are indicated by dashed lines.
Fig. 2.
A partial packing diagram, viewed approximately along the a axis, showing the hydrogen-bonded supramolecular ladder. Dashed lines show N—H···O and O—H···O hydrogen bonds (symmetry codes as given in Table 1).
Crystal data
| C6H6NO2+·C6HCl2O4− | F(000) = 672.00 |
| Mr = 332.10 | Dx = 1.795 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71075 Å |
| Hall symbol: -P 2ybc | Cell parameters from 7392 reflections |
| a = 9.4166 (8) Å | θ = 3.0–30.0° |
| b = 19.6900 (16) Å | µ = 0.56 mm−1 |
| c = 6.7089 (6) Å | T = 103 K |
| β = 99.043 (3)° | Platelet, dark purple |
| V = 1228.45 (18) Å3 | 0.30 × 0.30 × 0.23 mm |
| Z = 4 |
Data collection
| Rigaku R-AXIS RAPID-II diffractometer | 2228 reflections with I > 2σ(I) |
| Detector resolution: 10.00 pixels mm-1 | Rint = 0.047 |
| ω scans | θmax = 30.0° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −13→13 |
| Tmin = 0.847, Tmax = 0.880 | k = −27→27 |
| 9710 measured reflections | l = −8→9 |
| 3433 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.144 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0659P)2 + 0.8975P] where P = (Fo2 + 2Fc2)/3 |
| 3433 reflections | (Δ/σ)max < 0.001 |
| 202 parameters | Δρmax = 0.47 e Å−3 |
| 0 restraints | Δρmin = −0.92 e Å−3 |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | −0.17894 (6) | 0.28996 (3) | 0.27912 (9) | 0.02060 (16) | |
| Cl2 | 0.46528 (6) | 0.29140 (3) | 0.10812 (9) | 0.02077 (16) | |
| O1 | 0.00723 (19) | 0.40867 (8) | 0.2367 (3) | 0.0218 (4) | |
| O2 | 0.01147 (19) | 0.17042 (9) | 0.2397 (3) | 0.0184 (3) | |
| O3 | 0.27117 (18) | 0.17271 (8) | 0.1488 (3) | 0.0196 (4) | |
| O4 | 0.27772 (19) | 0.41277 (8) | 0.1577 (3) | 0.0209 (4) | |
| O5 | 0.12383 (18) | 0.45615 (9) | 0.6917 (3) | 0.0215 (4) | |
| O6 | 0.3466 (2) | 0.47658 (10) | 0.8601 (3) | 0.0265 (4) | |
| N1 | 0.1860 (2) | 0.52690 (11) | 0.3720 (3) | 0.0199 (4) | |
| C1 | 0.0667 (2) | 0.35416 (11) | 0.2174 (3) | 0.0164 (4) | |
| C2 | −0.0036 (2) | 0.28950 (11) | 0.2347 (3) | 0.0160 (4) | |
| C3 | 0.0678 (2) | 0.23079 (11) | 0.2188 (3) | 0.0154 (4) | |
| C4 | 0.2206 (2) | 0.22965 (11) | 0.1715 (3) | 0.0155 (4) | |
| C5 | 0.2899 (2) | 0.29272 (11) | 0.1544 (4) | 0.0170 (4) | |
| C6 | 0.2232 (2) | 0.35480 (12) | 0.1732 (3) | 0.0165 (4) | |
| C7 | 0.2794 (3) | 0.52837 (12) | 0.5464 (4) | 0.0198 (5) | |
| C8 | 0.3984 (3) | 0.56970 (13) | 0.5626 (4) | 0.0229 (5) | |
| H8 | 0.4656 | 0.5708 | 0.6840 | 0.027* | |
| C9 | 0.4194 (3) | 0.60996 (13) | 0.3987 (4) | 0.0262 (5) | |
| H9 | 0.5010 | 0.6388 | 0.4077 | 0.031* | |
| C10 | 0.3206 (3) | 0.60759 (13) | 0.2230 (4) | 0.0268 (5) | |
| H10 | 0.3332 | 0.6352 | 0.1109 | 0.032* | |
| C11 | 0.2035 (3) | 0.56488 (13) | 0.2118 (4) | 0.0239 (5) | |
| H11 | 0.1357 | 0.5624 | 0.0913 | 0.029* | |
| C12 | 0.2418 (3) | 0.48289 (12) | 0.7090 (4) | 0.0195 (5) | |
| H1 | 0.109 (5) | 0.498 (2) | 0.363 (6) | 0.053 (11)* | |
| H2 | 0.071 (4) | 0.1437 (17) | 0.227 (5) | 0.032 (9)* | |
| H6 | 0.319 (4) | 0.4534 (18) | 0.964 (6) | 0.043 (10)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0146 (3) | 0.0253 (3) | 0.0233 (3) | 0.0013 (2) | 0.0076 (2) | −0.0006 (2) |
| Cl2 | 0.0151 (3) | 0.0258 (3) | 0.0230 (3) | −0.0018 (2) | 0.0080 (2) | −0.0011 (2) |
| O1 | 0.0207 (8) | 0.0196 (8) | 0.0256 (9) | 0.0020 (7) | 0.0051 (7) | −0.0022 (7) |
| O2 | 0.0163 (8) | 0.0164 (7) | 0.0238 (9) | 0.0013 (7) | 0.0074 (7) | −0.0008 (7) |
| O3 | 0.0176 (8) | 0.0201 (8) | 0.0220 (8) | 0.0016 (7) | 0.0063 (7) | −0.0008 (7) |
| O4 | 0.0232 (9) | 0.0200 (8) | 0.0210 (9) | −0.0027 (7) | 0.0083 (7) | 0.0019 (6) |
| O5 | 0.0177 (8) | 0.0212 (8) | 0.0274 (9) | −0.0017 (7) | 0.0094 (7) | 0.0018 (7) |
| O6 | 0.0221 (9) | 0.0336 (10) | 0.0235 (9) | −0.0063 (8) | 0.0024 (7) | 0.0050 (8) |
| N1 | 0.0145 (9) | 0.0229 (10) | 0.0229 (10) | −0.0018 (8) | 0.0050 (8) | 0.0000 (8) |
| C1 | 0.0182 (10) | 0.0180 (10) | 0.0130 (10) | 0.0007 (9) | 0.0020 (8) | 0.0001 (8) |
| C2 | 0.0130 (10) | 0.0197 (10) | 0.0152 (10) | 0.0009 (8) | 0.0023 (8) | −0.0019 (8) |
| C3 | 0.0154 (10) | 0.0183 (10) | 0.0132 (10) | −0.0002 (8) | 0.0048 (8) | 0.0005 (8) |
| C4 | 0.0149 (10) | 0.0193 (10) | 0.0132 (10) | 0.0009 (8) | 0.0048 (8) | 0.0003 (8) |
| C5 | 0.0132 (10) | 0.0207 (10) | 0.0176 (10) | −0.0013 (9) | 0.0041 (8) | −0.0006 (9) |
| C6 | 0.0165 (10) | 0.0204 (10) | 0.0133 (10) | −0.0014 (9) | 0.0039 (8) | −0.0011 (8) |
| C7 | 0.0179 (11) | 0.0184 (11) | 0.0241 (12) | 0.0001 (9) | 0.0067 (9) | −0.0006 (9) |
| C8 | 0.0188 (11) | 0.0236 (12) | 0.0272 (13) | 0.0000 (10) | 0.0066 (10) | −0.0018 (10) |
| C9 | 0.0223 (12) | 0.0231 (12) | 0.0355 (14) | −0.0031 (10) | 0.0118 (11) | 0.0023 (11) |
| C10 | 0.0292 (13) | 0.0248 (12) | 0.0291 (13) | 0.0006 (11) | 0.0129 (11) | 0.0049 (10) |
| C11 | 0.0229 (12) | 0.0270 (12) | 0.0221 (12) | 0.0049 (10) | 0.0051 (10) | 0.0028 (10) |
| C12 | 0.0210 (11) | 0.0193 (10) | 0.0197 (11) | −0.0002 (9) | 0.0076 (9) | −0.0021 (9) |
Geometric parameters (Å, °)
| Cl1—C2 | 1.723 (2) | C1—C6 | 1.548 (3) |
| Cl2—C5 | 1.727 (2) | C2—C3 | 1.350 (3) |
| O1—C1 | 1.227 (3) | C3—C4 | 1.521 (3) |
| O2—C3 | 1.318 (3) | C4—C5 | 1.416 (3) |
| O2—H2 | 0.79 (3) | C5—C6 | 1.389 (3) |
| O3—C4 | 1.237 (3) | C7—C8 | 1.375 (3) |
| O4—C6 | 1.263 (3) | C7—C12 | 1.497 (3) |
| O5—C12 | 1.218 (3) | C8—C9 | 1.394 (4) |
| O6—C12 | 1.305 (3) | C8—H8 | 0.9500 |
| O6—H6 | 0.90 (4) | C9—C10 | 1.383 (4) |
| N1—C11 | 1.340 (3) | C9—H9 | 0.9500 |
| N1—C7 | 1.349 (3) | C10—C11 | 1.379 (4) |
| N1—H1 | 0.92 (4) | C10—H10 | 0.9500 |
| C1—C2 | 1.448 (3) | C11—H11 | 0.9500 |
| C3—O2—H2 | 107 (3) | O4—C6—C1 | 115.8 (2) |
| C12—O6—H6 | 112 (2) | C5—C6—C1 | 117.90 (19) |
| C11—N1—C7 | 122.5 (2) | N1—C7—C8 | 119.6 (2) |
| C11—N1—H1 | 119 (2) | N1—C7—C12 | 115.0 (2) |
| C7—N1—H1 | 118 (2) | C8—C7—C12 | 125.4 (2) |
| O1—C1—C2 | 122.6 (2) | C7—C8—C9 | 119.3 (2) |
| O1—C1—C6 | 118.5 (2) | C7—C8—H8 | 120.4 |
| C2—C1—C6 | 118.91 (19) | C9—C8—H8 | 120.4 |
| C3—C2—C1 | 120.4 (2) | C10—C9—C8 | 119.5 (2) |
| C3—C2—Cl1 | 121.40 (18) | C10—C9—H9 | 120.2 |
| C1—C2—Cl1 | 118.15 (17) | C8—C9—H9 | 120.2 |
| O2—C3—C2 | 123.3 (2) | C11—C10—C9 | 119.5 (2) |
| O2—C3—C4 | 114.75 (19) | C11—C10—H10 | 120.3 |
| C2—C3—C4 | 121.9 (2) | C9—C10—H10 | 120.3 |
| O3—C4—C5 | 126.4 (2) | N1—C11—C10 | 119.7 (2) |
| O3—C4—C3 | 115.7 (2) | N1—C11—H11 | 120.2 |
| C5—C4—C3 | 117.85 (19) | C10—C11—H11 | 120.2 |
| C6—C5—C4 | 122.9 (2) | O5—C12—O6 | 126.9 (2) |
| C6—C5—Cl2 | 119.23 (17) | O5—C12—C7 | 120.4 (2) |
| C4—C5—Cl2 | 117.86 (17) | O6—C12—C7 | 112.7 (2) |
| O4—C6—C5 | 126.3 (2) | ||
| O1—C1—C2—C3 | −177.9 (2) | C4—C5—C6—C1 | 0.7 (3) |
| C6—C1—C2—C3 | 2.1 (3) | Cl2—C5—C6—C1 | −179.21 (16) |
| O1—C1—C2—Cl1 | 1.3 (3) | O1—C1—C6—O4 | −0.9 (3) |
| C6—C1—C2—Cl1 | −178.77 (16) | C2—C1—C6—O4 | 179.2 (2) |
| C1—C2—C3—O2 | 177.7 (2) | O1—C1—C6—C5 | 179.2 (2) |
| Cl1—C2—C3—O2 | −1.4 (3) | C2—C1—C6—C5 | −0.7 (3) |
| C1—C2—C3—C4 | −3.3 (3) | C11—N1—C7—C8 | −0.6 (4) |
| Cl1—C2—C3—C4 | 177.58 (16) | C11—N1—C7—C12 | 179.6 (2) |
| O2—C3—C4—O3 | 3.3 (3) | N1—C7—C8—C9 | 0.7 (4) |
| C2—C3—C4—O3 | −175.8 (2) | C12—C7—C8—C9 | −179.5 (2) |
| O2—C3—C4—C5 | −177.73 (19) | C7—C8—C9—C10 | −0.1 (4) |
| C2—C3—C4—C5 | 3.2 (3) | C8—C9—C10—C11 | −0.7 (4) |
| O3—C4—C5—C6 | 177.0 (2) | C7—N1—C11—C10 | −0.2 (4) |
| C3—C4—C5—C6 | −1.8 (3) | C9—C10—C11—N1 | 0.8 (4) |
| O3—C4—C5—Cl2 | −3.0 (3) | N1—C7—C12—O5 | −11.4 (3) |
| C3—C4—C5—Cl2 | 178.11 (16) | C8—C7—C12—O5 | 168.8 (2) |
| C4—C5—C6—O4 | −179.2 (2) | N1—C7—C12—O6 | 168.4 (2) |
| Cl2—C5—C6—O4 | 0.8 (3) | C8—C7—C12—O6 | −11.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O1 | 0.92 (4) | 2.11 (3) | 2.932 (2) | 147 (3) |
| N1—H1···O5 | 0.92 (4) | 2.33 (4) | 2.698 (2) | 103 (2) |
| N1—H1···O5i | 0.92 (4) | 2.34 (4) | 2.900 (2) | 119 (3) |
| O2—H2···O3 | 0.79 (3) | 2.11 (3) | 2.612 (2) | 122 (3) |
| O2—H2···O5ii | 0.79 (3) | 2.05 (3) | 2.746 (2) | 148 (3) |
| O6—H6···O4iii | 0.90 (3) | 1.63 (3) | 2.528 (2) | 177.1 (15) |
| C8—H8···O4iv | 0.95 | 2.50 | 3.338 (3) | 147 |
| C9—H9···O3v | 0.95 | 2.33 | 3.227 (3) | 156 |
| C11—H11···O1vi | 0.95 | 2.46 | 3.374 (3) | 162 |
Symmetry codes: (i) −x, −y+1, −z+1; (ii) x, −y+1/2, z−1/2; (iii) x, y, z+1; (iv) −x+1, −y+1, −z+1; (v) −x+1, y+1/2, −z+1/2; (vi) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: TK2376).
References
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Gotoh, K., Tabuchi, Y., Akashi, H. & Ishida, H. (2006). Acta Cryst. E62, o4420–o4421.
- Higashi, T. (1995). ABSCOR Rigaku Corporation, Tokyo, Japan.
- Ishida, H. (2009). Private communication (deposition numbers CCDC 720198 and CCDC 720199). CCDC, Cambridge, England.
- Rigaku/MSC (2004). PROCESS-AUTO and CrystalStructure Rigaku/MSC Inc., The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Tabuchi, Y., Takahashi, A., Gotoh, K., Akashi, H. & Ishida, H. (2005). Acta Cryst. E61, o4215–o4217.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809006412/tk2376sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809006412/tk2376Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report